Abstract
Objective
To prospectively compare the safety of transcatheter embolization of superior rectal arteries in healthy pigs with multiple agents such as coils, spheres and liquids.
Materials and Methods
Nine adult domestic pigs (three males, mean weight: 60 kg [50–70]) were randomly assigned to the embolization group: copolymer of ethylene vinyl alcohol (EVOH)—Onyx® (group 1, n = 3), microspheres 500 µ (group 2, n = 3), 2-mm micro-coils (group 3, n = 3). After a selective angiogram has been acquired, the embolic agent was infused at the distal part of rectal arteries. An angio-CT was performed before and after each embolization. After one week, angiography was repeated prior to euthanasia. At necropsy, the anorectal juncture was removed for histopathologic examination.
Results
At necropsy, 100% of animals embolized with Onyx developed a significant necrosis zone of the distal part of the rectum. Histological examination revealed a mural infarction. For the micro-coil and microsphere groups, gross examination of the intestines did not reveal any evidence of ischaemia. The coils were found in the distal arterial vasculature of the meso-rectum, allowing a downstream revascularization by collaterals. The microspheres and onyx in the rectal wall, more distally.
Conclusion
Microspheres appear to induce fewer histologic complications than the liquid embolic agent and provide a more distal occlusion than micro-coils. These results suggest that, for superior rectal artery embolization, a super-selective embolization using spheres in human clinical conditions should be more effective and as safe as coil embolization. EVOH might be an unsafe embolization agent for haemorrhoids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Based on a pathophysiological concept that excessive arterial blood supply to the internal haemorrhoidal plexus is associated with the pathogenesis of haemorrhoids [1,2,3,4,5,6,7,8,9], new minimally invasive methods have emerged, such as haemorrhoidal artery embolization, termed emborrhoid by Vidal et al. [10]. This technique consists of an endovascular occlusion with micro-coils of the distal branches of the superior rectal artery (SRA). This technique has shown good short-term results on chronic haemorrhagic symptoms [11,12,13,14,15] with an average of 30% of recurrences, related to a reperfusion by collaterals of the corpus cavernosum recti downstream of the coil packing [15]. Those collaterals arise both from the SRA and from the middle rectal arteries that are branches of the internal iliac arteries. Micro-coils are known to be safe embolic agents for gastrointestinal haemorrhage [16]. A lack of efficacy can occur; however, if the coil placement is too proximal [17] or if there is an arteriovenous malformation-like appearance with a nidus, it has been described in haemorrhoid pathology [15, 18]. Liquids and microspheres are also effective embolic agents for acute gastrointestinal bleeding, especially in case of hyper-vascularization and distal target [19, 20]. Although they are less frequently used, the distal nature of the occlusions could lead to a higher risk of ischaemia [21]. Embolization with liquid agents and calibrated microspheres in haemorrhoidal pathology were not described in humans, according to the available literature when the study was designed. This more distal embolization should prevent haemorrhoidal revascularization by superior or middle rectal branches. Domestic pigs are becoming more frequently used in interventional radiology research because of many anatomical and physiological similarities with humans [22]. It has been demonstrated that the porcine model can be successfully used to perform mesenteric embolization [23]. Rectal arterial vascularization in pigs arises principally from the SRA, as described in humans. However, haemorrhoid pathology does not exist in pigs to according to knowledge.
The main objective of this study was to prospectively compare the safety of transcatheter embolization of superior rectal arteries in healthy pigs with multiple agents: coils, spheres and liquids.
Materials and Methods
Animals
The experimental protocols were approved by the Institutional Ethics Committee for Animal Research. Between February and March 2016, nine adult domestic Pietrain pigs (3 males, mean weight: 60 kg ± 10) were used. All the pigs were randomly assigned to an embolization group: liquid group (n = 3), microsphere group (n = 3) and micro-coils as a control group (n = 3).
Animal Preparation
The animals were kept fasting 24 h before the procedure.
On the day of embolization, a rectal enema with lukewarm water was performed at the animal facility. After initial sedation with intramuscular ketamine (20 mg/kg) and robinul (1 μg/kg), the animal was fitted with a peripheral venous line into an ear vein (propofol 2 mg/kg added), ECG electrodes and a saturation sensor positioned and oro-tracheal intubation. Anaesthesia was maintained with 1.5% sevoflurane.
Intramuscular antibiotic prophylaxis was performed during the course of the procedure (cefalexin 30 mg/kg).
Angiographic Procedure
All angiographies were performed in the experimental procedure radiology unit. The animal was positioned supine on an X-ray table equipped with a mobile digital C-arm (OEC Fluorostar 7900, General Electric Healthcare, Milwaukee, WI, USA). Under strict aseptic conditions and ultrasound control (SSA-700A Aplio 50, Toshiba, Tokyo, Japan) and using the Seldinger technique, a 6-French femoral arterial introducer (Ultimum St Jude Medical 23 cm, St Paul, MN, USA) was inserted. A 4-F multiperforated pigtail catheter (Pigtail Imager II Boston Scientific, Natick, Massachusetts, MA, USA) was then placed in the sub-renal abdominal aorta. The pig was then moved onto a CT scanner (Discovery PET/CT 710, General Electric Healthcare, Milwaukee, WI, USA) to perform a pelvic angio-CT: contrast medium (Visipaque 320 mg/ml, GE Healthcare, Milwaukee, WI, USA) diluted to 50% injected with an automatic injector at a flow rate of 8 cc/s (DUAL SHOT alpha, Nemoto, Tokyo, Japan). The image datasets were automatically transferred to a workstation (Advantage Windows, GE Medical Systems, Milwaukee, WI, USA) and reconstructed in 3D volume.
After returning to the angiography table, the catheterization of the inferior mesenteric artery was performed using a 4-F or 5-F catheter (SHK Imager II Boston Scientific), followed by subtracted digital angiography to identify the arteries of the lower rectum and anal canal.
A microcatheter (Rebar 2.4, Medtronic, Minneapolis, MN, USA) was then positioned distal to the SRA. A new angiography was performed to look for possible anastomoses in the internal iliac branches (Figure 1, supplementary online material).
Embolization
Embolization of the subpubic branches of both superior rectal arteries using the embolizing agent was selected at random (randomization with the schema 1:1:1, with the group number placed into sealed envelopes).
Group 1: Ethylene Vinyl Alcohol Copolymer (EVOH)—Onyx® (Medtronic, Minneapolis, MN, USA)
After rinsing the microcatheter with saline, the dead space in the microcatheter was then filled with 0.5 ml of dimethyl sulfoxide (DMSO, Medtronic, Minneapolis, MN, USA). Then, Onyx 34 (Medtronic, Minneapolis, MN, USA) was injected progressively with a 1-ml syringe. This injection was always done under fluoroscopy. If the embolic agent refluxed onto the tip of the microcatheter, the injection was stopped for an average of 1 min. In case of persistent reflux, the endpoint was considered to be achieved. The total duration of the Onyx injection varied between 3 and 5 min.
Group 2: Microspheres Embozene 500 µ® (Boston Scientific, Natick, MA, USA)
After rinsing the microcatheter with saline, particles suspended in iodinated contrast medium (Visipaque 320 mg/ml, GE Healthcare, Milwaukee, WI, USA) were injected using a 1-ml syringe in free flow until the flow slowed significantly. This injection was always done under fluoroscopy. If the embolic agent refluxed onto the tip of the microcatheter, the injection was stopped for an average of 1 min. The near stasis of contrast defined the endpoint. The total duration of microparticle injection varied between 5 and 10 min.
Group 3: 2-mm Axium® Micro-coils (Medtronic, Minneapolis, MN, USA)
The microcatheter was positioned in each of the right and left branches, and 2-mm-diameter micro-coils were released using the pusher guide under continuous fluoroscopic control. Repositioning of the micro-coils in case of incorrect packing was possible due to the mechanical detachment mode. After embolization, a control angiography was performed to confirm the definitive occlusion of the target branches. Again, the animal was placed on the CT scanner to perform a post-procedure pelvic angio-CT with the same parameters.
The material was then removed, and the puncture site closed by manual compression for 10 min. The animals were then extubated and transported to the laboratory animal facility for surveillance.
Post-embolization Animal Monitoring
The pigs were monitored clinically on a multi-day basis for 7 days. On the 7th day, a new procedure was performed under general anaesthesia following the same protocol as on inclusion. The animal was prepared with a rectal enema before the procedure.
The animal was positioned supine on a radiology table. A right femoral arterial puncture under ultrasound control using the Seldinger technique allowed the placement of a 6-F introducer. After the catheterization the inferior mesenteric artery using a 4-F catheter, a digital subtraction arteriography ensured that there was no early repermeabilization. An angiogram of the internal iliac arteries was performed to look for revascularization of the anorectal vasculature. A post-procedure pelvic angio-CT was then performed to look for localized complications and vascular re-management.
On the basis of this angiography and the angio-CT, a vascular revascularization score was established as follows: 0 for no revascularization, 1 for unilateral revascularization and 2 for bilateral revascularization. On completion of these control procedures, the animal was euthanized under general anaesthesia using the usual protocol (midazolam 15 mg, chlorpromazine 25 mg, KCL 15% (w/v) 20 ml).
Macroscopic Examination
As soon as the animal was euthanized, we proceeded with anorectal sampling by the lower route, allowing us to extract the anal margin, the anal canal and the lower rectum with its meso-rectum (about 15 cm high in total) in one piece. These samples were analysed in a cold room to look for necrosis, perforation or abscesses. They were then immersed in 10% buffered formalin (Tissue-Tek VIP, Sakura) for fixing. The samples were then embedded in paraffin (Tissue-Tek VIP5 Jr, Sakura) before being cut into 5-µm-thick slices and assembled on slides.
Histological Examination
After haematoxylin–eosin staining by the automated stainer (Tissue-Tek DRS 2000, Sakura), the slides were dehydrated and stabilized by the film splicer (Tissue Tek Film, Sakura).
The analysis was done on the optical microscopy station (Eclipse Ni, Nikon) by one pathologist looking for evidence of mucosal or transmural ischemia that may have occurred as a result of embolotherapy. Each specimen was assigned an ischemia score: 0: normal, vascular congestion, mild oedema; 1: moderate oedema, chronic inflammation; 2: marked oedema, chronic inflammation, epithelial atrophy; 3: early or massive epithelial necrosis.
Each specimen was also assigned a score for intramural penetrance in the rectal wall: 0 if penetrance does not extend beyond the muscularis and 1 if penetrance is beyond the muscularis in the section.
Results
Anatomy
The inferior mesenteric artery originated at the anterior aspect of the aorta between 2.5 and 3 cm above the aortic bifurcation. The superior rectal artery (SRA) of all nine pigs was the main rectal artery, measuring between 2.5 and 3 mm in diameter at its origin. The SRA runs vertically downwards and vascularizes the whole rectum up to the anal margin via perforating arteries. We noted a systematic division above the pubic symphysis into a left branch and a right branch.
In 90% of cases (8 pigs), there was a calibre asymmetry between the left and right branch. In 80% of the specimens (seven pigs), we identified large perianal anastomotic networks at the anal margin, connecting the distal branches of the SRA with the internal iliac arteries (Figure 2, supplementary online material).
An embolization of both superior rectal branches was achieved in all three groups. Technical success of embolization was 100% without off-target migration.
Group 1: A distal progression of Onyx was observed (5 cm on average ± 1 cm) occupying the proximal divisions of the branches but also multiple branches (n = 3 ± 1) towards perforating arteries to the submucosa (Fig. 1). Day 7 CT-scan and angiography controls showed no repermeabilization. Gross examination on day 7 showed areas of focal necrosis of the anorectal junction (20 mm ± 5 mm in diameter) in 100% of cases (Fig. 2). Histological analysis confirmed these findings with areas of transmural necrosis reaching the epithelial lining, the submucosa and the inner muscular layer. Onyx, characterized by its black colour, was located both superficially (meso-rectum) and deep in the submucosa and even in parts of the mucosa (Fig. 3).
Group 2: Day 7 CT scan and angiography controls showed no repermeabilization (Fig. 4). Gross examination and histological analysis on day 7 did not show any signs of macroscopic ischaemia (Fig. 5). One specimen had an area of focal necrosis on the surface epithelium, which was attributed to the fixing process. There was no oedema, congestion or infiltration suggestive of post embolization ischemia. The microparticles were found to be distributed both superficially (meso-rectum) and deep within the submucosa. They were located in the vascular lumen, associated with a moderate peripheral inflammatory reaction (Fig. 6).
Histological analysis with haematoxylin and eosin stain of ano-rectal specimen from microspheres group showing the absence of epithelial necrosis (A). Microspheres of 500 µ are seen into vascular spaces into muscularis (A, green arrow) and sub-mucosa (A, black arrows), surrounded by moderate inflammatory reaction (B, blue arrows). Note the spherical and unfragmented appearance of the beads (C)
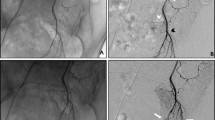
Group 3: Day 7 CT-scan and angiography showed a revascularization of the SRA branches downstream of the occlusions by the coil packing through collateral arteries from the internal iliac arteries (Fig. 7). Gross examination on day 7 showed no parietal necrosis, which was confirmed by histological analysis. One specimen had an area of focal necrosis on the surface epithelium which was attributed to the fixing process (Fig. 8).
Angio-CT 3D reformats before embolization of the superior rectal artery branches with micro-coils (A) and 7 days after the embolization showing a downstream reperfusion of the rectal branches by the internal iliac arteries after the embolization (B, white arrows), with no recanalization of the coil packing at angiography (C, black arrows). The anastomosis between superior rectal arteries and the left internal iliac artery was visible before the embolization (D, blue arrow)
Ano-rectal fixed specimen from micro-coils group showing a normal aspect at gross examination of the ano-rectal mucosa (A). Micro-coil is seen in a proximal branch of the SRA at the level of meso-rectum (B, black arrow). Histological analysis with haematoxylin and eosin stain of ano-rectal specimen group showing the absence of epithelial necrosis (C)
Histological findings are summarized in Table 1.
Complications
All animals survived the various anaesthetic and embolization procedures without any signs of poor clinical tolerance at the end of the week of monitoring.
A femoral puncture site haematoma was found in a pig from the Onyx group.
A distal arterial rupture per-embolization within the meso-rectum occurred in a pig in the micro-coil group without any consequences.
One case of cellulitis of the perineum and tail was identified in a pig from the microparticle group. Staged histological samples did not reveal any off-target embolization, pointing to an infectious cause.
Discussion
The results of this study suggest the absence of rectal ischemia after super-selective embolization with 500-µ calibrated microparticles, opening an additional therapeutic perspective in the treatment of chronic haemorrhoidal bleeding. These results are in agreement with those of the recent literature about haemorrhoid arterial embolization [24,25,26]. Recently, a study comparing the use of different sizes of microspheres (500–700 μm, 700–900 μm and 900–1200 μm) showed a clinical success rate of 93% on haemorrhoidal bleeding. Not surprisingly, the rate of minor complications was high, close to 50%, and consisted of small ischemic ulcerations of the anorectal junction [27]. In the present study, the size of the microspheres was based on Kusano’s study who used a microparticle diameter threshold of 400µ above which he did not observe intestinal ischemia in an animal model, by preserving the vasa recta [28].
As regards embolization with EVOH (Onyx), its use in the context of gastro-intestinal (GI) haemorrhage remains very limited. However, the literature argues for a low rate of ischemic complication in embolization of GI bleeding [19, 29, 30]. In this study, rectal necrosis was found in 100% of cases after distal Onyx embolization. A possible explanation for this discrepancy with the literature is the lack of systematic endoscopic or histological analysis in the reported clinical series [30, 31]. Another possible explanation is the absence of a pathological target in the study’s specimens. EVOH, which has a very high penetrative power, was then distributed throughout the downstream vascular bed up to the sub-mucosal arterioles, preventing any revascularization. Unlike microparticles, whose calibration makes it possible to control the level of distality in the occlusion, EVOH is a liquid agent that progresses to polymerization [32]. It is possible that blocked flow injection due to the small size of the rectal arteries at the level of the pubic symphysis favoured very distal migration, as described with balloon-assisted embolization [33].
Concerning the micro-coils, results are in agreement with those published in the literature concerning humans [13,14,15] and animals [23], as regards good tolerance without rectal ischemia. Occlusion on non-terminal branches involving extra-mural arteries (meso-rectum) allows revascularization by branches of the internal iliac arteries [15]. This mechanism tends to explain the recurrence rate of symptoms (28%) when using the emborrhoid technique with micro-coils [14, 15]. The involvement of the middle rectal arteries in the haemorrhoidal vasculature in up to 25% of cases [2, 15] suggests that combined embolization of the SRA and middle rectal arteries would improve the clinical success rate [15, 34]. In order to reach the corpus cavernosum recti, direct sclerotherapy injection has been proposed at early stage of haemorrhoids but is associated with high rate of recurrence [35].
This study has several limitations: (1) Limited number of animals does not allow for powerful statistical analysis. (2) The absence of a valid haemorrhoidal model in animals despite the pig rectal vascular anatomy being very similar to that of humans [23]. An experimental simian haemorrhoidal model using superior rectal vein ligation has been proposed [36], but it does not reflect the haemorrhoidal pathogenesis, whose link with venous hyper pressure has long been refuted [37].
In conclusion, embolization of the distal branches of superior rectal arteries on healthy pigs with calibrated microparticles (500 µ) was associated with the absence of rectal ischemia and a satisfactory occlusion. Occlusion with micro-coils was also well tolerated but with revascularization by the internal iliac collaterals. Onyx embolization in this experience resulted in focal rectal necrosis. These results suggest that, for SRA embolization, using spheres in human clinical conditions should be more effective and as safe as coil embolization. EVOH might be an unsafe embolization agent for haemorrhoids.
References
Ganz RA. The evaluation and treatment of hemorrhoids: a guide for the gastroenterologist. Clin Gastroenterol Hepatol. 2013;11:593–603.
Thompson HF. The nature of hemorrhoids. Br J Surg. 1975;62:542–52.
Schuurman JP, Go PM, Bleys RL. Anatomical branches of the superior rectal artery in the distal rectum. Colorectal Dis. 2009;11:967–71.
Stelzner F. The corpus cavernosum recti. Dis Colon Rectum. 1964;7:398–9.
Chung YC, Hou YC, Pan AC. Endoglin (CD105) expression in the development of haemorrhoids. J Clin Invest. 2004;34:107–12.
Aigner F, Bodner G, Gruber H, et al. The vascular nature of hemorrhoids. J Gastrointest Surg. 2006;10:1044–50.
Simillis C, Thoukididou SN, Slesser AAP, Rasheed S, Tan E, Tekkis PP. Systematic review and network meta-analysis comparing clinical outcomes and effectiveness of surgical treatments for haemorrhoids. Br J Surg. 2015;102:1603–18.
Watson AJ, Hudson J, Wood J, et al. Comparison of stapled haemorrhoidopexy with traditional excisional surgery for haemorrhoidal disease (eTHoS): a pragmatic, multicentre, randomised controlled trial. Lancet. 2016;388:2375–85.
Aigner F, Bodner G, Conrad F, Mbaka G, Kreczy A, Fritsch H. The superior rectal artery and its branching pattern with regard to its clinical influence on ligation techniques for internal hemorrhoids. Am J Surg. 2004;187:102–8.
Vidal V, Louis G, Bartoli JM, Sielezneff Y, et al. Embolization of the hemorrhoidal arteries (the emborrhoid technique): a new concept and challenge for interventional radiology. Diagn Interv Imaging. 2014;95:307–15.
Berczi V, Gopalan D, Cleveland TJ. Embolization of a hemorrhoid following 18 hours of life-threatening bleeding. Cardiovasc Interv Radiol. 2008;31:183–5.
Kim M, Song HJ, Kim S, Cho YK, Kim HU, Song BC, et al. Massive life-threatening lower gastrointestinal hemorrhage caused by an internal hemorrhoid in a patient receiving antiplatelet therapy: a case report. Korean J Gastroenterol. 2012;60:253–7.
Vidal V, Sapoval M, Sielezneff Y, et al. Emborrhoid: a new concept for the treatment of hemorrhoids with arterial embolization: the first 14 cases. Cardiovasc Intervent Radiol. 2015;38:72–8.
Moussa N, Sielezneff I, Sapoval M, et al. Embolization of the superior rectal arteries for chronic bleeding due to hemorrhoidal disease. Colorectal Dis. 2017;19:194–9.
Tradi F, Louis G, Giorgi R, Mege D, Bartoli JM, Sielezneff I, Vidal V. Embolization of the superior rectal arteries for hemorrhoidal disease: prospective results in 25 patients. J Vasc Interv Radiol. 2018;29:884–92.
Nicholson AA, Ettles DF, Hartley JE, et al. Transcatheter coil embolotherapy: a safe and effective option for major colonic hemorrhage. Gut. 1998;43:79–84.
Loffroy R, Guiu B, D’Athis P, et al. Arterial embolotherapy for endoscopically unmanageable acute gastroduodenal hemorrhage: predictors of early rebleeding. Clin Gastroenterol Hepatol. 2009;7:515–23.
Komekami Y, Konishi F, Makita K, Mijin T, Onogawa A, Chochi T, Lee C, Yoshida T, Maeda T, Mitsusada M, Hasegawa S. Rectal arterio-venous malformation (AVM) with bleeding of an internal hemorrhoid. Clin J Gastroenterol. 2016;9:22–6.
Lenhart M, Paetzel C, Sackmann M, et al. Superselective arterial embolisation with a liquid polyvinyl alcohol copolymer in patients with acute gastrointestinal haemorrhage. Eur Radiol. 2010;20:1994–9.
Guy EG, Shetty PC, Sharma RP, Burke MW, Burke TH. Acute lower gastrointestinal hemorrhage: treatment by superselective embolization with polyvinyl alcohol particles. AJR Am J Roentgenol. 1992;159:521–6.
Weldon DT, Burke SJ, Sun S, Mimura H, Golzarian J. Interventional management of lower gastrointestinal bleeding. Eur Radiol. 2008;18:857–67.
Dondelinger RF, Ghysels MP, Brisbois D, et al. Relevant radiological anatomy of the pig as a training model in interventional radiology. Eur Radiol. 1998;8:1254–73.
Chin AC, Singer MA, Mihalov M, et al. Superselective mesenteric embolization with microcoils in a porcine model. Dis Colon Rectum. 2002;45:212–8.
Zakharchenko A, Kaitoukov Y, Vinnik Y, et al. Safety and efficacy of superior rectal artery embolization with particles and metallic coils for the treatment of hemorrhoids (Emborrhoid technique). Diagn Interv Imaging. 2016;97:1079–84.
Moussa N, Bonnet B, Pereira H, Pechmajou L, Pellerin O, Abed A, Del Giudice C, Dean C, Bouda D, de Parades V, Fathallah N, Sapoval M. Mid-term results of superior rectal artery and coils for hemorrhoidal embolization with particles bleeding. Cardiovasc Intervent Radiol. 2020;43(7):1062–9.
Makris GC, Thulasidasan N, Malietzis G, Kontovounisios C, Saibudeen A, Uberoi R, Diamantopoulos A, Sapoval M, Vidal V. Catheter-directed hemorrhoidal dearterialization technique for the management of hemorrhoids: a meta-analysis of the clinical evidence. J Vasc Interv Radiol. 2021;S1051–0443(21):01079–84.
Küçükay MB, Küçükay F. Superior rectal artery embolization with tris-acryl gelatin microspheres: a randomized comparison of particle size. J Vasc Interv Radiol. 2021;32(6):819–25.
Kusano S, Murata K, Ohuchi H, et al. Low-dose particulate polyvinylalcohol embolization in massive small artery intestinal hemorrhage: experimental and clinical results. Invest Radiol. 1987;22:388–92.
Saeed Kilani M, Lepennec V, Petit P, Magalon G, Casanova D, Bartoli JM, et al. Embolization of peripheral high-flow arteriovenous malformations with Onyx. Diagn Interv Imaging. 2017;98:217–26.
Valek V, Husty J. Quality improvement guidelines for transcatheter embolization for acute gastrointestinal nonvariceal hemorrhage. Cardiovasc Intervent Radiol. 2013;36:608–12.
Urbano J, Manuel Cabrera J, Franco A, Alonso-Burgos A. Selective arterial embolization with ethylene-vinyl alcohol copolymer for control of massive lower gastrointestinal bleeding: deasibilitiy and initial experience. J Vasc Interv Radiol. 2014;25:839–46.
Saeed Kilani M, Izaaryene J, Cohen F, Varoquaux A, Gaubert JY, Louis G, et al. Ethylene vinyl alcohol copolymer (Onyx®) in peripheral interventional radiology: indications, advantages and limitations. Diagn Interv Imaging. 2015;96:319–26.
Jagadeesan BD, Grigoryan M, Hassan AE, et al. Endovascular balloon-assisted embolization of intracranial and cervical arteriovenous malformations using dual-lumen coaxial balloon microcatheters and Onyx: initial experience. Neurosurgery. 2013;73(2 Suppl Operative):ons238–ons234.
Sun X, Xu J, Zhang J, et al. Management of rectal bleeding due to internal haemorrhoids with arterial embolisation: a single-centre experience and protocol. Clin Radiol. 2018;73(11):985.e1-985.e6.
Kanellos I, Goulimaris I, Christoforidis E, Kelpis T, Betsis D. A comparison of the simultaneous application of sclerotherapy and rubber band ligation, with sclerotherapy and rubber band ligation applied separately, for the treatment of haemorrhoids: a prospective randomized trial. Colorectal Dis. 2003;5:133.
Plapler H. Hemorrhoids: an experimental model in monkeys. Acta Cirurgica Brasileira. 2006;21:354–6.
Bernstein WC. What are hemorrhoids and what is their relationship to the portal venous system? Dis Colon Rectum. 1983;26(12):829–34.
Acknowledgements
Our study was financially supported by the Research Committee of French Society of Radiology. This funding source had no role in the design of this study, during its execution, analyses, interpretation of the data, or decision to submit results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Figure 1
Angiography of the inferior mesenteric artery with a 4-French catheter (A). Distal placement of a 2.4-French micro-catheter above the pubic bone (B, black arrow) corresponding to the superior rectal artery bifurcation (C) (PNG 1503 KB)
Figure 2
3D reformats of pre-embolization angio-CT of a pig from group 1 (A), group 2 (B) and group 3 (C) and after CT scan from a human patient suffering from haemorrhoids (D). Similar course and bifurcation of the trunk and the bifurcation of the superior rectal artery between pig and human. Anastomoses with internal iliac arteries are well demonstrated (A and B, white arrows) (PNG 892 KB)
Rights and permissions
About this article
Cite this article
Tradi, F., Panneau, J., Brige, P. et al. Evaluation of Multiple Embolic Agents for Embolization of the Superior Rectal Artery in an Animal Model. Cardiovasc Intervent Radiol 45, 510–519 (2022). https://doi.org/10.1007/s00270-021-03041-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-021-03041-7