Abstract
Purpose
To evaluate the influence of continuous infusion of acetic acid 50% during radiofrequency ablation (RFA) on the size of the thermal lesion produced.
Methods
Radiofrequency (RF) was applied to excised bovine liver by using an expandable needle electrode with 10 retractable tines (LeVeen Needle Electrode, RadioTherapeutics, Sunnyvale, CA) connected to a commercially available RF generator (RF 2000, RadioTherapeutics, Sunnyvale, CA). Experiments were performed using three different treatment modalities: RF only (n = 15), RF with continuous saline 0.9% infusion (n = 15), and RF with continuous acetic acid 50% infusion (n = 15). RF duration, power output, tissue impedance, and time to a rapid rise in impedance were recorded. The ablated lesions were evaluated both macroscopically and histologically.
Results
The ablated lesions appeared as spherical or ellipsoid, well-demarcated pale areas with a surrounding brown rim with both RF only and RF plus saline 0.9% infusion. In contrast, thermolesions generated with RF in combination with acetic acid 50% infusion were irregular in shape and the central portion was jelly-like. Mean diameter of the coagulation necrosis was 22.3 ± 2.1 mm (RF only), 29.2 ± 4.8 mm (RF + saline 0.9%) and 30.7 ± 5.7 mm (RF + acetic acid 50%), with a significant increase in the RF plus saline 0.9% and RF plus acetic acid 50% groups compared with RF alone. Time to a rapid rise in impedance was significantly prolonged in the RF plus saline 0.9% and RF plus acetic acid 50% groups compared with RF alone.
Conclusions
A combination of RF plus acetic acid 50% infusion is able to generate larger thermolesions than RF only or RF combined with saline 0.9% infusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In recent years image-guided percutaneous thermotherapies, such as radiofrequency ablation (RFA), laser induced thermotherapy (LITT), and microwave coagulation therapy (PMCT), have emerged as promising minimally invasive treatment modalities for primary and secondary liver neoplasms in selected patients [1, 2]. However, all thermoablation techniques are limited by both the volume of the coagulation necrosis created in one treatment session without needle repositioning and the rate of postinterventional local recurrence, especially at the margin of the thermolesion [2]. Many technical developments and improved treatment strategies have been attempted to overcome these limitations [3–7]. Our goal was to evaluate whether simultaneous infusion of acetic acid 50% during RFA is able to increase the coagulation size and whether there are different effects on the margin of the thermal lesion due to acetic acid 50% that could have an impact on preventing local tumor recurrence.
Materials and Methods
RFA Setting
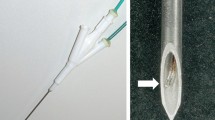
All ablations were done using a commercially available RFA unit (RF 2000, RadioTherapeutics, Sunnyvale, CA) and probes (LeVeen Needle Electrode, RadioTherapeutics, Sunnyvale, CA). The radiofrequency (RF) generator operated at 460 kHz and delivered variable power, up to a maximum of 100 W. It monitored tissue impedance, RF power applied, and time of application. The electrodes were hollow, 15-gauge needles equipped with 10 retractable, curved distal tines that when fully deployed encompassed an area 2 cm in diameter. As return electrode a dispersive electrode (Valleylab PolyHesive) plugged into the RF generator return receptacles was used in all trials. After connecting the needle electrode to the RF generator, the RF generator was switched on and, for all procedures, output was initially set to 30 W. Every 60 sec the wattage was increased by 10 W until 50 W were attained. The power was maintained at 50 W for 5 min or until a rapid rise in impedance which stopped current flow and the ablation. All experiments were limited to a single-phase ablation.
Study Design and Ex Vivo Experiments
Bovine livers were obtained from a local butcher, warmed to room temperature, and placed in a metallic basin. Then a return electrode was attached to the outer side of the bottom of the metallic basin and plugged into the RF generator return receptacles. Experiments were performed using three different treatment modalities:
-
1.
RF only (n = 15)
-
2.
RF with continuous saline 0.9% infusion (n = 15)
-
3.
RF with continuous acetic acid 50% infusion (n = 15).
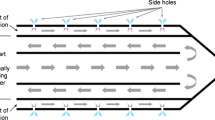
The RF electrode was inserted at least 2 cm into the ex vivo liver tissue and held in place by a rigid fixing. An additional infusion needle with a closed conical tip and three sideholes (Pflugbeil, Germany) was placed in the liver tissue with its tip directly adjacent to the RF electrode. According to the study protocol either saline 0.9% or acetic acid 50% was administered continuously at a flow rate of 0.5 ml/min during the ablation procedure.
Documentation and Pathologic Assessment
During the experiments parameters including RF duration, power output, impedance, and time to a rapid rise in impedance were monitored. After completion of the experiments, the liver was sectioned across the ablation site along the needle track. The ablated lesions were examined both macroscopically and histologically. Visual examination included photographic documentation and determination of the size and shape of the thermal lesions. The maximal transverse diameter of each visible thermal lesion was measured.
For pathologic examination the lesions were carefully excised, sliced into 5–10 mm sections and fixed in formalin for 1 week. The specimens then were serially cut into sections 4 μm thick and embedded in paraffin. Staining with hematoxylin–eosin followed.
Statistical Analysis
The primary endpoint of the experimental study was lesion diameter. Secondary endpoints were RF duration, power output, tissue impedance, and time to a rapid rise in impedance. The diameter of the coagulation necrosis achieved with RF only was compared with those achieved with RF in combination with saline 0.9% or acetic acid 50%. Forty-five complete datasets were evaluated. The data were displayed as means ± standard deviation. The two-tailed Student’s t-test was used to determine statistical significance. A p value of less than 0.05 was considered statistically significant. Data analysis was done using SPSS for Windows (SPSS, Chicago, IL).
Results
The results of the experiments are summarized in Tables 1, 2, and 3.
The ablated lesions generated with RF only and RF in combination with saline 0.9% both appeared as spherical or ellipsoid, well-demarcated areas within the liver tissue. There was a central charred zone with a small cavity corresponding to the needle tract surrounded by pale tissue representing coagulation necrosis. All thermolesions produced by RF only and RF in combination with saline 0.9% had a small brownish rim attributed to vascular congestion encompassing the lesion (Fig. 1). The mean diameter of the lesions produced with RF only was 22.3 ± 2.1 mm (range 18.8–25.5 mm) and for those produced by RF plus saline 0.9% was 29.2 ± 4.8 mm (range 21.5–41.4 mm). A rapid rise in impedance with RF only was registered in all experiments after a mean RF duration of 1.6 ± 0.4 min (range 1.2–2.5 min). In the RF plus saline 0.9% group a rapid rise in impedance was found in all but one experiment with a mean RF duration of 2.9 ± 1.2 min (range 1.2–5.00 min).
In contrast, lesions generated with RF together with acetic acid 50% infusion appeared as irregular-shaped areas with the central parts being brown and jelly-like. There was no clear demarcation of the thermolesion and in most cases only an incomplete brownish rim was present (Fig. 2). Histologic investigation revealed coagulation necrosis with incomplete vascular congestion and surrounding areas of coagulated tissue. The mean diameter of the lesions was 30.7 ± 5.7 mm (range 20.9–46.6 mm). A rapid rise in impedance was documented in all but one experiment with the mean RF duration being 2.7 ± 0.8 min (range 1.6–5.00 min).
Lesion diameters were significantly larger in the RF plus saline 0.9% and RF plus acetic acid 50% groups compared with RF alone. There was a tendency toward further enlargement of the thermal lesion when comparing the RF plus saline 0.9% and RF plus acetic acid 50% groups. Time to a rapid rise in impedance was significantly longer in the RF plus saline 0.9% and RF plus acetic acid 50% groups compared with RF alone. No difference was observed in RF duration between the RF plus saline 0.9% and RF plus acetic acid 50% groups.
Discussion
Percutaneous RFA will have an important role as an alternative treatment option of focal liver malignancies [1, 2]. Several clinical trials have outlined the efficacy of RFA concerning local tumor destruction and control in different small tumors [8–12]. However, key limitations of this minimally invasive treatment are the extent of the coagulation necrosis achievable in a single application session and the rate of local recurrence [13, 14]. In addition to the effects of different generator algorithms and needle designs, modulation of the biological environment of treated tissues has been shown to increase the volume of coagulation necrosis [4, 15]. One strategy is to inject saline in different concentrations into the tissue during RFA. The mechanisms of actions are alteration of local tissue electrical conductivity and energy deposition with consecutive increase in tissue heating and coagulation [4]. In addition liquids create improved tissue heat conduction which also results in a greater lesion size [5]. The goal of the present study was to evaluate whether RFA with simultaneous infusion of a liquid that also has marked antitumoral effect is able to increase coagulation size, too. The second intention was to evaluate whether there are different effects with this combined treatment option, especially regarding the margin of the thermal lesion compared with that produced by RF alone or RF plus infusion of saline 0.9%, that might have an impact on preventing local recurrence. Because acetic acid has been described as a potential alternative to percutaneous ethanol injection in the treatment of small hepatocellular carcinomas [16, 17], we decided to use acetic acid as the fluid agent. Ohnishi et al. [18] demonstrated acetic acid to be superior to ethanol, producing a higher degree of necrosis and having more homogeneous diffusion. The cytotoxic mechanisms of acetic acid are similar to those of ethanol and include protein denaturation, and dissolution of basement membranes and interstitial collagen resulting in coagulative necrosis of tumor cells [19–21]. Acetic acid has the advantage of infiltrating the septa and capsule of tumors, whereas ethanol does not. In addition, acetic acid appears to infiltrate through firm lesions more homogeneously than ethanol and therefore may hold promise in the treatment of liver metastases too [18, 19]. Our results showed that combined treatment, RFA plus acetic acid infusion, produces a significant enlargement of the ablated area compared with RFA alone. The mechanisms of actions are speculated to be the same as those responsible for modulation of the biological environment which has been demonstrated with saline in nonperfused livers: (1) alteration of local tissue electrical conductivity and energy deposition with consecutive increase in tissue heating and coagulation and (2) improved tissue heat conduction resulting in a greater coagulation necrosis [4, 5]. It is very important to differentiate between perfused and nonperfused livers because in perfused livers there is another effect that might contribute to an enlargement of lesion size: thrombosis of traversing vessels. Acetic acid, like ethanol, has the potential to induce thrombosis of small vessels within the ablated area resulting in a reduced heat sink effect with consecutive enlargement of the thermolesion [21, 22]. Compared with RFA plus saline 0.9%, a further tendency to increase coagulation diameter was demonstrated with RFA plus acetic acid 50% in our experiments. On the one hand this might be due to the limited number of experiments. On the other hand this is supposed to be due to the fact that acetic acid 50% itself has a marked cytotoxic effect which leads to a higher rate of tissue destruction and better diffusion of the liquid [23, 24]. This hypothesis is supported by the different macroscopic and microscopic findings in the groups treated with saline 0.9% or acetic acid 50% in addition to RF. In particular the margins of the ablated lesions were found to be irregular-shaped after RF plus acetic acid 50%, unlike those after RF plus saline 0.9%. Thermal lesions produced with RF plus simultaneous saline 0.9% infusion showed a complete brown rim around the central pale area, whereas lesions produced with RF plus acetic acid 50% infusion in most cases did not. Histologic investigation revealed devitalized necrotic lesions with patchy fibrosis not only within the central parts but also at the margins of the lesions, the surrounding rings of inflammatory tissue being incomplete. We conclude that the greater enlargement of coagulation necrosis after treatment with RFA plus acetic acid 50% compared with that produced by RF combined with saline 0.9% is attributable to diffusion of acetic acid 50% to the periphery of the ablated area with consecutive tissue destruction by the cytotoxic effect of the acid itself. Because of uneven diffusion of the acetic acid, the margins of the coagulation necrosis appeared irregular. Our data compare favorably with those recently published by Lee et al. [25, 26]. These authors found an enlarged and irregular shape of the RF thermolesion after tissue modulation with acetic acid. Concerning the clinical use of combined RF and acetic acid, one has to consider that the use of acetic acid could result in thermolesions of an unpredictable size and shape [26]. Especially in liver tumors, which are usually spherical lesions, an irregularly shaped ablation area would not seem to be helpful in achieving complete tumor necrosis [27].
Conclusion
Our data suggest that RFA combined with infusion of acetic acid 50% holds the promise to treat even larger tumorous focal lesions in a single treatment session and to reduce peripheral local recurrence because of the additional cytotoxic effect of acetic acid at the periphery of the thermal lesion. However, it needs to be considered that acetic acid 50% will not be controllable when used as a tissue modulator during RFA because of its highly cytotoxic effects with consecutive uneven diffusion. This might result in a lower rate of complete tumor necrosis and higher rate of complications in a clinical setting, especially as a result of tissue destruction of sensitive structures adjacent to the ablated area. Further experimental evaluation of acetic acid as a tissue modulator for RFA is mandatory to determine clear recommendations for its clinical use.
References
Dodd GD III, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, Gillams AR, Karahan OI, Rhim H (2000) Minimally invasive treatment of malignant hepatic tumors: At the threshold of a major breakthrough. RadioGraphics 20:9–27
de Tucker Sanctis J, Goldberg SN, Mueller PR (1998) Percutaneous treatment of hepatic neoplasms: A review of current techniques. Cardiovasc Intervent Radiol 21:273–296
Goldberg SN, Solbiati L, Hahn PF, Cosman E, Conrad JE, Fogle R, Gazelle GS (1998) Large-volume tissue ablation with radio frequency by using a clustered, internally cooled electrode technique: Laboratory and clinical experience in liver metastases. Radiology 209:371–379
Goldberg SN, Ahmed M, Gazelle GS, Kruskal JB, Huertas JC, Halpern EF, Oliver BS, Lenkinski RE (2001) Radio-frequency thermal ablation with NaCl solution injection: Effect of electrical conductivity on tissue heating and coagulation—phantom and porcine liver study. Radiology 219:157–165
Livraghi T, Goldberg SN, Monti F, Bizzini A, Lazzaroni S, Meloni F, Pellicano S, Solbiati L, Gazelle GS (1997) Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology 202:205–210
De Baere T, Denys A, Wood BJ, Lassau N, Kardache M, Vilgrain V, Menu Y, Roche A (2001) Radiofrequency liver ablation: Experimental comparative study of water-cooled versus expandable systems. AJR Am J Roentgenol 176:187–192
Ni Y, Miao Y, Mulier S, Yu J, Baert AL, Marchal G (2000) A novel “cooled-wet” electrode for radiofrequency ablation. Eur Radiol 10:852–854
Goldberg NS, Gazelle GS, Compton CC, Mueller PR, Tanabe KK (2000) Treatment of intrahepatic malignancy with radiofrequency ablation. Radiologic-pathologic correlation. Cancer 88:2452–2463
Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, Gazelle GS (2000) Hepatocellular carcinoma: Radio-frequency ablation of medium and large lesions Radiology 214:761–768
Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L (1996) Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol 167:759–768
Dupuy DE, Zagoria RJ, Akerley W, Mayo-Smith WW, Kavanagh PV, Safran H (2000) Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol 174:57–59
Wood BJ, Bates S (2001) Radiofrequency thermal ablation of a splenic metastasis. J Vasc Interv Radiol 12:261–263
McGahan JP, Dodd III GD (2001) Radiofrequency ablation of the liver: Current status. AJR Am J Roentgenol 176:3–16
Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, Zangrandi A, Andreola S, Silverman D, Buscarini L (1998) Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol 170:1015–1022
Goldberg SN, Hahn PF, Halpern EF, Fogle RM, Gazelle GS (1998) Radio-frequency tissue ablation: Effect of pharmacologic modulation of blood flow on coagulation diameter. Radiology 209:761–767
Ohnishi K, Yoshioka H, Ito S, Fujiwara K (1996) Treatment of nodular hepatocellular carcinoma larger than 3 cm with ultrasound-guided percutaneous acetic acid injection. Hepatology 24:1379–1385
Liang H-L, Yang C-F, Pan H-B, Lai K-H, Cheng J-S, Lo G-H, Chen CKH, Lai P-H (2000) Small hepatocellular carcinoma: Safety and efficacy of single high-dose percutaneous acetic acid injection for treatment. Radiology 214:769–774
Ohnishi K, Yoshioka K, Ito S, Fujiwara K (1998) Prospective randomized controlled trial comparing percutaneous acetic acid injection and percutaneous ethanol injection for small hepatocellular carcinoma. Hepatology 27:67–72
Ohnishi K (1998) Comparison of percutaneous acetic acid injection and percutaneous ethanol injection for small hepatocellular carcinoma. Hepato-Gastroenterology 45:1254–1258
Lee JM, Lee YH, Kim YK, Kim SW, Kim CS (2004) Combined therapy of radiofrequency ablation and ethanol injection of rabbit liver: An in vivo feasibility study. Cardiovasc Intervent Radiol 27:151–157
Goldberg SN, Kruskal JB, Oliver BS, Clouse ME, Gazelle GS (2000) Percutaneous tumor ablation: Increased coagulation by combining radio-frequency ablation and ethanol instillation in a rat breast tumor model. Radiology 217:827–831
Shiina S, Tagawa K, Unuma T, Takanashi R, Yoshiura K, Komatsu Y, Hata Y, Niwa Y, Shiratori Y, Terano A (1991) Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer 68:1524–1530
Koda M, Okamoto K, Miyoshi Y, Kawasaki H (1992) Hepatic vascular and bile duct injury after ethanol injection therapy for hepatocellular carcinoma. Gastrointest Radiol 17:167–169
Shah SS, Jacobs DL, Krasinkas AM, Furth EE, Itkin M, Clark TW (2004) Percutaneous ablation of VX2 carcinoma-induced liver tumors with use of ethanol versus acetic acid: Pilot study in a rabbit model. J Vasc Interv Radiol 15:63–67
Lee JM, Kim YK, Kim SW, Han JK, Kim SH, Choi BI (2004) Combined radiofrequency ablation and acetic acid hypertonic saline solution instillation: An in vivo study of rabbit liver. Korean J Radiol 5:31–38
Lee JM, Lee YH, Kim YK, Kim SW, Kim SH, Han JK, Choi BI (2004) Combined treatment of radiofrequency ablation and acetic acid injection: An in vivo feasibility study in rabbit liver. Eur Radiol 14:1303–1310
Dodd GD, Frank MS, Aribandi M, Chopra S, Chintapalli KN (2002) Radiofrequency thermal ablation: Computer analysis created by overlapping ablations. AJR Am J Roentgenol 177:777–782
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lubienski, A., Düx, M., Lubienski, K. et al. Radiofrequency Thermal Ablation: Increase in Lesion Diameter with Continuous Acetic Acid Infusion. Cardiovasc Intervent Radiol 28, 789–794 (2005). https://doi.org/10.1007/s00270-005-0022-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-005-0022-3