Abstract
Purpose
To evaluate the efficacy of cisplatin-infused and normal saline-infused radiofrequency ablation (RFA) with internally cooled perfusion (ICP) electrode.
Materials and Methods
Using a 200 W generator, thirty ablation zones were created and divided into three groups of 10 each as follows: group A, RFA alone with 16 gauge monopolar internally cooled (IC) electrode; group B, cisplatin-infused RFA with 16 gauge ICP electrode; and group C, normal saline-infused RFA with 16 gauge ICP electrode. Radiofrequency was applied to the explanted bovine liver for 12 min. During RFA, cisplatin and normal saline were injected into tissue at a rate of 0.5 mL/min through the ICP electrode by injection pump. Dimensions of the ablation zone and technical parameters were compared between the three groups.
Result
In the cisplatin-infused RFA group, the ablation zone size was significantly larger than that of the RFA-alone group but significantly smaller than normal saline-infused RFA group. The width of longitudinal section and volume were 3.39 ± 0.22 cm2 and 26.55 ± 4.62 cm3 in RFA-alone group, 3.88 ± 0.32 cm2 and 36.45 ± 5.46 cm3 in cisplatin-infused RFA group, and 4.52 ± 0.50 cm2 and 49.44 ± 7.55 cm3 in normal saline-infused RFA group, respectively (p < 0.05 between any two groups). The mean impedance in group A, B, and C were 60.0 ± 7.2, 50.3 ± 2.5, and 40.3 ± 4.0 Ω, respectively (p < 0.05 between any two groups).
Conclusion
Cisplatin-infused RFA with ICP electrode created the larger size of ablation zone than that of monopolar RFA with an IC electrode, but created the smaller size of ablation zone than that of normal saline-infused RFA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Image-guided radiofrequency ablation (RFA) has become a worldwide accepted minimally invasive strategy for effective treatment of the hepatocellular carcinoma and metastatic liver tumor [1,2,3]. However, when the tumor is larger than 2 cm, it can cause incomplete coagulation necrosis of RFA, which is an important cause of high local tumor progression rate [4, 5]. Several attempts have been made to overcome local tumor progression of RFA, which are the use of combination therapies to increase the size of the ablation zone, including a combination of RFA with chemical ablation, transarterial chemoembolization, intravenous liposomal chemotherapy, and vascular occlusion [6,7,8,9,10]. However, such combined treatment may increase patient discomfort, costs, and procedure time. Alternatively, there are methods of increasing the volume of coagulation necrosis using an electrode designed to modify tissue-energy interaction. Among them, continuous infusion of various fluids was attempted using a perfusion electrode during the RFA, especially saline infusion [11,12,13]. Besides saline solution, several other fluids such as acetic acid or hydrochloric acid-infused RFA have been suggested to create larger lesions than RFA alone [14, 15]. Previously, there was a study on the intratumoral injection of anticancer agent immediately followed by RFA [16]. However, this is an experimental model for breast cancer, and the intratumoral injection of the anticancer drug was not a continuous injection during the RFA. There were a few attempts to continuously infuse anticancer agent into tumors during RFA with perfusion electrode. We hypothesized that significant increase in coagulation necrosis could also be achieved by anticancer agent-infused RFA with perfusion electrode. As a broad-spectrum anticancer drug, cisplatin is an effective agent for the treatment of many malignant tumors and is mainly administered through intraarterial or intravenous route. Moreover, direct intratumoral injection of cisplatin has been used to treat a variety of malignant tumors, including lung cancer [17], head and neck cancer [18], and liver tumors [19].
The purpose of this experimental study was to evaluate the in vitro efficacy of cisplatin-infused radiofrequency ablation with internally cooled perfusion (ICP) electrode by assessing the dimensions and technical parameters of ablated lesions in the bovine liver. We then compared the efficacy of RFA alone using internally cooled (IC) electrode, cisplatin-infused RFA using ICP electrode, and normal saline-infused RFA using ICP electrode.
Materials and Methods
RFA Setting and Ablation Protocol
In our institution, in vitro experimental study did not require institutional review board approval. The IC RF system is a 200W RF generator (VIVA RF generator; STARmed; Goyang-si, Gyunggi-do, Republic of Korea) with a conventional 16-gauge electrode (Proteus RF electrode, STARmed) with the 3 cm active tip. The electrode was cooled internally by delivering chilled saline to maintain the electrode temperature < 25 °C with a peristaltic pump (VIVA pump; STARmed).
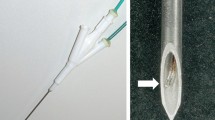
The ICP RF system uses the same generator and peristaltic pump as the IC electrode. A 16-gauge ICP electrode consist of an inner part of internally cooling water (18-gauge) with an outer part of infusion (16-gauge) separately (Injectable RF electrode, STARmed). An ICP electrode with 3 cm active tip has 20 side holes (5 holes × 4 lines; each holes 0.25–0.35 mm in diameter) at the exposure area so that the saline or cisplatin is used for infusion to the tissue at a rate of 0.5 mL/min by independent injection pump (BYZ-810, Changsha BEYOND Medical Device, Hunan, China) (Fig. 1). Normal saline-infused RFA involved a continuous infusion of 0.9% normal saline and cisplatin-infused RFA involved a continuous infusion of cisplatin (CISPLAN INJ 50 mg/100 ml, Seoul, Korea). RF was applied to the livers in the monopolar mode, and RF energy was applied to both systems for 12 min. Tissue impedance was monitored using the circuitry incorporated in the generator. The applied current, power output, and impedance were continuously monitored during the RF ablation, and these parameters were recorded automatically using a computer program (Real Time Graphics software V 2.0; Radionics, Burlington, Mass., U.S.A.).
Schema shows longitudinal section of the internally cooled perfusion electrode. The electrode consists of a part of internally cooling water (18-gauge) with a part of infusion (16-gauge) separately. There are 20 side holes (5 holes × 4 lines; each holes 0.25–0.35 mm in diameter) on the active exposure portion of the part of infusion. A part of infusion separates from a part of internally cooling water, and can inject useful solution such as hypertonic saline for increasing electrical conductivity
In Vitro Preparation
RF ablation (RFA) was performed in ten freshly excised bovine livers that were cut into about 10 × 10 × 10 cm3 blocks and partially immersed in a 50 × 20 × 20 cm3 saline-filled bath. A custom-made metal plate grounding pad was placed to one sidewall of the bath. The electrode was inserted at least 5 cm into the liver tissue to ensure that no active tip surface was exposed to the air and there was enough tissue to contain a lesion. From ten freshly excised bovine livers, thirty blocks were created and subdivided into three different groups based on the RFA procedure to be followed: group A, 10 RFA with IC electrode; group B, 10 cisplatin-infused RFA with ICP electrode; group C, 10 normal saline-infused RFA with ICP electrode.
Lesion Size Measurement
The liver blocks containing the ablated area were dissected along the electrode insertion axis (longitudinal plane, L-plane) and then cut transversely perpendicular to the L-plane (transverse plane, T-plane). Previously, the central white area of the RF-induced ablation zone had been shown to correspond to the zone of coagulation necrosis [20]. Thus, two investigators measured the depth of longitudinal section (Dv) along the electrode axis and the width of longitudinal section (Dw) perpendicular to the Dv in the L-plane. After measuring these two diameters of the ablation zone in the L-plane, the long axis diameter (Dt1) and the short axis diameter (Dt2) of the ablation zone in the T-plane were measured. The ablation area volume was determined by converting the lesion to a sphere using the formula: π(Dv × Dt1 × Dt2)/6.
Statistical Analysis
The dimensions of the ablation zones and the technical parameters of the three groups were averaged for each group and then compared using one-way analysis of variance (ANOVA) with the Turkey test (p = 0.05, two-tailed test). The values were expressed as means ± standard deviation. Statistically significant differences were defined as p values < 0.05. The statistical analysis was performed using STATA ver. 14.0 (Stata Corp LP., College Station, TX, USA).
Results
Size of Ablation Zone
The four diameters and the calculated volume are summarized in Table 1. The mean Dw, Dv, Dt1, and Dt2 of each group were 3.39 ± 0.22, 4.22 ± 0.22, 3.56 ± 0.25, and 3.36 ± 0.28 cm in group A, 3.88 ± 0.32, 4.67 ± 0.14, 4.03 ± 0.32, and 3.68 ± 0.23 cm in B, and 4.52 ± 0.50, 4.74 ± 0.19, 4.62 ± 0.46, and 4.30 ± 0.37 cm in C, respectively. In the cisplatin-infused RFA group (group B), the mean Dw, Dt1, and Dt2 of ablation zones was significantly larger than those of the RFA-alone group (group A), but significantly smaller than those of the normal saline-infused RFA group (group C). The mean Dv of the ablation zones in group B and C was significantly larger than that in group A (p < 0.05). There were no significant differences in the mean Dv between groups B and C (p > 0.05).
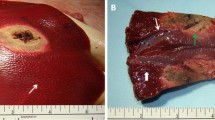
The mean calculated volume of the ablation zones in cisplatin-infused RFA group (group B) was significantly larger than that of RFA-alone group (group A), but significantly smaller than that of the normal saline-infused RFA group (group C) (p < 0.05): 22.55 ± 4.62 cm3 in group A, 36.45 ± 5.46 cm3 in group B, and 49.44 ± 7.55 cm3 in group C (Fig. 2).
Comparison of radiofrequency ablation (RFA)-induced coagulation in the three groups. The mean volume of the ablation zone in group B was significantly larger than that of group A, but significantly smaller than that of group C. A. Photograph of specimen from group A (RFA alone) B. Photograph of specimen from group B (cisplatin-infused RFA) C. Photograph of specimen from group C (normal saline-infused RFA)
Technical Parameters
The mean impedance in group A, B, and C was 60.0 ± 7.2, 50.3 ± 2.5, and 40.3 ± 4.0 Ω, respectively (p < 0.05 between any two groups). The mean current in group A, B, and C was 1148 ± 115, 1330 ± 64, and 1495 ± 112 mA, respectively (p < 0.05 between any two groups). The mean of the total delivered energy in group A, B, and C were 8.52 ± 1.08, 10.10 ± 1.04, and 9.30 ± 1.38 kcal, respectively (p < 0.05 between groups A and B) (Table 2).
Discussion
Percutaneous RFA is widely used as an alternative to surgical treatment of liver tumors, but the major limitation of RFA is the small ablation volume [1,2,3,4, 21, 22]. Several studies have been tried to increase coagulation volume, among which RFA with chemoablation using ethanol or acetic acid and RFA with continuous fluid infusion can be performed at the same time and same place [6,7,8,9,10,11,12,13,14,15]. Several investigators used saline-infused RFA, which improved the electrical and heat conduction and energy delivery, with a resulting in an increased ablation volume [11, 12]. However, there has been no study about the continuous infusion of the anticancer agent during RFA. Cisplatin is composed of a platinum ion surrounded by four ligands and has a molecular weight of 301.1 g/mol, and a density of 3.74 g/cm3 [23]. Cisplatin is known as a broad-spectrum anticancer agent that kills tumor cells by directly destroying DNA duplication and causes an immune response [24]. In the treatment of hepatocellular carcinoma, cisplatin is used for transarterial chemoembolization and hepatic arterial infusion while reducing systemic toxicity and side effects [25, 26]. The response rate was reported to be 33.8% [26] with a hepatic arterial infusion of cisplatin and only 9% [27] with systemic cisplatin chemotherapy, suggesting that the anticancer effect of the cisplatin is directly related to the local concentration of cisplatin. Recently, some studies on intratumoral chemotherapy have shown that local injection of anticancer drugs into tumors minimizes systemic side effects and increases the therapeutic dose of tumors by 6–10 fold [28]. According to the literature, the anticancer agents used in intratumoral chemotherapy are cisplatin, methotrexate, bleomycin, mitoxantrone, mitomycin, and 5-fluorouracil [29, 30]. Cisplatin has been used as an intratumoral injection in hepatic tumors as one of the above anticancer drugs [19]. We have designed a method of radiofrequency ablation with continuous infusion of cisplatin into a tumor using a perfusion electrode, considering that cisplatin has an enhanced anticancer effect at high local concentration and can be used for intratumoral chemotherapy.
Our result shows that the ablation size of cisplatin-infused RFA was larger than that of RFA alone but smaller than that of normal saline-infused RFA. The impedance was the smallest in normal saline-infused RFA group and the largest in RFA-alone group, and the current was opposite. Several factors may explain this difference. Continuous infusion of fluid during RFA prevents the loss of moisture around the tissue, which reduces impedance and increases electrical and heat conductivity and increases the size of ablation, compared to RFA alone. This can be supported by previous studies that injection of a fluid containing no ions such as distilled water, ethanol, or 5% glucose solution led to an increase in coagulation volume [13]. Saline is an ion-containing liquid and has a higher electrical conductivity than non-ionic solution. Cisplatin can reduce the impedance and increase the ablation volume during RFA as an ionic fluid property, but it is not as good as saline.
Previous studies have reported that combination of RFA and intratumoral doxorubicin injection in an animal model and the combination of RFA and IV liposomal doxorubicin in animal and human tumor model have been shown to increase the extent of coagulation [9, 16, 31]. Ahmed et al. [32] showed that similar extent of tumor coagulation was observed between liposomal doxorubicin and cisplatin chemotherapy combined with RFA in an animal tumor model. This can be explained by the synergy of sublethal thermal damage induced by RFA and cytotoxic effect of the chemotherapeutic agent. Although thermal coagulation by RFA alone usually occurs above 50 °C, the effect of anticancer agents is enhanced by hyperthermia at the periphery of ablation zone, which is about 45–50 °C [33, 34]. Thus, a combination of RFA and chemotherapy can lower the threshold temperature of cell death, thereby increasing the extent of coagulation. Our study is an in vitro study applied to the explanted bovine liver, which is significantly different from animal tumor model described above. If cisplatin-infused RFA with perfusion electrode is applied to an animal tumor model, it may have resulted in higher coagulation volume than that obtained in our in vitro study. Therefore, further studies in animal tumor models are needed to demonstrate the combined effect of thermal ablation and anticancer agent.
Our experimental study has certain limitations. First, it was performed with normal liver parenchyma in vitro, not with tumors in situ; hence, the results obtained cannot be readily adapted for use in a clinical setting. Living tumor tissues benefit from a cooling “sink” effect due to the blood flow, resulting in rapid heat exchange. Also, it is difficult to assess cytotoxic effect of cisplatin in our in vitro study, considering that cisplatin kills cancer cells by damaging their DNA. We did not attempt to evaluate the biological effects of cisplatin infusion. We demonstrated an increase in the ablation volume due to the increased electrical and thermal conductivity by cisplatin infusion. The results of this experiment may serve as a reference for estimating the ablation area due to the physical/chemical property of cisplatin. This study can be a stepping stone for future animal experiments in vivo, and the combination of our study and future in vivo study will help determine additional ablation volume increases by pure cytotoxic effects other than the physical/chemical properties of cisplatin. Second, the present study was performed with relatively small sample size. Additional multi-institutional studies with larger sample size are needed to validate our findings. Third, the diameter of the ablation zone was measured on the gross specimens instead of using an imaging modality such as computed tomography, which can more accurately determine the volume and shape of the RFA zone.
In conclusion, cisplatin-infused RFA with ICP electrode created a larger size of ablation zone than RFA alone with an IC electrode but was less effective than normal saline-infused RFA. Further studies in animal tumor models are needed to demonstrate the combined effect of thermal ablation and anticancer agent.
References
McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001;176(1):3–16.
Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000;1(4):175–84.
Decadt B, Siriwardena AK. Radiofrequency ablation of liver tumours: systematic review. Lancet Oncol. 2004;5(9):550–60.
Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Gwak GY, Yoo BC. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58(1):89–97.
Kim YS, Lim HK, Rhim H, Lee MW. Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2014;28(5):897–908.
Lee JM, Lee YH, Kim YK, Kim SW, Kim SH, Han JK, Choi BI. Combined treatment of radiofrequency ablation and acetic acid injection: an in vivo feasibility study in rabbit liver. Eur Radiol. 2004;14(7):1303–10.
Kurokohchi K, Watanabe S, Masaki T, Hosomi N, Miyauchi Y, Himoto T, Kimura Y, Nakai S, Deguchi A, Yoneyama H, Yoshida S, Kuriyama S. Comparison between combination therapy of percutaneous ethanol injection and radiofrequency ablation and radiofrequency ablation alone for patients with hepatocellular carcinoma. World J Gastroenterol. 2005;11(10):1426–32.
Veltri A, Moretto P, Doriguzzi A, Pagano E, Carrara G, Gandini G. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol. 2006;16(3):661–9.
Goldberg SN, Kamel IR, Kruskal JB, Reynolds K, Monsky WL, Stuart KE, Ahmed M, Raptopoulos V. Radiofrequency ablation of hepatic tumors: increased tumor destruction with adjuvant liposomal doxorubicin therapy. AJR Am J Roentgenol. 2002;179(1):93–101.
Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchiano A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V, Blum HE, Bartolozzi C. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217(1):119–26.
Luo RG, Gao F, Gu YK, Huang JH, Li CL. Radioablation settings affecting the size of lesions created ex vivo in porcine livers with monopolar perfusion electrodes. Acad Radiol. 2010;17(8):980–4.
Kettenbach J, Kostler W, Rucklinger E, Gustorff B, Hupfl M, Wolf F, Peer K, Weigner M, Lammer J, Muller W, Goldberg SN. Percutaneous saline-enhanced radiofrequency ablation of unresectable hepatic tumors: initial experience in 26 patients. AJR Am J Roentgenol. 2003;180(6):1537–45.
Bruners P, Muller H, Gunther RW, Schmitz-Rode T, Mahnken AH. Fluid-modulated bipolar radiofrequency ablation: an ex vivo evaluation study. Acta Radiol. 2008;49(3):258–66.
Lubienski A, Dux M, Lubienski K, Grenacher L, Kauffmann G. Radiofrequency thermal ablation: increase in lesion diameter with continuous acetic acid infusion. Cardiovasc Intervent Radiol. 2005;28(6):789–94.
Luo RG, Fao F, Huang JH, Gu YK, Jiang XY, Huang YJ. Diluted hydrochloric acid generates larger radiofrequency ablation lesions in excised porcine livers. Diagn Interv Radiol. 2013;19(2):145–9.
Goldberg SN, Saldinger PF, Gazelle GS, Huertas JC, Stuart KE, Jacobs T, Kruskal JB. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intratumoral doxorubicin injection in a rat breast tumor model. Radiology. 2001;220(2):420–7.
Celikoglu F, Celikoglu SI, York AM, Goldberg EP. Intratumoral administration of cisplatin through a bronchoscope followed by irradiation for treatment of inoperable non-small cell obstructive lung cancer. Lung Cancer. 2006;51(2):225–36.
Burris HA 3rd, Vogel CL, Castro D, Mishra L, Schwarz M, Spencer S, Oakes DD, Korey A, Orenberg EK. Intratumoral cisplatin/epinephrine-injectable gel as a palliative treatment for accessible solid tumors: a multicenter pilot study. Otolaryngol Head Neck Surg. 1998;118(4):496–503.
Vogl TJ, Engelmann K, Mack MG, Straub R, Zangos S, Eichler K, Hochmuth K, Orenberg E. CT-guided intratumoural administration of cisplatin/epinephrine gel for treatment of malignant liver tumours. Br J Cancer. 2002;86(4):524–9.
Lee JD, Lee JM, Kim SW, Kim CS, Mun WS. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: observation during acute and chronic stages. Korean J Radiol. 2001;2(3):151–8.
Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities–part II. J Vasc Interv Radiol. 2001;12(10):1135–48.
Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000;214(3):761–8.
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78.
Wu PF, Lin CH, Kuo CH, Chen WW, Yeh DC, Liao HW, Huang SM, Cheng AL, Lu YS. A pilot study of bevacizumab combined with etoposide and cisplatin in breast cancer patients with leptomeningeal carcinomatosis. BMC Cancer. 2015;15:299.
Maeda N, Osuga K, Higashihara H, Tomoda K, Mikami K, Nakazawa T, Nakamura H, Tomiyama N. Transarterial chemoembolization with cisplatin as second-line treatment for hepatocellular carcinoma unresponsive to chemoembolization with epirubicin-Lipiodol emulsion. Cardiovasc Intervent Radiol. 2012;35(1):82–9.
Yoshikawa M, Ono N, Yodono H, Ichida T, Nakamura H. Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Hepatol Res. 2008;38(5):474–83.
Okada S, Okazaki N, Nose H, Shimada Y, Yoshimori M, Aoki K. A phase 2 study of cisplatin in patients with hepatocellular carcinoma. Oncology. 1993;50(1):22–6.
Goldberg EP, Hadba AR, Almond BA, Marotta JS. Intratumoral cancer chemotherapy and immunotherapy: opportunities for nonsystemic preoperative drug delivery. J Pharm Pharmacol. 2002;54(2):159–80.
Celikoglu SI, Karayel T, Demirci S, Celikoglu F, Cagatay T. Direct injection of anti-cancer drugs into endobronchial tumours for palliation of major airway obstruction. Postgrad Med J. 1997;73(857):159–62.
Celikoglu F, Celikoglu SI. Intratumoural chemotherapy with 5-fluorouracil for palliation of bronchial cancer in patients with severe airway obstruction. J Pharm Pharmacol. 2003;55(10):1441–8.
Goldberg SN, Girnan GD, Lukyanov AN, Ahmed M, Monsky WL, Gazelle GS, Huertas JC, Stuart KE, Jacobs T, Torchillin VP, Halpern EF, Kruskal JB. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intravenous liposomal doxorubicin in a rat breast tumor model. Radiology. 2002;222(3):797–804.
Ahmed M, Lukyanov AN, Torchilin V, Tournier H, Schneider AN, Goldberg SN. Combined radiofrequency ablation and adjuvant liposomal chemotherapy: effect of chemotherapeutic agent, nanoparticle size, and circulation time. J Vasc Interv Radiol. 2005;16(10):1365–71.
Bull JM. An update on the anticancer effects of a combination of chemotherapy and hyperthermia. Cancer Res. 1984;44(10 Suppl):4853s–6s.
Kawai H, Minamiya Y, Kitamura M, Matsuzaki I, Hashimoto M, Suzuki H, Abo S. Direct measurement of doxorubicin concentration in the intact, living single cancer cell during hyperthermia. Cancer. 1997;79(2):214–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, M.S., Hong, H.P., Park, K. et al. In Vitro Bovine Liver Experiment of Cisplatin-Infused and Normal Saline-Infused Radiofrequency Ablation with an Internally Cooled Perfusion Electrode. Cardiovasc Intervent Radiol 42, 886–892 (2019). https://doi.org/10.1007/s00270-019-02178-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-019-02178-w