Abstract
Purpose
The purpose of this retrospective study was to investigate the efficacy of transarterial chemoembolization (TACE) using cisplatin as a second-line treatment for advanced hepatocellular carcinoma (HCC) unresponsive to TACE using epirubicin–Lipiodol emulsion at our institution.
Materials and Methods
Between January 2006 and March 2009, 51 patients with unresectable HCC underwent TACE using cisplatin. All patients had shown persistent viable tumor or tumor progression after at least 2 sessions of TACE using epirubicin–Lipiodol emulsion. TACE procedures consisted of arterial injection of a mixture of Lipiodol and cisplatin (30–100 mg [mean 57 ± 21]) (n = 29) or arterial infusion of cisplatin (30–100 mg [mean 87 ± 19]) solution (n = 22) followed by injection of 1-mm porous gelatin particles. Early tumor response was assessed by contrast-enhanced computed tomography (CT) according to Response Evaluation Criteria in Solid Tumors (RECIST) and European Association for the Study of the Liver (EASL) criteria. Overall survival and progression-free survival was calculated using the Kaplan–Meier method. Toxicity was assessed according to NCI-CTCAE version 3 criteria.
Results
Response rates were 11.8 and 27.5% by RECIST and EASL criteria, respectively. Overall survival rates were 61.9, 48.2, and 28.9% at 1, 2, and 3 years, respectively, and the median survival time was 15.4 months. Progression-free survival rate was 35.2% at 1 year, and median progression-free survival time was 3.1 months. No major complications were observed, and the occurrence of postembolization syndrome was minimal. Grade 3 to 4 toxicities included thrombocytopenia (5.8%), increased aspartate aminotransferase (AST) level (35.3%), and increased alanine aminotransferase (ALT) level (23.5%).
Conclusion
Switching the TACE anticancer drug from epirubicin to cisplatin might be the feasible option for advanced HCC, even when considered resistant to the initial form of TACE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide, hepatocellular carcinoma (HCC) is one of the most common causes of cancer death, and the largest concentration of cases is in Asia [1]. Because most HCCs are associated with underlying liver cirrhosis caused by chronic infection with hepatitis B or C virus and alcoholic liver injury, intensive screening for cirrhotic patients at high risk using imaging studies and tumor markers could potentially lead to the detection of small HCCs; however, eligibility for potentially curative treatments, such as surgery and ablation, is limited according to tumor extent, tumor multiplicity, or underlying cirrhosis. Thus, transarterial chemoembolization (TACE) is the mainstay option for palliative treatment of unresectable HCC with proven survival benefits [2, 3].
Although many chemotherapeutic agents, such as doxorubicin, epirubicin, cisplatin, and mitomycin, are often used in TACE [2], confusion remains concerning the efficacy of chemotherapy added to TACE [4, 5]. There is no unified protocol using single or multiple combined anticancer drugs. In our country, epirubicin is the most popular drug used in TACE. Recently, a new cisplatin powder (IA-Call; Nippon Kayaku, Tokyo, Japan), designed for hepatic arterial infusion, has become available; however, the actual first-choice drug remains uncertain. To our knowledge, there are only a few reports about the efficacy of TACE with cisplatin as a second-line treatment for advanced HCC unresponsive to epirubicin–Lipiodol emulsion [6, 7].
In this retrospective study, we investigated the clinical outcomes of the efficacy of TACE with the new cisplatin powder as a second-line treatment for advanced HCC unresponsive to epirubicin–Lipiodol emulsion at our institution.
Materials and Methods
Study Population
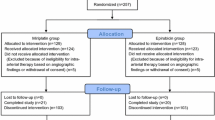
Between January 2006 and March 2009, 456 patients with unresectable HCC underwent TACE with 973 sessions in our hospital. Of these patients, 51 were judged as having HCC resistant to TACE with epirubicin–Lipiodol emulsion by a multidisciplinary HCC panel, including radiologists, hepatologists, and surgeons at our hospital, and received TACE with cisplatin. The multidisciplinary panel reached a consensus that TACE was the appropriate treatment option at the time of initial diagnosis in all 51 patients, who had undergone TACE at least twice before being considered as having HCC resistant to TACE with epirubicin–Lipiodol emulsion. HCC resistant to TACE with epirubicin–Lipiodol emulsion was defined as HCC of increased size and/or number in the treated segment and/or extended to other segments despite repeated courses of TACE with epirubicin–Lipiodol emulsion, as described by Kawamura et al. [6]. Thus, these 51 consecutive patients with HCC resistant to TACE with epirubicin–Lipiodol emulsion were enrolled in this retrospective cohort study. All patients gave written informed consent to undergo TACE with cisplatin, and this retrospective study was approved by the Institutional Review Board.
All 51 patients met the following inclusion criteria: (1) hypervascular HCC diagnosed by all imaging modalities and tumor markers; (2) no history of treatment with anticancer drugs except epirubicin; (3) Eastern Cooperative Oncology Group performance status score 0 to 2; (4) sufficient hematopoietic function with a platelet count >30,000/mm3 and leukocyte count >2,000/mm3; and (5) adequate liver function classified as Child-Pugh class A or B. They also did not meet the following exclusion criteria: (1) imaging findings with major portal venous tumor invasion; (2) evidence of extrahepatic metastasis of HCC; (3) history of iodine contrast medium allergy; and (4) serious comorbid disorders.
Patient Background
Tables 1 and 2 list the patient characteristics. There were 43 men and 8 women with a median age of 73 years (range 49–85). All patients had underlying cirrhosis and as the dominant cause, 37 (73%) had hepatitis C virus infection, 4 (8%) had hepatitis B virus infection, 1 (2%) had both hepatitis B and C virus infection, 3 (6%) had alcohol-induced hepatitis, 1 (2%) had non–alcoholic steatohepatitis (NASH), and 5 (10%) were of unknown etiology. Child-Pugh class was class A in 29 (57%) patients and class B in 22 (43%) patients.
The clinical diagnosis of HCC was made based on the combination of imaging findings and increased serum level of tumor markers, such as alpha-fetoprotein (AFP) and/or protein-induced vitamin K antagonist-II (PIVKA-II; des-γ-carboxy prothrombin). Although the imaging studies included dynamic computed tomography (CT) or magnetic resonance imaging (MRI), in addition to CT during arterial portography (CTAP) and hepatic arteriography (CTHA), the lesion was diagnosed as HCC basically with dynamic CT at the time of initial TACE with cisplatin. Seven patients (14%) had a single main nodule, 2 (4%) had a single main nodule with satellite nodules, 6 (12%) had 2 distinct nodules, 1 (2%) had 3 distinct nodules, and 35 (69%) had >5 distinct nodules. The median diameter of the largest tumor was 38 mm (range 13 to 140). Serum AFP exceeded the upper limit (5 ng/mL) in 49 patients (96%), and serum PIVKA-II exceeded the upper limit (40 ng/mL) in 39 patients (76%). Fifty-one patients underwent TACE with epirubicin–Lipiodol emulsion, and there was an average of 3.7 sessions (range 2–7) before the initial TACE with cisplatin. One patient received hemodialysis because of chronic renal failure.
TACE Procedures
In all TACE procedures, hepatic angiography was performed by the femoral approach using a 4Fr catheter and 1.8Fr to 2.4Fr microcatheter. CTHA and CTAP were performed using a united angio-CT system to assess tumor extension, hemodynamics of the tumor, and portal blood flow. Before 2008, the angio-CT system consisted of a single-detector helical computed tomograph (ProSeed SA; General Electric Healthcare, Milwaukee, WI) and a C-arm angiograph (Advantx LCA; General Electric Healthcare), which shared a common table without the need to transfer patients to a separate CT room. After 2009, the angio-CT system consisted of a 40-detector helical computed tomograph (Artis Zee; Siemens AG, Munich, Germany) and a C-arm angiograph with a flat-panel detector (SOMATOM Sensation Open; Siemens AG). After confirming the hepatic arteries supplying the target tumor, TACE with iodized oil (Lipiodol Ultra-Fluide; Andre Guerbet, Aulnay-sous-Bois, France) procedures consisted of arterial injection of a mixture of Lipiodol and cisplatin (IA-Call; Nippon Kayaku) followed by the injection of gelatin sponge particles (Spongel; Astellas, Tokyo, Japan) or porous gelatin particles (Gelpart; Nippon Kayaku) using a microcatheter in a selective manner, whereas TACE without Lipiodol procedures consisted of arterial infusion of cisplatin solution (IA-Call; Nippon Kayaku) followed by the injection of gelatin sponge particles or porous gelatin particles. The end point of embolization was blood flow cessation of the tumor-feeding artery. The ratio of cisplatin : Lipiodol : nonionic contrast media (Iomeprol 300 mg/mL; Iomeron; Eisai, Tokyo, Japan) were 10 mg : 0.7 mL : 0.3 mL or 10 mg : 1 mL : 0.5 mL, according to the tumor extent and its vascularity. The maximum dose of cisplatin was 100 mg/session. The total dose of Lipiodol (mL) was almost equal to the sum of the target tumor diameter (cm); however, it was limited to ≤5 mL to minimize liver damage. For arterial infusion of the cisplatin solution, the total cisplatin dose was no more than 65 mg/m2 or 100 mg/session.
Chemotherapeutic Agent
In this study, highly soluble cisplatin powder (IA-Call; Nippon Kayaku) was used as the chemotherapeutic agent. This cisplatin powder was developed for hepatic arterial infusion chemotherapy and can be used to create a high-concentration solution [8, 9]. A mixture of Lipiodol and cisplatin can be also be made without difficulty [10]. To prepare the mixture of Lipiodol and cisplatin, we added 3 or 5 mL nonionic contrast media (Iomeprol 300 mg/mL; Iomeron; Eisai) into the IA-Call vial (Nippon Kayaku) containing 100 mg cisplatin and shook the vial by hand. Then we mixed the solution with Lipiodol by pumping two syringes. To prepare the cisplatin solution without Lipiodol, we added 70 mL warm saline to the IA-Call vial.
Selection Criteria for TACE Procedures
The treatment course of each patient was discussed by the multidisciplinary HCC panel in our hospital. The decision to use TACE with or without Lipiodol depended on liver function, condition of portal venous tumor invasion, and extent of the tumors. Generally, TACE without Lipiodol was performed for the patients with worse conditions, such as diffuse and infiltrative tumors or those with segmental portal venous invasion, whereas TACE with Lipiodol was used for multinodular HCCs.
Assessment
Early tumor response was evaluated by dynamic CT in principle 1 to 3 months after the initial TACE with cisplatin based on the change in the maximum diameter of the whole tumor, including the necrotic part induced by TACE, according to the criteria of Response Evaluation Criteria in Solid Tumors (RECIST) [11]. Early tumor response was also assessed based on the change in the bidimensional diameters of the viable part (intra- or peripheral enhancement in the arterial phase), according to the criteria adopted by the European Association for the Study of the Liver (EASL) [12, 13]. Best overall response of the tumor at baseline was evaluated during the observation period according to RECIST criteria. As an exception, dynamic MRI was alternatively used in one patient who underwent TACE with a mixture of Lipiodol and cisplatin and in two patients who underwent TACE after hepatic arterial infusion of cisplatin because of their history of side effects from iodinated contrast material.
Complications were classified as major or minor according to the Society of Interventional Radiology reporting standards [5]. Major complications resulted in an unplanned increase in the level of care, permanent adverse sequelae, or death. Minor complications resulted in no sequelae with or without nominal therapy requirement.
Toxicity was assessed according to the National Cancer Institute—Common Terminology Criteria for Adverse Effects (NCI-CTCAE), Version 3.0 [14]. Hematological toxicity was evaluated by measuring the number of leukocytes and thrombocytes, serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), albumin, total bilirubin, creatinine, and prothrombin activity within 2 weeks before, once during 3 to 7 days after, and 1 month after initial TACE with cisplatin.
Statistical Analysis
Early tumor response and best overall response were compared between groups with and without Lipiodol using Mann–Whitney U-test. Overall survival and progression-free survival of all patients was calculated from the date of initial TACE with cisplatin to the end of August 2009 using the Kaplan–Meier method and compared by the log-rank test.
To identify predictors of the survival period, univariate and multivariate analysis with Cox proportional hazards regression models was performed for the following factors: age, sex, use conditions of Lipiodol, Child-Pugh class, dominant cause of cirrhosis, number of tumors, diameter of the largest tumor, location of tumors, extent of portal venous, hepatic venous, and intrahepatic biliary tumor invasion, level of embolization, presence of an extrahepatic feeding artery, dose of cisplatin, and serum levels of total bilirubin, AFP, and PIVKA-II. Patients lost to follow-up or alive at the time of analysis were censored.
Statistical significance was defined as two tailed with a p < 0.05. All analyses were performed using a statistical software package (SPSS 11.0 for Windows; SPSS Japan, Tokyo, Japan).
Results
Twenty-nine of 51 patients (56.9%) underwent TACE with a mixture of Lipiodol and cisplatin, and 22 of 51 patients (43.1%) underwent TACE after hepatic arterial infusion of cisplatin as the initial TACE with cisplatin. In TACE with a mixture of Lipiodol and cisplatin, the dose of cisplatin per patient ranged from 30 to 100 mg (average 57 ± 21), and the dose of Lipiodol per patient ranged 1 to 5 mL (average 3.4 ± 1.3). In TACE after hepatic arterial infusion of cisplatin, the dose of cisplatin per patient ranged from 30 to 100 mg (average 87 ± 19 mg).
Tumor Response
Early tumor response was assessed by imaging outcomes at 1- to 3-month follow-up after initial TACE with cisplatin. According to RECIST criteria, the objective tumor response rate was 11.8% (0% complete response [CR] and 11.8% partial response [PR]) (Table 3). According to EASL criteria, the response rate increased to 27.5% (2% CR and 25.5% PR) (Table 4). The objective best overall tumor response rate, according to RECIST criteria, was 21.6% (0% CR and 21.6% PR) (Table 5). There were neither significant differences in tumor response according EASL or RECIST criteria between groups with and without Lipiodol (p = 0.17, 0.17, and 0.40).
Follow-Up
The overall mean follow-up period was 11 months (range 2 to 37). The follow-up period exceeded 6, 12, 18, 24, 30, and 36 months in 37 (72.5%), 18 (35.3%), 8 (15.7%), 6 (11.8%), 2 (3.9%), and 1 (2.0%) patient(s), respectively. Of 29 patients undergoing TACE with a mixture of Lipiodol and cisplatin, TACE with the same regimen was repeated in 16 patients (55.2%), and TACE with the other regimen was repeated in 1 patient (3.4%). Of 22 patients undergoing TACE after hepatic arterial infusion of cisplatin, the same regimen was repeated in 3 patients (13.6%), and TACE with the other regimen was repeated in 5 patients (22.7%).
Overall survival rates were 61.9, 48.2, and 28.9% at 1, 2, and 3 years, respectively, and the median survival time was 15.4 months (Fig. 1A). Overall progression-free survival rates were 35.2 and 26.4% at 1 and 2 years, respectively, and the median progression-free survival time was 3.1 months (Fig. 2A). There were no significant differences in overall or progression-free survival between groups with and without Lipiodol (p = 0.66 and 0.14, respectively) (Figs. 1B, 2B).
A Cumulative survival rates for all 51 patients. The 1-, 2-, 3-year survival rates were 61.9, 48.2, and 28.9%, respectively. B Cumulative survival rates were 69.1, 53.3, and 17.8% at 1, 2, and 3 years, respectively, in 29 patients who underwent TACE with a mixture of Lipiodol and cisplatin and 51.5, 41.2, and 41.2% at 1, 2, and 3 years, respectively, in 22 patients who underwent TACE after hepatic arterial infusion of cisplatin. There was significant difference between the two groups (p = 0.66)
A One- and 2-year progression-free survival rates were 35.2 and 26.4%, respectively. B Cumulative progression-free survival rates were 44.8 and 29.9% at 1 and 2 years, respectively, in 29 patients who underwent TACE with a mixture of Lipiodol and cisplatin and 19.1 and 19.1% at 1 and 2 years, respectively, in 22 patients who underwent TACE after hepatic arterial infusion of cisplatin. There was significant difference between the two groups (p = 0.14)
During the observation period, 19 (37.2%) patients died. The causes of death were progression of HCC in 13 patients, liver failure in 3 patients, heart failure in 2 patients, and interstitial pneumonia in 1 patient.
Toxic Effects
No major complications, including liver failure, liver abscess, biloma, surgical cholecystitis, gastrointestinal hemorrhage, pulmonary embolism, and death, were observed within 30 days after each TACE procedure with cisplatin. Postembolization syndrome was minimal, if present, and was categorized as grade 1 or 2 after initial TACE with cisplatin (Table 6). Grade 3 toxicities after TACE with cisplatin included thrombocytopenia in 3 patients (5.9%), increased AST in 18 patients (35.3%), and increased ALT in 11 patients (21.6%). Grade 4 toxicities included increased ALT in 1 patient (2.0%) (Table 7).
Predictors of Survival Period
The results of univariate and multivariate analysis with Cox proportional hazards regression models are listed in (Tables 8, 9). Only the extent of intrahepatic biliary tumor invasion was a statistically significant predictor of the survival period (p = 0.003) (Table 9, Fig. 3).
Discussion
TACE is most widely performed for patients with HCC who are not eligible for curative surgery or ablation as the main palliative treatment to delay tumor progression. The survival benefit of TACE has been also confirmed by randomized controlled trials and meta-analysis [2, 3, 15]; however, there is no clear evidence identifying the best chemotherapeutic agent or the optimal retreatment schedule for TACE.
As drugs for TACE, anthracycline anticancer drugs, such as doxorubicin and epirubicin, are most commonly used in Asia. Other chemotherapeutic agents, such as cisplatin and mitomycin, are also often used. In this study, cisplatin was used as the second-line chemotherapeutic agent because HCC is considered to be relatively sensitive to cisplatin [11, 16–18].
Although TACE can be repeated in most patients, therapeutic efficacy cannot be expected by repetitive TACE with the same protocol if HCCs are considered to be resistant to it.
In this study, the objective tumor response rate according to RECIST and ESAL criteria, and the best overall response rate according to the RECIST criteria, were relatively low (11.8, 27.5, and 21.6%, respectively) This may be partly because the target disease was advanced HCC uncontrolled by TACE with epirubicin–Lipiodol emulsion in this study, and there were a greater proportion of advanced conditions: Child-Pugh class B in 22 patients (43%), multiple tumors in 35 patients (69%), and bilobar disease in 32 patients (63%).
To our knowledge, there is little information on the efficacy of retreatment with TACE using a different anticancer drug substituted for the former agent because of the former’s lack of effectiveness. There have been only a few papers regarding the efficacy of TACE with cisplatin as a second-line treatment for advanced HCC unresponsive to TACE with epirubicin–Lipiodol emulsion [6, 7].
Kawamura et al. reported the result of 59 TACE procedures with cisplatin unresponsive to TACE with epirubicin–Lipiodol emulsion [6]. The objective tumor response rate was 33.8%, and the 1-, 2- and 3-year survival rates were 60.8, 40.0, and 21.7%, respectively. They also reported the results of platinum analogue (TACE or hepatic arterial infusion) in 152 patients with unresectable HCCs unresponsive to TACE with epirubicin–Lipiodol emulsion, and the objective tumor response rate was 22.4%. These data are similar to our results.
Seki et al. reported the results of 14 TACE procedures with cisplatin unresponsive to TACE with epirubicin–Lipiodol emulsion [7]. The objective tumor response rate was 64.3% and the 1-year progression-free survival rate was 33.4%. Their objective tumor response rate was superior to ours; however, there was a difference in the background factors, especially tumor number, because a greater proportion of patients had a solitary nodule in their study than did in ours. Nevertheless, their progression-free survival rate was similar to that of our study.
We could not know whether second-line TACE using cisplatin had a survival benefit compared with the clinical course with conservative management alone because this study was a nonrandomized controlled trial. Although we cannot directly compare it with our results, the survival rate with conservative management alone has been reported in a few randomized controlled trials; the 1-, 2- and 3-year survival rates were 63, 27 and 17%, respectively, in the study by Llovet et al. and 32, 11 and 3%, respectively, in the study by Lo et al. [3, 15].
Intrahepatic biliary tumor invasion was the only significant poor prognostic factor in multivariate analysis. Hepatic functional reserve, which generally shows the relation to prognosis, was not a prognostic factor in this study because patients with HCC resistant to TACE with epirubicin–Lipiodol emulsion were enrolled, and most had multiple progressive HCCs.
Morimoto et al. reported that in rabbit models, the platinum concentration in tumor tissue after 60 min in the TACE group with cisplatin and Lipiodol was significantly higher than in the TACE group without Lipiodol consisting of arterial infusion of cisplatin solution followed by injection of gelatin sponge particles [19]. Kawaoka et al. reported in their study that longer survival was expected for patients with a high rate of Lipiodol accumulation in the tumor [18]. In the present study, many patients had multiple HCC nodules; therefore, only a small amount of Lipiodol and cisplatin might have comparatively accumulated in the tumor tissue. There was no significant difference in tumor response or survival rate between groups with and without Lipiodol. In contrast, Lu et al. concluded in their report that superselective TACE with low- and high-dose chemotherapeutic agents included similar degrees of cellular apoptosis and necrosis [20]. Even if more Lipiodol and cisplatin were used with TACE, the results might not be so different.
Although some patients developed grade 3 and 4 toxicities, including thrombocytopenia and increased liver transaminases, this protocol was thought to be tolerable because all parameters recovered to baseline levels and patient performance status and liver function reserve were also preserved.
Limitations
There are some limitations to this study. First, it was a retrospective study with a small number of patients in a single institution. Therefore, we could not regulate the evaluation periods among the subjects, and the tumor response was assessed at different intervals (between 1 and 3 months after TACE). The retreatment schedule was also inhomogeneous among patients, and TACE was repeated when there was definite tumor progression based on the consensus of the multidisciplinary panel. These inhomogeneities could have influenced the results. In addition, it is difficult to compare our results with those of other studies because each study used different criteria to evaluate the treatment response.
To clarify the true benefit of TACE with such a second-line regimen, a randomized controlled trial will be necessary that compares outcomes after switching to TACE with the second-line drug regimen versus repeating TACE with the original drug regimen using end points such as time to progression and overall survival.
In conclusion, switching the TACE anticancer drug from epirubicin to cisplatin might be a feasible option for advanced HCC, even when considered resistant to the initial form of TACE.
References
Bosch FX, Ribes J, Diaz M et al (2004) Primary liver cancer: worldwide incidence and trends. Gastroenterology 127(5 Suppl 1):S5–S16
Llovet JM, Bruix J (2003) Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37(2):429–442
Llovet JM, Real MI, Montana X et al (2002) Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359(9319):1734–1739
Cammà C, Schepis F, Orlando A et al (2002) Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 224(1):47–54
Brown DB, Cardella JF, Sacks D et al (2009) Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 20(7 Suppl):S219–S226, S226.e1–10
Kawamura Y, Ikeda K, Hirakawa M et al (2009) Efficacy of platinum analogue for advanced hepatocellular carcinoma unresponsive to transcatheter arterial chemoembolization with epirubicin. Hepatol Res 39(4):346–354
Seki A, Igarashi T, Kawauchi T et al (2009) Transcatheter arterial chemoembolization with cisplatin-Lipiodol emulsion for epirubicin-Lipiodol TACE resistant unresectable hepatocellular carcinoma: Initial evaluation of efficacy and toxicity [in Japanese]. Jpn J Intervent Radiol 24(3):252–257
Yoshikawa M, Ono N, Yodono H et al (2008) Phase II study of hepatic arterial infusion of a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma. Hepatol Res 38(5):474–483
Maeda N, Osuga K, Higashihara H et al (2010) In vitro characterization of cisplatin-loaded superabsorbent polymer microspheres designed for chemoembolization. J Vasc Interv Radiol 21(6):877–881
Takaki Y, Kaminou T, Shabana M et al (2008) Suitable blending method of lipiodol-cisplatin in transcatheter arterial embolization for hepatocellular carcinoma: evaluation of sustained release and accumulation nature. Hepatogastroenterology 55(81):202–206
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Bruix J, Sherman M, Llovet JM et al (2001) Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35(3):21–30
Llovet JM, Beaugrand M (2003) Hepatocellular carcinoma: present status and future prospects. J Hepatol 38(Suppl 1):S136–S149
Trotti A, Colevas D, Setser A et al (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13(13):176–781
Lo CM, Ngan H, Tso WK et al (2002) Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35(5):1164–1171
Shen DW, Akiyama S, Schoenlein P et al (1995) Characterisation of high-level cisplatin-resistant cell lines established from a human hepatoma cell line and human KB adenocarcinoma cells: cross-resistance and protein changes. Br J Cancer 71(4):676–683
Ono Y, Yoshimasu T, Ashikaga R et al (2000) Long-term results of lipiodol-transcatheter arterial embolization with cisplatin or doxorubicin for unresectable hepatocellular carcinoma. Am J Clin Oncol 23(6):564–548
Kawaoka T, Aikata H, Takaki S et al (2009) Transarterial infusion chemotherapy using cisplatin-lipiodol suspension with or without embolization for unresectable hepatocellular carcinoma. Cardiovasc Intervent Radiol 32(4):687–694
Morimoto K, Sakaguchi H, Tanaka T et al (2008) Transarterial chemoembolization using cisplatin powder in a rabbit model of liver cancer. Cardiovasc Intervent Radiol 31(5):981–985
Lu K, Li YH, He XF et al (2008) Necrosis and apoptosis in hepatocellular carcinoma following low-dose versus high-dose preoperative chemoembolization. Cardiovasc Intervent Radiol 31(6):1133–1140
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maeda, N., Osuga, K., Higashihara, H. et al. Transarterial Chemoembolization With Cisplatin as Second-Line Treatment for Hepatocellular Carcinoma Unresponsive to Chemoembolization With Epirubicin-Lipiodol Emulsion. Cardiovasc Intervent Radiol 35, 82–89 (2012). https://doi.org/10.1007/s00270-010-0086-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-010-0086-6