Abstract
The adverse effects of iron overload have raised more concerns as a growing number of studies reported its association with immune disorders. This study aimed to investigate alterations in the immune system by iron overload in patients with myelodysplastic syndrome (MDS) and an iron-overloaded mouse model. The peripheral blood from patients was harvested to test the effect of iron overload on the subsets of T lymphocytes, and the level of reactive oxygen species (ROS) was also evaluated. The data showed that iron-overloaded patients had a lower percentage of CD3+ T cells and disrupted T cell subsets, concomitant with higher ROS level in lymphocytes. In order to explore the mechanism, male C57Bl/6 mice were intraperitoneally injected with iron dextran at a dose of 250 mg/kg every 3 days for 4 weeks to establish an iron-overloaded mouse model and the blood of each mouse was collected for the analysis of the T lymphocyte subsets and T cell apoptosis. The results showed that iron overload could reduce the percentage of CD3+ T cells and the ratio of Th1/Th2 and Tc1/Tc2 but increase the percentage of regulatory T (Treg) cells and the ratio of CD4/CD8. We also found that iron overload induced the apoptosis of T lymphocytes and increased its ROS level. Furthermore, these effects could be partially recovered after treating with antioxidant N-acetyl-l-cysteine (NAC) or iron chelator deferasirox (DFX). Taken together, these observations indicated that iron overload could selectively affect peripheral T lymphocytes and induce an impaired cellular immunity by increasing ROS level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron is an important component of hemoglobin. It plays an important role in the body’s oxygen transport, DNA synthesis, respiration, and cell metabolism [1, 2]. However, a variety of factors such as increased intestinal iron absorption or repeated blood transfusions can lead to an excessive amount of body iron deposits, causing parenchymal organ dysfunction [3–5]. Previous studies have shown that iron overload can directly damage hematopoietic stem/progenitor cells (HSPCs) and bone marrow microenvironment [6, 7]. Immunological abnormalities have also been observed in clinical and experimental iron overload, and disruption of proteins of adaptive immunological system could lead to or exacerbate iron overload [8, 9]. However, the effects of iron overload on the immune system have not been elucidated, and the exact mechanism is still uncertain.
Generally, T cell represents a key sentinel, acting in the innate immune and adaptive immune response. It can be divided into different subsets according to the different surface molecules, intracellular molecules expression, cytokine production, and state of immunity [10, 11]. Helper T (Th) cells and cytotoxic T (Tc) cells play a central role in regulating immune responses to the foreign insults, and they response by secreting cytokines that modulate the development and activity of immune effectors. The dominance of either of the Th or Tc subsets correlates directly with the outcome and severity of infection [12, 13].
Numerous studies have shown that the existence of an increase of the cytoplasmic reactive oxygen species (ROS) in iron overload conditions [14–18]. ROS are a group of highly reactive chemicals containing oxygen produced either exogenously or endogenously. Endogenous ROS are produced by the body’s own aerobic metabolism, while exogenous ROS are produced by either ionizing radiation or chemicals. They are related to a wide variety of human disorders, such as chronic inflammation, age-related diseases, and cancers [19–21]. ROS are also essential for various biological functions, including cell survival, growth, proliferation, differentiation, and immune response [22, 23]. These molecules function as important participants in immune regulation in distinct suppressive cell population. Now, it is becoming clear that ROS play an important role in the immune system and are intimately involved in various aspects of the immune response [23–27].
In this study, we set out to prove our hypothesis that iron overload could affect the proliferation, development, and survival of peripheral T lymphocytes by increasing ROS production. We first established iron overload, iron chelation, and antioxidative mouse models and then analyzed components of immune cells in the peripheral blood. Our data showed that iron overload resulted in fewer leukocytes, abnormal T lymphocyte subsets, and increased cell apoptosis, which effects could be partially reversed by antioxidant N-acetyl-l-cysteine (NAC) or iron chelator deferasirox (DFX).
Materials and methods
Patients and controls
Twenty myelodysplastic syndrome (MDS) patients with iron overload were recruited from Tianjin First Central Hospital. Ten MDS patients without iron overload, matched for age and gender with the examined patients, were enrolled as controls. Their characteristics are listed in Table 1. The diagnosis and classification of MDS were performed according to the World Health Organization (WHO) recommendations. Iron overload was evaluated by serum ferritin and transferrin saturation [28, 29]. Patients with acute leukemia transformation or severe infection, undergoing chemotherapy or cytokine therapy, were excluded from this study. The blood routine and serum ferritin tests were performed before iron chelation therapy. This study was approved by the ethical committee of Tianjin First Central Hospital. Informed consent was obtained from all participants.
Sample collection
The peripheral blood from patients and donors was collected in ethylenediaminetetraacetic acid (K3EDTA) tubes.
Reagents
Anti-mouse-CD3-percp, CD8-PE, CD8-percp, CD3-APC, CD3-percp, CD25-PE, FoxP3-APC, CD4-FITC, B220-APC, IFN-γ-FITC, and IL-4-APC were purchased from BioLegend Company (San Diego, CA, USA); anti-human-CD3-APC, CD8-PE, CD3-percp, CD8-percp, IFN-γ-FITC, IL-4-APC, and CD19-APC were purchased from BD Company (San Diego, CA, USA); iron dextran was purchased from Pharmacosmos A/S Company (Denmark); the ROS staining kit (S0033) and NAC were purchased from the Beyotime Institute of Biotechnology; calcein-acetoxymethyl ester (AM) fluorescent dye was purchased from Sigma-Aldrich Company (USA); DFX was purchased from Novartis; RPMI 1640 was purchased from Gibco Company (USA); annexin/propidium iodide (PI) kit was purchased from BD Biosciences Company; carboxyfluorescein succinimidyl ester (CFSE) was purchased from Molecular Probe Company (USA).
Animal and treatment
C57BL/6-Ly-5.1 (Ly5.1) male mice were obtained from the Institute of Peking University Health Science Center (Beijing, China). The mice were bred at the certified animal care facility in the Institute of Radiation Medicine of Peking Union Medical College (PUMC). All animal care and experimental procedures were conducted according to ethical standards of animal use and were approved by the Institutional Committee of Animal Care and Use of PUMC.
All mice were used at approximately 6–8 weeks of age, and the average weight was (20 ± 0.24 mg). Forty male mice were randomly divided into four groups: (a) a control group, (b) an iron-overloaded (IO) group (250 mg/kg), (c) an IO + NAC group, and (d) an IO + DFX group. The IO groups were injected with iron dextran intraperitoneally every 3 days for 4 weeks. The IO + NAC group mice were given NAC in drinking water (40 mM). The water bottles were changed twice per week with a freshly made NAC solution. The IO + DFX group mice received 2.5 mg DFX via gavage twice every 3 days for 4 weeks.
Identification of iron-overloaded mice model
Labile iron pool (LIP) level of lymphocytes was measured by calcein-AM fluorescent dye. The peripheral blood cells were washed twice in PBS and then incubated (5 × 105 cells/well) for 15 min at 37 °C with 0.125 μM calcein-AM and analyzed by a flow cytometer with the mean fluorescence intensity (MFI) calculated by the CellQuest software.
Hematological examination
Peripheral blood (150 μl/mouse) was harvested from the retro-orbital venous plexus and collected in RPMI 1640 containing 2% EDTA. Complete blood counts were obtained using a pocH-100i hematology analyzer (Sysmex, Japan). The cell counts included white blood cells (WBCs), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), the percentages of ANC (ANC%) and ALC (ALC%), red blood cells (RBCs), hemoglobin (HGB), and platelets (PLTs).
Subsets of lymphocytes
Surface staining and intracellular cytokine staining were performed as previously described [26, 27]. Briefly, surface staining was performed in 100 μl PBS with 3% (v/v) FBS and different antibody cocktails for surface markers (anti-CD3, anti-CD4, anti-CD8, and anti-B220) at room temperature for 15 min. Post-staining for surface markers, the intracellular cytokines were stained using intracellular staining kits from BD Biosciences. Following staining, the cells were washed and run through a BD Calibur flow cytometer (BD Biosciences) and 100,000 cells were sampled.
To detect the ratio of Th1/Th2 and Tc1/Tc2, peripheral blood mononuclear cells (PBMCs) were cultured with PMA (50 ng/mL), ionomycin (500 ng/mL), and GolgiStop (1.0 mL/mL) for 5 h. After surface staining, the cells were incubated with anti-IFN-γ and anti-IL-4 for 40 min at room temperature. In order to detect the percentage of regulatory T (Treg) cells, after surface staining, PBMCs were labeled with FoxP3 for 30 min at room temperature. Finally, cells were washed and analyzed by a flow cytometer (BD Calibur).
Assess the apoptosis of T cells
Apoptosis were determined using an annexin V-FITC/PI apoptosis detection kit (BD Biosciences) according to the manufacturer’s instruction. Briefly, the peripheral blood cells were washed twice with PBS and then washed once with annexin binding buffer. Samples were incubated in annexin V-FITC at routine temperature for 10 min in the dark and then labeled with PI at routine temperature for 5 min. Finally, cells were washed and analyzed by a flow cytometer (BD Calibur).
Flow cytometer measurements of ROS
ROS staining was performed using a ROS staining kit following the manufacturer’s protocol. The peripheral blood cells were incubated with 10 μM DCFH-DA and anti-CD3 antibody in a humidified atmosphere of 5% CO2 in air at 37 °C for 15 min. The levels of intracellular ROS were analyzed by measuring the MFI of 2′,7′-dichlorofluorescein (DCF) using a flow cytometer.
Statistical analyses
All experiments were performed at least three times. Analyses of flow cytometry data were conducted using CellQuest software. Continuous variables were presented as mean ± standard deviation (SD) or median. They were compared by a parametric (Student’s t test, ANOVA) or non-parametric (Mann–Whitney U, Kruskal–Wallis) test as appropriate according to each variable distribution. Categorical variables were compared using Fisher’s exact tests. Differences were considered to be statistically significant at P <0.05. The statistical analyses were performed with the GraphPad Prism program (GraphPad Software, Inc., San Diego, CA, USA) or SPSS software (version 19.0).
Results
Lymphocyte immunophenotyping, T cell subsets, and ROS level in iron-overloaded patients
We found that LIP levels of lymphocytes from MDS patients with iron overload were higher than those of patients without iron overload (1068.83 ± 64.27 vs. 2047.32 ± 228.94, P < 0.001). The percentage of CD3+ T cells and the ratio of Th1/Th2, Tc1/Tc2, and CD4/CD8 were evaluated in controls and 20 MDS patients with iron overload. Compared to the controls, the percentage of CD3+ T cell and the ratio of Th1/Th2 and Tc1/Tc2 were significantly lower in iron-overloaded patients, whereas those of CD4/CD8 were higher (Fig. 1). We also found that ROS levels of CD3+ T lymphocytes from iron-overloaded patients were higher than control (107.49 ± 10.58 vs. 29.09 ± 3.04, P < 0.001), which indicated that ROS might participate in the pathogenesis of iron overload.
Abnormal T cell subsets in peripheral blood cells from iron-overloaded patients. a A representative FACS analysis of CD3+ T cells in lymphocyte. b A representative FACS analysis of CD4+ and CD8+ cells in CD3+ T cells. c A representative FACS analysis of CD4+IFN-γ+Th1 cells and CD4+IL-4+Th2 cells from an IO patient and a control patient. d A representative FACS analysis of CD8+IFN-γ+Tc1 cells and CD8+IL-4+Tc2 cells from an IO patient and a control patient. e The percentage of CD3+ T cells in lymphocytes was significantly decreased in IO patients compared with control. f The ratio of CD4/CD8 cells was increased in IO patients compared with control. The ratios of Th1/Th2 (g) and Tc1/Tc2 (h) were significantly decreased in IO patients compared with control (control, n = 10; IO, n = 20). Statistical analyses were performed with Student’s t test or Mann–Whitney U test as appropriate. ## P < 0.01, vs. control; ### P < 0.001, vs. control
The establishment of an iron-overloaded mouse model
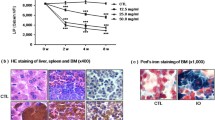
According to our previous studies, we injected mice intraperitoneally with iron dextran for 4 weeks to establish an iron-overloaded mouse model, and iron deposits in the liver were easily observed [6, 7] (Fig. 2a). To confirm the iron accumulation in the immune system, LIP levels of lymphocytes were evaluated (Fig. 2b). Our results demonstrated that this experimental murine model reflected an iron-overloaded pathogenic condition.
The establishment of an iron-overloaded mouse model. a Iron deposition in the liver from the IO group mice and the controls (Perl’s iron staining, scale bar = 50 μm). b The LIP level of lymphocytes in the IO group was increased compared with the control group, and concomitant treatment with DFX significantly attenuated this effects. Statistical analyses were performed with ANOVA test. The data represent the means ± SD; n = 10 per group. ### P < 0.001, vs. control; ***P < 0.001, vs. IO
Iron overload affects peripheral T lymphocytes
Compared to the control, significantly lower numbers of lymphocytes were observed in the peripheral blood of experimental mice after receiving iron dextran for 4 weeks (P < 0.05). The platelet, RBC counts, and HGB levels were also decreased after iron overload. Although the frequency of ANC was increased after iron overload, the absolute number of ANC did not show a significant difference between different groups. After treating with NAC, the percentage of lymphocytes in the IO group mice was significantly increased, while the absolute number of lymphocytes did not show a significant difference (Table 2).
T lymphocytes play a pivotal role in the host immune defense against infection and tumor. A flow cytometer was used to examine the effects of iron overload exposure on T cells. Our results showed that after 4 weeks of iron dextran treatment, the frequency of T cells in the peripheral blood was significantly decreased (Fig. 3a). Compared to the IO group, the frequency of T cells in the peripheral blood from IO + NAC and IO + DFX groups was increased (Fig. 3a), but there was no significant difference in the frequency of B cells in the peripheral blood between different groups at these time points (Fig. 3b).
Iron overload decreased the percentage of T cells. a The percentage of CD3+ T cells in lymphocytes was decreased in IO group mice compared with control, and it was increased by DFX and NAC treatment. b Iron overload has no effect on the percentage of B220+ B cells in mice peripheral blood lymphocytes. Statistical analyses were performed with ANOVA test as appropriate. The data represent the means ± SD; n = 10 per group. ### P < 0.001, vs. control; ***P < 0.001, vs. IO
Effects of iron overload on the T lymphocyte subsets
We examined the subsets of T lymphocyte in different groups, and our results showed that the ratio of CD4/CD8 and the percentage of Treg cells were substantially increased after iron overload (Figs. 4 and 5a, b). However, the ratio of Th1/Th2 and Tc1/Tc2 was decreased, which suggested the balance between type 1 and type 2 cytokine-producing cells was disrupted after excessive iron deposition (Figs. 4 and 5c, d). Furthermore, these effects were partly reversed after treating with NAC and DFX.
Flow cytometry analysis of the subsets of T cells from different groups mice. a A representative FACS analysis of CD4+ and CD8+ cells in CD3+ T cells. b A representative FACS analysis of CD25highFoxp3+ Treg cells in CD4+ cells from different group mice. c A representative FACS analysis of CD4+IFN-γ+Th1 cells and CD4+IL-4+Th2 cells from different group mice. d A representative FACS analysis of CD8+IFN-γ+Tc1 cells and CD8+IL-4+Tc2 cells from different group mice
Iron overload affects the subsets of T cells. Compared to the controls, iron overload increased the ratio of CD4/CD8 (a) and the frequency of Treg cells (b), which effect could be reversed by treating with NAC or DFX. The ratios of Th1/Th2 (c) and Tc1/Tc2 (d) in the peripheral blood of IO group mice were significantly decreased. Statistical analyses were performed with Kruskal–Wallis or ANOVA test as appropriate. n = 10 per group. ## P < 0.01, vs. control group; ### P < 0.001, vs. control group; *P < 0.05 ,vs. IO group; **P < 0.01, vs. IO group; ***P < 0.001, vs. IO group
Iron overload induces the apoptosis of CD4+ and CD8+ T cells
We further tested the effect of iron overload on the apoptosis of CD4+ T and CD8+ T cells. As shown in Fig. 6, the apoptosis of CD8+ T cells and CD4+ T cells (annexin V+PI+) in iron-overloaded mice was significantly higher than that in control group mice, and DFX or NAC treatment could reverse this effect.
Iron overload induces the apoptosis of CD4+ and CD8+ T cells. A representative FACS analysis of the apoptosis of CD4+ T cells (a) and CD8+ T cells (b) from different group mice. Compared to the controls, iron overload increased the apoptosis of CD4+ T cells (c) and CD8+ T cells (d), and the ratios were decreased by treating with NAC or DFX. Statistical analyses were performed with Kruskal–Wallis or ANOVA test as appropriate. n = 10 per group. ### P < 0.001, vs. control group; *P < 0.05, vs. IO group; **P < 0.01, vs. IO group; ***P < 0.001, vs. IO group
Iron overload increases ROS production in T cells
It is well known that iron is the main catalyst of ROS in an organism, and a growing body of evidence demonstrates that there is a positive correlation between ROS and LIP levels. In this study, we investigated the levels of ROS in CD3+ T cells in iron-overloaded mice. There was a significant increase in ROS levels in the IO group compared to the controls, which could be reversed by DFX and NAC treatment (Fig. 7).
Iron overload increases ROS production of T cells. a A representative FACS analysis of the level of ROS in CD3+ T cells from different group mice. b Compared to the controls, IO increased the levels of ROS in CD3+ T cells, and the elevated ROS level could be suppressed by NAC and DFX. Statistical analyses were performed with ANOVA test. The data represent the means ± SD; n = 10 per group. ### P < 0.001, vs. control group; ***P < 0.001, vs. IO group
Discussions
It is well known that a link exists between cell-mediated immunity and iron metabolism [13]. Indeed, both iron deficiency and iron overload can exert subtle effects on the immune status [30, 31]. Our data showed that iron overload could reduce the percentage of T cells, especially CD8+ T cells. Moreover, the ratios of Th1/Th2 and Tc1/Tc2 were dramatically reduced among iron-overloaded patients and iron-overloaded mice, while the percentage of Treg cells was increased. In addition, it also resulted in ROS accumulation in T cells and induced T cell apoptosis. Importantly, these effects could be partly reversed after treating with an antioxidant agent or iron chelator.
Recent studies suggest that iron overload can lead to immune disorders and increase the risk of bacterial and fungal infections, but the specific effects and mechanism of action remain unclear [32, 33]. In our preliminary studies, we successfully established the iron-overloaded mouse model and showed the inhibitory effects on peripheral blood cell counts [7]. On the basis of the iron-overloaded model, we assessed whether iron overload injured the immune system. We first counted the peripheral blood cells, and our results showed that iron overload resulted in reduced lymphocytes. Furthermore, iron overload significantly decreased the frequency of CD3+ T cells compared with the control group, while the frequency of B220+ cells was not altered.
We further examined the effects of iron overload on T cell subsets and T cell apoptosis in our iron-overloaded mouse model. It reduced the percentage of T cells, especially CD8+ T cells, but increased the percentage of Treg cells and the ratio of CD4/CD8. These results were consistent with previously published results, which showed decreased CD8+ numbers and increased CD4/CD8 ratios in hereditary hemochromatosis patients [34, 35]. Furthermore, the lower number of CD8+ T lymphocyte in hereditary hemochromatosis is associated with decreased CD8+ effector memory, which indicated an inability of CD8+ lymphocyte to differentiate into mature phenotype [35]. Thus, iron overload might affect the ability of the host to mount an effective response of antigen-specific CD8+ T cells to infection. This also supports the hypothesis that iron overload increased the risk of bacterial and fungal infections. However, other studies have shown that iron overload decreased CD4+ T cells and increased CD8+ T cells, which resulted in a reduced CD4/CD8 T cell ratio [31, 36]. These apparently inconsistent observations may be related to different conditions used in different studies, including iron dosage, model of administration, and treatment duration time.
CD4+ Th cells are divided into Th1 and Th2 cells based on their cytokine secretion patterns. CD8+ T cells are vital for the antiviral cellular immune response which directly kill the target cells infected by viruses, bacteria, and other pathogens. Similar to CD4+ Th cells, CD8+ T cells can be divided into Tc1 and Tc2 subsets. Tc1 and Th1 secrete IFN-γ, IL-2, IL-12, and several other cytokines which mediate cellular immunity. Similarly, Tc2 and Th2 secrete IL-4, IL-5, IL-6, IL-10, and several other cytokines which mediate humoral immunity. Our data showed that iron-overloaded mice had a decreased ratio of Th1/Th2 and Tc1/Tc2, which indicated a defective type 1 immune response [37]. Type 1 immune response, which is involved in cellular immunity, is the main immune response following infection. Thus, our results may explain the increased susceptibility of iron overload patients to infection.
As the need for repeated RBC transfusion, MDS patients often have secondary iron overload. To confirm our findings in clinical samples, we examined the lymphocyte subsets of MDS patients with or without iron overload. We found that patients with iron overload had a lower percentage of CD3+ T cells and disrupted T cell subsets, which was consistent with the results of animal experiment. However, our study has some limitations. Several studies revealed that the lymphocytes of MDS patients tend to exhibit numerical and qualitative abnormalities. Thus, it is difficult to separate the MDS-related factor and iron overload. Although we confirmed our conclusion in animal experiment, further study including non-MDS patients is still needed.
On the basis of the previous data, we tried to explain the mechanism of iron overload in regulating the immune cells. It was reported that T lymphocytes can take up the toxic non-transferrin-bound iron (NTBI) in the plasma through some specific transporter. Increased intracellular NTBI can catalyze the production of ROS through Fenton’s reaction and subsequently stimulate a series of cell signaling pathways to induce cell apoptosis [38–42]. Our data also show that ROS level in CD3+ T cells was significantly increased. It is known that ROS play an important regulatory role in the activation and differentiation of T cells [43–45]. Therefore, we speculated that iron overload may induce T lymphocyte abnormality by increasing the ROS production. We used a ROS scavenger NAC to evaluate whether the effects of excessive iron on T lymphocytes could be reversed. Our data showed that disrupted T cell subsets and apoptosis could be partially reversed after treating iron-overloaded mice with NAC. These results indicate that iron overload may regulate T lymphocytes by increasing ROS production.
Taken together, the present study showed that iron overload could induce T lymphocyte subset abnormalities and increase T cell apoptosis, suggesting that the aberrant T cell responses may increase the probability of infection after iron overload. Furthermore, our results also revealed that ROS was an important mediator in this process, which provided a new strategy to treat iron overload-induced immune abnormalities. Further studies are needed to investigate the mechanisms by which iron overload increases the ROS level.
References
Dunn LL, Suryo Rahmanto Y, Richardson DR (2007) Iron uptake and metabolism in the new millennium. Trends Cell Biol 17(2):93–100. doi:10.1016/j.tcb.2006.12.003
Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117(3):285–297
Shenoy N, Vallumsetla N, Rachmilewitz E, Verma A, Ginzburg Y (2014) Impact of iron overload and potential benefit from iron chelation in low-risk myelodysplastic syndrome. Blood 124(6):873–881. doi:10.1182/blood-2014-03-563221
Porter JB, Garbowski M (2014) The pathophysiology of transfusional iron overload. Hematol Oncol Clin North Am 28(4):683–701. doi:10.1016/j.hoc.2014.04.003
Remacha AF, Arrizabalaga B, Villegas A, Duran MS, Hermosin L, de Paz R, Garcia M, Diez Campelo M, Sanz G, Iron-Study Group (2015) Evolution of iron overload in patients with low-risk myelodysplastic syndrome: iron chelation therapy and organ complications. Ann Hematol 94(5):779–787. doi:10.1007/s00277-014-2274-y
Chai X, Li D, Cao X, Zhang Y, Mu J, Lu W, Xiao X, Li C, Meng J, Chen J, Li Q, Wang J, Meng A, Zhao M (2015) ROS-mediated iron overload injures the hematopoiesis of bone marrow by damaging hematopoietic stem/progenitor cells in mice. Sci Rep 13(5):10181. doi:10.1038/srep 10181
Zhang Y, Zhai W, Zhao M, Li D, Chai X, Cao X, Meng J, Chen J, Xiao X, Li Q, Mu J, Shen J, Meng A (2015) Effects of iron overload on the bone marrow microenvironment in mice. PLoS One 10(3):e0120457. doi:10.1371/journal. pone.0124246 eCollection 2015
Costa M, Cruz E, Oliveira S, Benes V, Ivacevic T, Silva MJ, Vieira I, Dias F, Fonseca S, Gonçalves M, Lima M, Leitão C, Muckenthaler MU, Pinto J, Porto G (2015) Lymphocyte gene expression signatures from patients and mouse models of hereditary hemochromatosis reveal a function of HFE as a negative regulator of CD8+ T-lymphocyte activation and differentiation in vivo. PLoS One 10(4):e0124246. doi:10.1371/journal. pone.0120219 eCollection 2015
Van Den Ham KM, Shio MT, Rainone A, Fournier S, Krawczyk CM, Olivier M (2015) Iron prevents the development of experimental cerebral malaria by attenuating CXCR3-mediated T cell chemotaxis. PLoS One 10(3):e0118451. doi:10.1371/journal. pone.0118451 eCollection 2015
Dzielak DJ (1992) The immune system and hypertension. Hypertension 19(1 Suppl):136–144
Mencacci A, Cenci E, Boelaert JR, Bucci P, Mosci P, Fè d’Ostiani C, Bistoni F, Romani L (1997) Iron overload alters innate and T helper cell responses to Candida albicans in mice. J Infect Dis 175(6):1467–1476
Romani L, Puccetti P, Bistoni F (1995) Biological role of helper T-cell subsets in candidiasis. Chem Immunol 63:115–137
Puccetti P, Romani L, Bistoni F (1995) A Th1-Th2-like switch in candidiasis: new perspectives for therapy. Trends Microbiol 3(6):237–240
Jacobs A (1976) Metabolic consequences of iron overload. Br J Haematol 34(1):1–4
Wills ED (1972) Effects of iron overload on lipid peroxide formation and oxidative dimethylation by the liver endoplasmic reticulum. Biochem Pharmacol 21(2):239–247
Rachmilewitz EA, Shohet SB, Lubin BH (1976) Lipid membrane peroxidation in B-thalassemia major. Blood 47(3):495–505
Dormandy TL (1978) Free radical oxidations and antioxidants. Lancet 1(8065):647–650
Ghoti H, Amer J, Winder A, Rachmilewitz E, Fibach E (2007) Oxidative stress in red blood cells, platelets and polymorphonuclear leukocytes from patients with myelodysplastic syndrome. Eur J Haematol 79(6):463–467. doi:10.1111/i.1600-0609.2007.00972.x
Liou GYSP, Storz P (2010) Reactive oxygen species in cancer. Free Radic Res 44(5):479–496. doi:10.3109/10715761003667554
Freinbichler W, Colivicchi MA, Stefanini C, Bianchi L, BalliniC MB, Weinberger P, Linert W, Varešlija D, Tipton KF, Della Corte L (2011) Highly reactive oxygen species: detection, formation and possible functions. Cell Mol Life Sci 68(12):2067–2079. doi:10.1007/s00018- 011-0682-x
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95. doi:10.1152/physrev.00018.2001
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194(1):7–15. doi:10.1083/jcb.201102095
Miller EW, Dickinson BC, Chang CJ (2010) Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc Natl Acad Sci U S A 107(36):15681–15686. doi:10.1073/pnas.1005776107
Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM (2011) Mitochondrial reactive oxygen species promote production of pro-inflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 208(3):519–533. doi:10.1084/jem.20102049
Malhotra A, Shanker A (2011) NK cells: immune cross-talk and therapeutic implications. Immunotherapy 3(10):1143–1166. doi:10.2217/imt.11.102
Devadas S, Zaritskaya L, Rhee SG, Oberley L, Williams MS (2002) Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J Exp Med 195(1):59–70
Wentworth AD, Jones LH, Wentworth P Jr, Janda KD, Lerner RA (2000) Antibodies have the intrinsic capacity to destroy antigens. Proc Natl Acad Sci U S A 97(20):10930–10935
Piperno A (1998) Classification and diagnosis of iron overload. Haematologica 83(5):447–455
Porter JB, Lin KH, Beris P, Forni GL, Taher A, Habr D, Domokos G, Roubert B, Thein SL, EPIC study investigators (2011) Response of iron overload to deferasirox in rare transfusion- dependent anaemias: equivalent effects on serum ferritin and labile plasma iron for haemolytic or production anaemias. Eur J Haematol 87(4):338–348. doi:10.1111/j.1600-0609.2011.01660.x
Walker EM Jr, Walker SM (2000) Effects of iron overload on the immune system. Ann Clin Lab Sci 30(4):354–365
Cherayli BJ (2010) Iron and immunity: immunological consequences of iron deficiency and overload. Arch Immunol Ther Exp (Warsz) 58(6):407–415. doi:10.1007/s00005-010-0095-9
Brock JH, Halliday JW, Pippard MJ, Powell LW (1994) Iron metabolism in health and disease. WB Saunders, Philadelphia, pp 353–389
Boelaert JR (1996) Iron and infection. Acta Clin Belg 51(4):213–221
Porto G, Reimao R, Goncalves C, Vicente C, Justica B, de Sousa M (1994) Haemochromatosis as a window into the study of the immunological system: a novel correlation between CD8+ lymphocytes and iron overload. Eur J Haematol 52(5):283–290
Macedo MF, Porto G, Costa M, Vieira CP, Rocha B, Cruz E (2010) Low numbers of CD8+ T lymphocytes in hereditary haemochromatosis are explained by a decrease of the most mature CD8+ effector memory T cells. Clin Exp Immunol 159(3):363–371. doi:10.1111/j.1365-2249.2009.04066x
Cardier JE, Romano E, Soyano A (1997) T lymphocytes subsets in experimental iron overload. Immunopharmacol Immunotoxicol 19(1):75–87. doi:10.3109/08923979709038534
Toma A, Fenaux P, Dreyfus F, Cordonnier C (2012) Infections in myelodysplastic syndromes. Haematologica 97(10):1459–1470. doi:10.3324/haematol.2012.063420
Areze J, Costa M, Vieira I, Dias V, Kong XL, Fernander R, Vos M, Carlsson A, Rikers Y, Porto G, Rangel M, Hider RC, Pinto JP (2013) Non-transferrin-bound iron (NTBI) uptake by T lymphocytes: evidence for the selective acquisition of oligomeric ferric citrate species. PLoS One 8(11):e79870
Thorson JA, Smith KM, Gomez F, Naumann PW, Kemp JD (1991) Role of iron in T cell activation: Th1 clones differ from Th2 clones in their sensitivity to inhibition of DNA synthesis caused by IgG Mabs against the transferrin receptor and the iron chelator deferoxamine. Cell Immunol 134(1):126–137
Zhang DY, Wang HJ, Tan YZ (2011) Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway. PLoS One 6(6):e21397. doi:10.1371/journal.pone.0021397
Clopton DA, Saltman P (1995) Low-level oxidative stress causes cell-cycle specific arrest in cultured cells. Biochem Biophy Res Commun 210(1):189–196. doi:10.1006/bbrc.1995.1645
Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T (2006) Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med 12:446–451. doi:10.1038/nm1388
Lam GY, Huang J, Brumell JH (2010) The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin Immunopathol 32(4):415–430. doi:10.1007/s00281-010-0021-0
Yang Y, Bazhin AV, Werner J, Karakhanova S (2013) Reactive oxygen species in the immune system. International Review of Immunology 32(3):249–270. doi:10.3109/08830185.2012.755176
Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K (2014) Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int 2014:761264. doi:10.1155/2014/761264
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All procedures performed in studies involving animals were in accordance with the guidelines for the care and use of animals and the ethical standards of the Institutional Committee of Animal Care and Use of PUMC. Informed consent was obtained from all participants for being included in this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by the National Natural Science Foundation of China (81400092, 81400083), the National Natural Science Foundation of Tianjin (13JCYBJC23400), the Science and Technique Foundation of Tianjin (13KG106, 16KG110), and the Tianjin Municipal Health and Family Planning Commission (2015KR15).
Additional information
Jie Chen and Wen-yi Lu share the first authorship.
Rights and permissions
About this article
Cite this article
Chen, J., Lu, Wy., Zhao, Mf. et al. Reactive oxygen species mediated T lymphocyte abnormalities in an iron-overloaded mouse model and iron-overloaded patients with myelodysplastic syndromes. Ann Hematol 96, 1085–1095 (2017). https://doi.org/10.1007/s00277-017-2985-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-2985-y