Abstract
Limited information is available from developing countries about complications, pattern of infections, and long-term outcome of patients following high-dose chemotherapy (HDCT) and autologous blood stem cell transplantation (ASCT). Between April, 1990 and December 2009, 228 patients underwent ASCT. Patients’ median age was 48 years, ranging from 11 to 68 years. There were 158 males and 70 females. Indications for transplant included multiple myeloma, n = 143; lymphoma, n = 44 (Hodgkin’s, n = 25 and non-Hodgkin’s, n = 19); leukemia, n = 22; and solid tumors, n = 18. Patients received HDCT as per standard protocols. Following ASCT, 175 (76.7%) patients responded; complete, 98 (43%); very good partial response, 37 (16.2%); and partial response, 40 (17.5%). Response rate was higher for patients with good Eastern Cooperative Oncology Group (ECOG) performance status (0–2 vs. 3–4, p < 0.001), pretransplant chemo-sensitive disease (p < 0.001) and those with diagnosis of hematological malignancies (p < 0.003). Mucositis, gastrointestinal, renal, and liver dysfunctions were major nonhematologic toxicities, 3.1% of patients died of regimen-related toxicities. Infections accounted for 5.3% of deaths seen before day 30. At a median follow-up of 66 months (range, 9–234 months), median overall (OS) and event-free survival (EFS) were 72 months (95% CI 52.4–91.6) and 24 months (95% CI 17.15–30.9), respectively. For myeloma, OS and EFS were 79 months (95% CI 52.3–105.7) and 30 months (95% CI 22.6–37.4), respectively. Pretransplant good performance status and achievement of significant response following transplant were major predictors of survival. Our analysis demonstrates that such procedure can be successfully performed in a developing country with results comparable to developed countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (ASCT) is a standard treatment approach for eligible, young patients of multiple myeloma (MM), chemo-sensitive relapsed Hodgkin’s (HL) and non-Hodgkin’s lymphoma (NHL) and children with high-risk neuroblastoma [1–8]. ASCT is also conducted in other malignant disorders including acute myeloid leukemia (AML) [9] testicular germ cell tumors, etc [10]. Infections and nonhematological toxicities are common complications seen in early posttransplant period (0–30 days) and are primarily related to high-dose chemotherapy (HDCT). Relapse and secondary malignancy are late complications [2–4]. While results of ASCT have been reported from many centers in the West, such data is not readily available from countries with limited resources [11–14]. In India, about 500 patients are transplanted per year in 11 centers; transplant rate is two transplants per million, compared to 30–42 transplants per million in developed countries [1]. In fact, developing hemopoietic stem cell transplant program in countries with limited resources is a challenge where food, sanitation, immunization, control of communicable diseases, and population control take priorities. We have recently analyzed the data on patients who underwent ASCT at our center. This report describes the results.
Patients and methods
Between April 1990 and December 2009, 228 consecutive patients underwent ASCT. Patients’ characteristics are shown in Table 1. Briefly, patients’ median age was 48 years, ranging from 11 to 68 years. Three patients were below 15 years and 7% were above 60 years of age; 158 were males and 70 females (M/F, 2.2:1). Indications for transplant included multiple myeloma (n = 143), HL (n = 25), NHL (n = 19), AML (n = 15), acute lymphoblastic leukemia (ALL; n = 3), chronic myeloid leukemia (CML, n = 5), and solid tumors (n = 18; five breast cancer, six epithelial ovarian cancer, five germ cell tumors of testis, one small cell lung cancer, and one peripheral neuro-ectodermal tumor). Data on initial myeloma patients has been reported earlier [11]. Patients were defined to have chemo-sensitive disease if they had complete (CR) or partial response at the time of ASCT. This included patients of HL; NHL; acute leukemia (AML, ALL) in first, second, or third CR; and solid tumors who had achieved CR or partial response (PR) following salvage chemotherapy. Myeloma patients with CR, PR, or very good partial response (VGPR) to pretransplant therapy were also included in chemo-sensitive disease category. Patients with minimal response (25–50% response) or for MM patients with 25–50% reduction in paraprotein or those with progressive or refractory disease were defined to have chemo-resistant disease.
Institute
Our institute is a tertiary care, referral, government hospital (total bed strength: 2,400 beds) with 150 beds dedicated to cancer patients. We initially started with two bed transplant unit in 1990 and now have a separate floor with nine bed transplant unit. Many patients are treated in periphery hospitals and are referred for subsequent management. All patients are initially reviewed in the organ-based clinics, treated as per institute-based protocols. Candidates for ASCT are registered in the weekly “Bone marrow/Stem cell transplant clinic” in which patients and family members are explained about the procedure, potential risks, and benefits. Transplant cost is met by the individuals, government support, medical insurance, and charitable organizations. During follow-up, patients are seen in the “Bone Marrow/Stem cell Transplant Clinic” initially monthly, then bi- to tri-monthly for 3 years, then every 6 months thereafter. Some of our patients come from very far away or from neighboring countries. These are followed locally by referring oncologist/hematologists as per guidelines provided and are seen once in 3 months at our institute. Follow-up information is available on all patients.
Transplant protocol
Pretransplant evaluation included history and physical examination; details of prior treatment were recorded. Investigations including hemogram, renal and liver function tests, bone marrow biopsy, echocardiography or MUGA scan, pulmonary function tests, and viral markers were done to assess overall fitness prior to ASCT. Central line (Hickman’s catheter) was inserted. All patients were admitted in a single room and reverse barrier nursing was practiced. Written informed consent was obtained prior to transplant.
Stem cell graft
Bone marrow was source of stem cells for first 10 patients which were harvested under general anesthesia. For the next 218 patients, mobilized peripheral blood stem cells (PBSCs) were harvested. For mobilization, patients received injected granulocyte colony-stimulating factor (G-CSF) 5 mcg/kg twice daily subcutaneously for 6 days. Stem cells were harvested on day 5 and 7 using Hemonetics cell separator-MCS 3p (Haemonetics, Braintree, MA, USA). PBSC harvest was done from median cubital vein in 199 (91.3%) patients, from central line (subclavian or internal jugular vein) in 16 (7.3%) and from femoral or internal jugular vein in three (1.4%) patients using dialysis catheter. The mean numbers of collections were two per patient (range, 1–6). Mononuclear cells were counted manually by doing differential count on stem cell preparation. For CD 34 counts, cells were labeled with florescein-conjugated anti-CD34 and analyzed using a FACS scan flow cytometer to yield absolute CD34+ counts [15]. Stem cells were kept at 4°C (for multiple myeloma patients) or cryopreserved at −80°C using cryoprotectant mixture consisting of 7.5% dimethyl sulfoxide (DMSO), albumin, and saline [16]. Viability of stem cells was done with trypan blue dye test.
High-dose chemotherapy
For multiple myeloma, patients received high-dose melphalan (n = 143) [11]. For HL and NHL, BCNU, cyclophosphamide, VP-–16 (etoposide) (CBV; n = 39), BCNU, etoposide, cytosine arabinoside and melphalan protocol (BEAM, n = 3) was administered [3]. busulphan and cyclophosphamide (Bu-Cy2) was used for acute leukemia (AML, ALL) and CML (n = 5). For solid tumors, carboplatin-cyclophosphamide and VP-16 (n = 6) or carboplatin + cyclophosphamide (n = 4) or paclitaxel, cyclophosphamide, and carboplatin (n = 2) or high-dose melphalan was used. HDCT was administered as per the standard guidelines. Autologous stem cells were reinfused intravenously on day 0 through a central venous catheter preceded by IV injection pheneramine maleate 50 mg. Posttransplant patients received injected G-CSF 5 mcg/Kg daily SC until engraftment. All the blood products transfused during posttransplant period were irradiated with 25 Gys.
Antimicrobial prophylaxis
Patients were admitted in a single room without laminar airflow or high efficiency particulate air (HEPA) filter, reverse barrier nursing was practiced. All patients received prophylaxis against fungi initially with fluconazole till 1998 and later with itraconazole. Ciprofloxacin was used for antibacterial prophylaxis. Routine acyclovir prophylaxis was given to patients with myeloma, lymphoma, and acute leukemia. Patients were advised to avoid raw, uncooked food over next 4 weeks. Once febrile, evaluation and treatment was done as per standard guidelines [17].
Hematological recovery
Engraftment was defined as achievement of absolute neutrophil count of ≥500/cm3 for three consecutive days. Platelet engraftment was defined as platelet counts of ≥20,000/cm3 for three consecutive days with transfusion independence.
Toxicity
All cases of nonhematological dysfunction were considered “regimen related” unless these could be clearly explained by another cause. A grading scale described by Bearman et al. [18] was used for toxic complications of transplant. Briefly, grade 0 represented no toxicity; grade I toxicity was fully reversible without specific intervention; grade II toxicity was not life threatening, but required specific measures to be reverted; grade 3 was life threatening but reversible; and grade 4 toxicity was fatal. The diagnosis of veno-occlusive disease (sinusoidal obstruction syndrome, SOS) was based on clinical criteria originally proposed by McDonald et al. Two of the following criteria had to be present within 20 days after transplantation, and not explained by other reasons: hyperbilirubinemia (bilirubin ≥2.0 mg/dL), painful hepatomegaly, and unexplained weight gain (≥2% from baseline) [19].
Response evaluation
Patients were evaluated for response as per WHO criteria [20] 4 weeks after transplant on outpatient basis and subsequently were kept on the follow-up. For myeloma patients, response was assessed 6 weeks after transplant as per European Group for Blood and Marrow Transplantation (EBMT) criteria described by Blade et al. [21].
Statistical analysis
All patients are evaluable for response and survival analysis. Posttransplant period was stratified in the standard manner as early (<30 days), intermediate (30–100 days), and late (>100 days). The prognostic factors for response to transplant were analyzed by Pearson chi-square test. All survival times were calculated from date of transplant. Overall survival was defined as the time from date of transplant until death or date of censoring. Event-free survival was calculated from date of transplant to disease progression or death (regardless of cause of death). Curves for overall and event-free survival were plotted according to method of Kaplan and Meier and were compared by the log-rank test. The prognostic factors for survival were analyzed by Cox regression analysis. The median follow-up for the whole group is 66 months (range, 9–234 months). The data has been censored on September 30, 2010. Statistical analysis was performed with SPSS software (version 16).
Results
Multiple myeloma was most common indication (62.7%) followed by HL (11%), NHL (8.3%), AML (6.6%), and solid tumors (8%). Prior to transplant, 166 (72.8%) patients had chemo-sensitive disease; 54 were in either first or second CR and 112 were in either first or second PR. The remaining 62 (27.2%) patients had chemo-resistant disease (minimal response, n =12 and relapse/progressive disease, n = 50; Table 1).
Engraftment
The median number of mononuclear and CD 34 + cells transfused was 4.78 × 108/kg (range, 0.39–11.8 × 108/kg) and 2.80 × 106/kg (range, 0.70–19.11 × 106/kg), respectively. Stem cells viability (after thawing) ranged from 88% to 98%, cell loss due to cryopreservation ranged from 2% to 12%.
Hematological Recovery
The median time to engraftment was 11 days (range, 9–24 days) and median time for platelet transfusion independence was 12 days (range, 8–36 days). Median duration of fever and antibiotics therapy was 10 and 11 days, respectively. Median duration of hospitalization posttransplant was 19 days. Fifteen patients (6.6%) failed to engraft. Following transplant, patients received a median of two units of red cells and three units of single donor platelet transfusion. Posttransplant patients received G-CSF for a median of 12 days (range,9–30 days).
In the myeloma group (n = 143), 70 patients had received stem cells cryopreserved at −80°C using a mixture of DMSO, albumin and saline. Sixty-three patients received stem cells kept at 4°C. There was no difference in the number of CD 34+ cells infused and hemopoietic recovery in two groups.
Response to transplant
One hundred seventy-five of 228 patients (76.7%) responded to transplant; complete, 98 (43%) and PR, 77 (33.7%) including VGPR in 37 (16.2%) patients (of myeloma). Eighteen patients (7.9%) had stable disease and 12 patients (5.3%) had either no response or progressed (Table 2). Among 166 patients with pretransplant chemo-sensitive disease, 150 (90.3%) responded; CR in 93 (56%), VGPR in 34 (20.5%), and PR in 23 (13.8%) patients. Among 62 patients with chemo-resistant disease, 25 (40.3%) patients responded; CR in five (8.1%), VGPR in three (4.8%), PR in 17(27.4%). CR rate was significantly higher in patients with pretransplant chemo-sensitive disease, 93/166 (56%) vs. 5/62(8.1%), p < 0.001.
Multiple myeloma (n = 143)
The overall response rate was 83.3%; CR in 58 (40.6%), VGPR in 37 (25.9%), and PR in 24 (16.8%) patients. Eight (5.6%) patients had stable disease and four (2.8%) patients had progressed.
Hodgkin’s lymphoma
The overall response rate was 88%; CR in 17 (68%), PR in five (20%). CR rate was higher among patients who had chemo-sensitive disease compared to chemo-resistant disease; 88% vs. 12%, p < 0.007.
Non hodgkin’s lymphoma
This group was heterogeneous in view of varied histology subtypes (diffuse large cell in nine, mantle cell in five, indolent in two, NK cell in one, peripheral T cell NOS in one, lymphoblastic in one). Overall response rate was 52.6%; CR in 42.1% (8/19), PR in 10.5% (2/19). Seven of eight CRs were among patients with chemo-sensitive disease (7/12), p < 0.05.
Acute myeloid leukemia (n = 15)
Among 15 patients with AML, two were in first CR, eight in CR2, three had border line remission, and two had refractory disease. Cytogenetically, 12 had intermediate risk and two had high risk. Seven of 15 patients achieved CR (53.3%), one PR, and four patients died of transplant-related complications.
Solid tumors (n = 18)
Eleven of 18 patients responded; CR in five (27.7%), PR in six (33.3%). Four patients had progressive disease (27.7%; Table 2).
Factors affecting response to transplant
Response rate was significantly higher for patients with good ECOG performance status (0–2 vs. 3–4, p < 0.001), pretransplant chemo-sensitive disease (p < 0.001), younger patients (age, ≤48; p < 0.03), and for those with diagnosis of hematologic malignancies (p = 0.003). Response rate was higher for patients transplanted between 2006 and 2009 compared to those treated in earlier periods, p < 0.004 (Table 3).
Toxicity to conditioning chemotherapy
GI toxicity
Grade III–IV mucositis, grade II–III nausea/vomiting, and grade II diarrhea were common nonhematological toxicities (Table 4). Risk of grade III–IV nausea/vomiting (p < 0.02), diarrhea (p < 0.007), and mucositis (p < 0.001) was higher among patients who received HD melphalan for conditioning compared to those receiving CBV and Bu-Cy2 regimen. In the myeloma group, 44 of 143 patients received inj. amifostine (a cytoprotector) 740 mg/m2 over 20 min just prior to high-dose melphalan; grade III and IV mucositis was not significantly different between two groups; 72.1% vs. 72%, p = 0.56.
Renal toxicity
Renal dysfunction was noted in 95 (41.7%) patients; grade I in 70 (30.7%), grade II in 17 (7.5%), and grade III in eight (3.5%) patients. Causes included medication(s) related in 51/95, high-dose chemotherapy in one, and tumor lysis in 15/95, sepsis ± medication in 10/95 and of indeterminate cause in 18 patients. Common medications attributed for renal dysfunction were antibiotics, amikacin, vancomycin, and amphotericin-B. Two patients died of acute renal failure; one was secondary to HD carboplatin. Another patient with refractory Hodgkin’s lymphoma (more than five regimens prior to transplant) died of acute renal failure on day +3 following HD chemotherapy with BEAM protocol. There was no evidence of sepsis on autopsy. Among eight patients with grade III renal toxicity, two had grade III VOD and four patients were cases of myeloma with end-stage renal disease.
Liver dysfunction and SOS
Fifty-eight (25.4%) patients had liver dysfunction, grade I in 49 (21.5%), grade II in six (2.6%), and grade III in three patients (1.3%). Liver dysfunction was attributed to medication in 14/58 (26%), sepsis in nine (15.5%), high-dose chemotherapy (cyclophosphamide, carboplatin) in 5/58, hepatitis in 3/58 (hepatitis B1, hepatitis C1, CMV-1), veno-occlusive disease (SOS) in 21/58, recurrent disease in 1/58, and indeterminate cause in five patients. The common medications attributed for liver dysfunction included fluconazole, itraconazole, amoxyclav, and amphotericin-B.
SOS was seen in 21 patients; grade I in 17, grade II in two, and grade III in two patients each. One patient with grade III SOS died; he was a case of refractory NHL and had received BEAM protocol for conditioning.
Cardiac toxicity
Four (1.7%) patients had evidence of cardiac toxicity, being mild in one, moderate in two, and one patient died of severe cardiac toxicity. The latter was considered secondary to high-dose cyclophosphamide induced acute myocarditis. This was a case of low-grade NHL with single functioning kidney.
Lung toxicity
Acute grade III pulmonary dysfunction was seen in six patients, in five due to pulmonary alveolar hemorrhage (PAH). Three patients died of severe PAH; these included two patients of AML in second CR and third patient with refractory myeloma. Three patients recovered following methyl prednisolone and supportive treatment. Grade III pulmonary toxicity was higher with Bu-Cy2 protocol.
Hemorrhagic cystitis
Four patients (1.7%) patients had evidence of drug induced cystitis possibly secondary to cyclophosphamide. This was self-limiting and resolved with conservative management.
CNS toxicity
Seventeen (8.2%) patients had evidence of central nervous system (CNS) toxicity characterized by somnolence, delirium, and tremors. This was grade I in 16 (7.7%) and grade III in one (0.5%) patient. There was no evidence of metabolic abnormalities at the time of CNS toxicity and were considered unrelated to medication.
Engraftment syndrome
Thirty three (14.9%) patients had evidence of engraftment syndrome (ES). Findings included weight gain (32/33), fever (21/33), dyspnoea (23/33), pleural effusion (12/33), skin rash (10/33), impaired liver functions (16/33), and renal functions (6/33). The median time for onset of engraftment syndrome was 11 days (range, 9–22 days). This required investigations to rule out other potential cause, e.g., infection. In most patients, findings gradually improved after stopping growth factors and with diuretics and after steroid use in two patients.
Infections
A total of 293 febrile episodes (mean 1.3) were recorded; neutropenic, 97.3%. Infection could be documented clinically and radiologically in 33.5%; clinical, radiological, and microbiologically in 12.8%; and clinical + microbiologically in 9.3% and microbiologically alone in 5.7% of febrile episodes. The remaining 34.4% of episodes were defined as isolated febrile episodes (Table 5).
The chest was the most common site of infection (24.2%) followed by GIT (11.9%; neutropenic enterocolitis and perianal, two patients had enterocolitis due to clostridium difficle), upper respiratory tract (3.5%), and skin infections (2.2%). Microbiologically, organisms could be isolated in 68 patients (31.6%). Isolates were Gram-negative in 17.2%, Gram-positive 10.6%, and polymicrobial in 3.5% of patients. Organisms could be isolated in 18.6% of patients from central line, these being Gram-positive in 9.3%, Gram-negative in 7.1%, polymicrobial in 1.5%, and fungal in 0.9% of isolates.
Ninety-four patients received amphotericin-B therapy in view of persistent fever. Fungal infection was suspected in 19 of them on basis of chest X-ray and high-resolution CT scan of chest [20, 21] but could be confirmed in 11 patients either on broncho-alveolar lavage, biopsy/cytology, or culture. Isolated organisms were aspergillus in six, candida in three, mucor in one, and penicillium in one patient. Viral infections were seen in 5% of recipients; herpes zoster in 2.2%, herpes simplex in 1.8%, and cytomegalovirus infection in two (0.9%) recipients.
Five patients (2.4%) received empirical anti-tubercular (ATT) treatment based on CT scan finding (mediastinal lymph nodes enlargement in two, pleural effusion in one, pleural effusion + pulmonary nodule in one, and bone marrow PCR + ve for mycobacterium tuberculosis in one patient). In all the five patients, fever and radiological findings resolved following ATT.
Day 30 and 100 mortality
Twenty three (10.1%) patients died before day 30, including 15 patients with graft failure. Causes of day 30 mortality included infection-related deaths in 12 (5.3%) patients, regimen related (n = 7; 3.1%), progressive disease in three (1.3%), and pulmonary embolism in one patient. Infection-related deaths included fungal infection in four (pulmonary aspergillosis in two, mucormycosis with hemophagocytosis in one, and candidimia in one) and Pneumocystis jiroveci (PCP) in one patient. The remaining seven patients had sepsis with or without multiorgan failure (Table 5). Regimen-related mortality was due to pulmonary alveolar hemorrhage in three patients, acute cardiac toxicity secondary to high-dose cyclophosphamide in one, and acute renal failure in two patients including one secondary to high-dose carboplatin and grade III SOS in one patient. Another six patients died between day 30 and 100; causes included relapse/refractory disease in four patients (2/4 also had fungal pneumonia) and sepsis with multi-organ failure in two patients, both were on prolonged ventilation. Risk of day 30 mortality was higher for patients with ECOG performance status 3–4 (13/35 vs. 10/193; p < 0.001), chemo-refractory disease (12/62 vs. 11/166; p < 0.007), female patients (12/58 vs. 11/158; p < 0.02), and those who failed to engraft by day +18 (p < 0.001). There was no difference in the day 30 mortality according to the diagnosis subtype.
Current status and survival
One hundred ninety-nine patients (87.3%) were alive on day +100 onwards; of these, 108 (54.3%) patients are currently alive at a median follow-up of 66 months, 74 disease-free and 34 patients are alive with disease. Ninety one (45.7%) patients have died; relapse being the main cause (84/199 = 42.2%). Other causes (n = 7) were hepatitis B and C in one case each, P. jiroveci (PCP) in one, acute myocardial infarction in one, suicide in one, and secondary malignancy (myelodysplastic syndrome, n = 1), and graft versus host disease (GVHD) in one patient. The latter patient, a 26-year-old male with myeloma underwent allogeneic peripheral blood stem cell transplant following relapse 2 years after ASCT; he died of acute GVHD on day +116, his myeloma was in remission at the time of death.
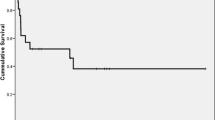
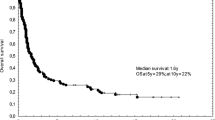
At a median follow-up of 66 months, median OS and EFS for all patients is 65 months (95% CI 44.78–85.22) and 22 months (95% CI, 15.69–28.31), respectively. For multiple myeloma, median OS and EFS are 79 months (95% CI 52.3–105.7) and 30 months (95% CI 22.6–32.7), respectively. Estimated 5-year OS and EFS is 72% ± .03 (SE) and 32.7% ± 04 (SE), respectively. The corresponding figures for 10-year OS and EFS are 48% ± .05 (SE) and 17.2% ± .05 (SE), respectively (Figs. 1, 2). For Hodgkin’s lymphoma, median OS is 81 months (95% CI 69.7–92.3). Median EFS has not reached yet. Corresponding figures for NHL are 12 months (95% CI 0–24.8) and 5 months (95% CI 2.5–7.5), respectively (Figs. 3 and 4). For acute myeloid leukemia patients, median OS and EFS are 8 months (95% CI, 1.4–14.6) and 6 months (95% CI 1.5–10.5), respectively.
Overall survival was superior for patients who were in CR1 or PR1 at the time of transplant compared to those in CR2 and PR2 which was higher than those with stable or progressive disease prior to transplant; 88 vs. 84 (95% CI 55.2–112.8) vs. 14 months (95% CI 3.39–24.61), p < 0.001. Corresponding figures for EFS are 52 months (95% CI 19.86–84.13) vs. 31 months (95% CI 21.50–40.5) vs. 5 months (95% CI 2.43–7.57), p < 0.001.
Prognostic factors
Patients with pretransplant chemo-sensitive disease (p < 0.001, Fig. 5), good ECOG performance status (0–2 vs. 3–4, p < 0.001) at the time of transplant, and those with diagnosis of hematologic malignancies (vs. solid tumors, p < 0.004) had a significantly longer OS and EFS. Both OS (p < 0.001) and EFS (p < 0.002) were higher for patients treated between 2006 and 2009 compared to those treated between 1990–2000 and 2001–2005. Patients who responded to transplant had a significantly longer overall and event-free survival. On Cox regression multivariate analysis, good ECOG performance status at the time of transplant, treatment period (2006 and 2009) and response to transplant emerged as the most significant predictors of both OS and EFS (Table 6).
Discussion
A periodic audit of stem cell transplant data is important to get insight into the transplant-related toxicities, infections, and overall outcome. In line with the ASCT experience as reported from Center for International Blood and Marrow Transplant Research (CIBMTR) and EBMT, we too observed that (1) multiple myeloma and lymphoma were two main indications for ASCT, (2) infections and regimen-related toxicities (mucositis, GI toxicity, renal and liver dysfunctions) were important cause of morbidity and mortality; these being higher in patients with pretransplant chemo-resistant disease, and (3) event-free and overall survival was higher for patients with good ECOG performance status and those who responded to transplant.
In the current study, patients’ median age was 48 years, 38.6% and 7 % of patients were older than 50 and 60 years, respectively. With improved supportive care, more and more eligible patients in the higher age group are being transplanted. This is also reflected in data from CIBMTR; between the year 2003 and 2007, 65% and 32% of patients were above age of 50 and 60 years, respectively, compared to lower percentage in earlier years [2]. Among 228 recipients, myeloma and lymphoma together accounted for 82% of all cases. In fact, both these conditions are currently the most common indications for ASCT internationally too [1–4].
Grade III–IV nausea/vomiting, diarrhea, and oral mucositis were major GI toxicities and were significantly higher with high-dose melphalan compared to CBV and Bu-Cy2 regimen. We did not observe reduction in grade 3–4 mucositis with inj. amifostine administered prior to high-dose melphalan in 61 of 143 myeloma patients as suggested by Spencer et al. [22]. Frequency and severity of renal dysfunction [23], liver dysfunction [24, 25], SOS (VOD) [26], pulmonary toxicity [27, 28], hemorrhagic cystitis [29], cardiac [30], and CNS toxicity [31] is similar to previous studies and was not significantly different among patients receiving HD melphalan, CBV or Bu-Cy2 regimen.
In the present study, 3.1% of patients died of regimen-related toxicities. This compares favorably to 8% in the CIBMTR data [2]. Of the patients, 14.9% had evidence of ES at a median of 11 days posttransplant. High index of suspicion for ES around day of engraftment (weight gain ≥5% was present in almost all patients with ES), stopping growth factors, liberal use of diuretics and steroids in two patients might be possible reasons for lack of mortality due to ES. Similar to present study, higher frequency of ES was also observed in a recent study among patients receiving HD melphalan [32].
Patients with pretransplant chemo-sensitive disease (p < 0.001), good ECOG performance status (p < 0.001), age ≤48 years (p < 0.03), and those with hematological malignancies (p < 0.003) had a significantly higher response to transplant (Table 3). These observations are similar to those reported in international [1–4] and singlecenter [33] studies.
Risk of transplant-related morbidity and mortality is directly proportional to the recipient’s disease status (chemo-sensitive vs. chemo-resistant/refractory) and performance status (ECOG, 0–2 vs. 3–4) at the time of transplant [1–4, 13, 33]. Early (day 30) transplant-related mortality was significantly higher among patients with pretransplant chemo-resistant disease 19.3% (12/62) vs.6.62% (11/166), p < 0.007. Most of these deaths were in the initial starting years of transplant program and once learning curve is over, mortality rate has come down; day 30 mortality was 2.7% (3/72) during the period 2006–2009, compared to 13% (10/77) and 12.6% (10/79) in previous years 1990–2000 and 2001–2005, respectively.
Infections (secondary to severe myelosuppression) remain the major cause of morbidity and mortality in early posttransplant (day 0–30) period. Important observations in present study are (1) higher frequency of Gram-negative bacterial isolates, (2) use of amphotericin-B in 43.6% of ASCT recipients, and (3) antitubercular treatment in five (2.4%) patients. Higher frequency of Gram-negative organisms has also been observed among patients of AML at our center [17]. It is important to reiterate here that all these transplants were carried out in single rooms without any HEPA filter or laminar air flow facilities [34, 35]. Due to increased frequency of possible fungal infections, we have now adopted a policy in our unit to start amphotericin-B early by day 4 or 5, if fever does not resolve or if there are radiological signs suggestive of fungal infection [36]. Mycobacterium tubercular infection has been reported occasionally in both autologous and allogeneic transplant recipients, possibly due to reactivation following severe myelosuppression and immune suppression [37].
Higher response rates and reduced morbidity and mortality among patients with pretransplant chemo-sensitive disease will argue in favor of adequate pretransplant therapy and developing criteria for proper case selection for ASCT for optimum outcome. After learning from this experience, we now transplant patients (e.g., myeloma) in CR or very good PR. Patients with stable or progressive disease are offered salvage therapy and ASCT is considered in responders. Improved overall and event-free survival in two major subgroups—myeloma and Hodgkin’s lymphoma (possibly a result of better case selection and improved management of infections associated with reduced early mortality over a period of time) has been encouraging and suggest that it is possible to develop transplant programs in developing countries [1, 11–13] and achieve results similar to international data.
References
Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A et al (2010) Hematopoietic stem cell transplantation. A global perspective. JAMA 303:1717–1724
CIBMTR Center for International Blood and Marrow Transplant. Research newsletter. August 2009; 15(1): 7–8 (Summary slides)
Ljungman P, Urbano-ispizua A, Cavazzana Calvo M, European Group for Blood and Marrow et al (2006) Allogeneic and autologous transplantation for hematological diseases, solid tumours and immune disorders: definitions and current practice in Europe. Bone Marrow Transplant 37:439–449
Nivison-Smith I, Bradstock KF, Dodds AJ et al (2007) Hematoloietic stem cell transplantation in Australia and New Zealand,1992–2004. Biol Blood Marrow Transplant 13(8):905–912
Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M et al (2002) Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet 359(9323):2065–2071
Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A et al (1996) Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet 347(8998):353–357
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, Intergroupe Francais du Myelome et al (1996) A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. N Engl J Med 11(335(2)):91–97
Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Children’s Cancer Group et al (1999) Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. N Eng J Med 341:1165–1173
Breems DA, Boogaerts MA, Dekker AW, Van Putten WL, Sonneveld P, Huijgens PC et al (2005) Autologous bone marrow transplantation as consolidation therapy in the treatment of adult patients under 60 years with acute myeloid leukemia in first complete remission: a prospective randomized Dutch–Belgian haemato-Oncology Co-operative Group (HOVON) and Swiss Group for Clinical Research (SAKK) trial. Br J Haematol 128:59–65
Lorch A, Kollmannsberger C, Hartmann JT, Metzner B, Schmidt-Wolf IG, Berdel WE, German Testicular Cancer Study Group et al (2007) Single versus sequential high-dose chemotherapy in patients with relapsed or refractory germ cell tumors: a prospective randomized multicenter trial of the German Testicular Cancer Study Group. J Clin Oncol 25(19):2778–2784
Kumar L, Ghosh J, Ganessan P, Gupta A, Hariprasad R, Kochupillai V (2009) High dose chemotherapy with autologous stem cell transplantation for multiple myeloma: what predicts the outcome? Experience from a developing country. Bone Marrow Transplant 43:481–489
Chandy M (2008) Stem cell transplantation in India. Bone Marrow Transplant 42(Suppl):S81–S84
Mahmoud H, El-Haddad A, Fahmy O, El-Emary M, Nassar A, Abdel-Mooti M et al (2008) Hematopoietic stem cell transplantation in Egypt. Bone Marrow Transplant 42(Suppl 1):S76–S80
Ruiz-Argüelles GJ, Gómez-Rangel D, Ruiz-Delgado GJ, Ruiz-Argüelles A, Pérez-Romano B, Rivadeneyra L (2003) Results of an autologous noncryopreserved, unmanipulated peripheral blood hematopoietic stem cell transplant program: a single-institution, 10-year experience. Acta Haematol 110(4):179–183
Bedi R, Kumar L, Kochupillai V (2003) Autologous peripheral blood stem cell transplantation: predictors for haemapoietic reconstitution. Nat Med J India 16:209–213
Raju GMK, Kochupillai V, Kumar L (1995) Storage of haematopoietic stem cells for autologous bone marrow transplantation. Nat Med J India 8:216–221
Gupta A, Singh M, Singh H, Kumar L, Sharma A, Bakhshi S et al (2010) Infections in acute myeloid leukemia: an analysis of 382 febrile episodes. Med Oncol 27(4):1037–1045
Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Buckner CD, Sullivan KM et al (1988) Regimen related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 6:1562–1568
McDonald GB, Hinds MS, Lloyd RN, Schoch HG, Wolford JL, Banaji M et al (1993) Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Int Med 118:255–267
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting the results of cancer treatment. Cancer 47:207–211
Blade J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G et al (1998) Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol 102:1115–1123
Spencer A, Horvath N, Gibson J, Prince HM, Herrmann R, Bashford J et al (2005) Prospective randomized trial of amifostine cytoprotection in myeloma patients undergoing high-dose melphalan conditioned autologous stem cell transplantation. Bone Marrow Transplant 35:971–977
MerouaniA SEJ, Jones RB, Archer PG, Schrier RW (1996) Renal function in high dose chemotherapy and autologous hematopoietic cell support treatment for breast cancer. Kidney Int 50(3):1026–1031
Ozdogan O, Ratip S, Ahdab YA, Dane F, Ahdab HA, Imeryüz N, Tözün N (2003) Causes and risk factors for liver injury following bone marrow transplantation. J Clin Gastroenterol 36:421–426
Ho GT, Parker A, MacKenzie JF, Morris AJ, Stanley AJ (2004) Abnormal liver function tests following bone marrow transplantation: aetiology and role of liver biopsy. Eur J Gastroenterol Hepatol 16:157–162
Carreras E, Bertz H, Arcese W, Vernant JP, Tomás JF, Hagglund H et al (1998) Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood 92(10):3599–3604
Afessa B, Tefferi A, Litzow MR, Peters SG (2002) Outcome of diffuse alveolar hemorrhage in hematopoietic stem cell transplant recipients. Am J Respir Crit Care Med 166:1364–1368
Majhail NS, Parks K, Defor TE, Weisdorf DJ (2006) Diffuse alveolar hemorrhage and infection-associated alveolar hemorrhage following hematopoietic stem cell transplantation: related and high-risk clinical syndromes. Biol Blood Marrow Transplant 12(10):1038–1046
Leung AY, Mak R, Lie AK, Yuen KY, Cheng VC, Liang R, Kwong YL (2002) Clinicopathological features and risk factors of clinically overt haemorrhagic cystitis complicating bone marrow transplantation. Bone Marrow Transplant 29:509–513
Mileshkin LR, Seymour JF, Wolf MM, Gates P, Januszewicz EH, Joyce P, Prince HM (2005) Cardiovascular toxicity is increased, but manageable, during high dose chemotherapy and autologous peripheral blood stem cell transplantation for patients aged 60 years and older. Leuk Lymphoma 46:1575–1579
Saiz A, Graus F (2004) Neurological complications of hematopoietic cell transplantation. Semin Neurol 24(4):427–434
Carreras E, Fernandez-Aviles F, Guerrero SM, Guerrero M, de Larrea CF, Martínez C et al (2010) Engraftment syndrome after auto SCT: analysis of diagnostic criteria and risk factors in a large series from a single center. Bone Marrow Transplant 45(9):1417–1422
Sorror M (2009) Impacts of pretransplant comorbidities on allogeneic hematopoietic cell transplantation (HCT) outcomes. Biol Blood Marrow Transplant 15(1 Suppl):149–153
Jantunen E, Salonen J, Juvonen E, Koivunen E, Siitonen T, Lehtinen T et al (2004) Invasive fungal infections in autologous stem cell transplant recipients: a nation-wide study of 1,188 transplanted patients. Eur J Haematol 73:174–178
Passweg JR, Rowlings PA, Atkinson KA, Barrett AJ, Gale RP, Gratwohl A et al (1998) Influence of protective isolation on outcome of allogeneic bone marrow transplantation for leukemia. Bone Marrow Transplant 21(12):1231–1238
Russell JA, Chaudhry A, Booth K, Brown C, Woodman RC, Valentine K et al (2000) Early outcomes after allogeneic stem cell transplantation for leukemia and myelodysplasia without protective isolation: a 10-year experience. Biol Blood Marrow Transplant 6(2):109–114
George B, Mathews V, Srivastava V, Srivastava A, Chandy M et al (2001) Tuberculosis among allogeneic bone marrow transplant recipients in India. Bone Marrow Transplant 27:973–975
Acknowledgments
We acknowledge the excellent care and dedication of residents and nursing staff of Medical Oncology Department. Mr. Shanti Swaroop and Narendra Singh helped in harvesting of stem cells and Ms. Mercy Thomas, Dr Sujata Mohanti, and Dr. Sobuhi Iqbal helped in cryopreservation of stem cells.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, L., Malik, P.S., Prakash, G. et al. Autologous hematopoietic stem cell transplantation—what determines the outcome: an experience from North India. Ann Hematol 90, 1317–1328 (2011). https://doi.org/10.1007/s00277-011-1205-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-011-1205-4