Abstract
Purpose
To evaluate the aneurysm neck angle changes and post-endovascular aneurysm repair (EVAR) complications.
Methods
We retrospectively analyzed 72 cases of elective EVAR for abdominal aortic aneurysm among 109 consecutive cases from December 2005 to April 2014. Patients were divided into angulated and non-angulated groups. The angulated group was defined as neck angulation ≥60°. Neck angle was evaluated pre- and post-EVAR during short- (within 1 month), mid- (3–6 months), and long-term (>1 year) follow-up. Aneurysm sac diameter change, aneurysm neck morphology other than angulation, endoleaks, and other post-procedural complications were also documented.
Results
A total of 34 patients were enrolled in the angulated group. There were no statistical differences in age, sex, follow-up duration, and aneurysm neck profile between the two groups (p > 0.05). Both groups showed statistically significant and consistent decreases in angulation during the follow-up period (p < 0.01). The angulated group revealed 22.45 % more straightening than the non-angulated group. Recoil of the Endurant device occurred in the angulated group. No statistically significant intergroup differences were observed in any endoleaks, complications, or re-intervention rates (p > 0.05). Pre-EVAR angle was the only predictor for post-procedural angle change (p < 0.001).
Conclusion
EVAR is applicable for patients with highly angulated aneurysm neck and provides consistent neck straightening over long-term follow-up. Recoil was evident in the angulated group using the Endurant device.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abdominal aortic aneurysm (AAA) is one of the underlying causes of sudden death worldwide with the reported prevalence of 1.7–12.7 % [1]. Since it was first introduced by Parodi et al. in 1991, endovascular aneurysm repair (EVAR) has become a revolutionary approach to the treatment of infrarenal AAA. EVAR has proven salient advantages in perioperative mortality and morbidity, hospital stay length, operative time, and blood loss compared to open repair [2–6]. However, EVAR may not always be the optimal treatment option since not all patients are eligible for it. A hostile neck, consisting of severe angulation, a short, reverse taper and severe calcification and thrombus, remains a leading anatomical limitation of EVAR.
The proximal neck anatomy is a major limiting factor in the determination of a patient’s suitability for EVAR [1, 3, 7]. These factors were initially estimated to exclude 20–40 % of patients [8–11]. Among them, angulation is possibly the most important characteristic of the aneurysmal neck [12]. In fact, the implantation of endografts in patients with highly angulated proximal neck anatomy results in considerable intraprocedural technical problems and adverse short-term clinical outcomes [1]. However, recent stent-graft design improvements and diversifications as well as the availability of operators who are more skilled in stent grafting techniques allow standard stent grafts to be implanted for shorter, more highly angulated, and wider aortic necks. Changes in aortic angulation over time after EVAR may affect the proximal sealing and fixation zone; therefore, they are considered a potential risk of late complications and adverse outcomes [12]. This raises the need to understand the post-procedural configuration of the angulated neck and identify complications during follow-up. In this series, we aimed to compare the proximal aneurysm neck angulation changes and EVAR clinical outcomes in patients with angulated and non-angulated necks.
Materials and Methods
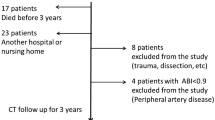
This study was approved by our institution’s institutional review board. Data were retrieved from the hospital’s database. Between December 2005 and June 2014, 109 consecutive patients with EVAR were treated for infrarenal AAA. Exclusion criteria included EVAR for pseudoaneurysm, ruptured AAA, aortic dissection, penetrating ulcer, and previous aortic surgery. Ultimately, 72 patients (60 men) who underwent elective EVAR for AAA were enrolled in this study. The patients were then divided into the angulated (n = 34) and non-angulated (n = 38) groups according to the aneurysmal neck angulation (≥60° or <60°, respectively). Follow-up duration was 1–85 months (mean, 18 months). Patient demographics, aneurysm profile (including aneurysm diameter, neck length, and neck diameter), endoleaks, complications, and re-intervention rate in both groups were also documented and compared. Four types of stent grafts were used in our series, including 31 Zenith (Cook Medical, Bloomington, IN, USA), 21 Endurant (Medtronic Vascular, Santa Rosa, CA, USA), 11 Excluder (WL Gore and Associates, Flagstaff, AZ, USA), and 9 Seal (S&G Biotech, Seongnam, Korea). Subgroups of the Zenith and Endurant device groups were also analyzed, while those of the Excluder and Seal were not due to small sample sizes. The indications for EVAR required consensus between vascular surgeons and interventional radiologists and considered each patient’s age, clinical condition, and imaging findings as well as the instructions for use (IFU) of specific stent grafts. Informed consent was obtained from all patients. All of the procedures were performed by one interventional radiologist with over 10 years of experience of EVAR.
The definition and measurements of aneurysm neck angulation (Fig. 1) were based on the method described by Hoshina et al. [6]. All patients underwent baseline computed tomography angiography (CTA) for the aneurysm anatomical evaluation before EVAR and follow-up CTA at 1 month, 6 months, 1 year, and every year after EVAR. The CTA and 3-D reconstruction images were obtained according to standard institutional protocols using 64-slice multidetector CT. Helical scan data were acquired from the xyphoid process to feet in the supine position with a detector coverage of 40 mm, a gantry rotation time of 0.6 s, a scan thickness of 1.25 mm, image reconstruction interval of 2.5 mm, and an effective tube current–time product of 300–400 mAs and 140 kVp. Nonionic contrast media (Bonorex Iohexol 300, CMS, Seoul, Korea) were used in all patients with the average dose was 2 ml/kg of body weight. The contrast medium was administered intravenously through a mechanical power injector (Stellant, Medrad, Pittsburgh, PA, USA) at a rate of 5 ml/s. The computer-assisted bolus-tracking software was used to determine the optimal scan delay for the arterial phase in each patient. All the CT images were reviewed at a workstation with the PACS (Maroview 5.4, Infinitt, Seoul, Korea). 3D images were reconstructed using AW Volume Share 4 software (GE, Milwaukee, WI, USA).
Statistical Analysis
Continuous data are presented as mean ± standard deviation, while categorical data are given as counts and percentages. Independent two-sample t test and repeated measure analysis of covariance were used to compare data between the two groups. All analyses were performed using R software version 3.1.2 (R Core Team, R Foundation for Statistical Computing). p values <0.05 indicate statistical significance for all comparisons.
Results
There were no differences in age, aneurysm neck morphology, number of comorbidities, or follow-up duration between the two groups. A short aneurysmal neck (<15 mm) was observed in 4 patients of the non-angulated group (mean neck length, 36.36 ± 15.41 mm), while it was not evident in any patients of the angulated group (mean neck length, 33.59 ± 17.14 mm). The mean follow-up duration was 14.38 ± 17.86 months (range, 1–67 months) for the angulated group and 21.74 ± 24.04 months (range, 1–85 months) for the non-angulated group (Table 1). Both groups showed significant decreases in neck angulation immediately after EVAR and remained consistent during the follow-up period (Table 2). The angulated group had a 22.45 % greater average degree of straightening than the non-angulated group (Fig. 2); however, the final angulations were still higher in the angulated group. Regarding device-specific evaluation, recoil was noted in 5 patients of the angulated group in whom the Endurant was used, whereas straightening was consistently seen in patients in the angulated group in whom the Zenith was used. No difference was seen in late configuration between the two devices in the non-angulated group (Fig. 3). The mean aneurysmal sac diameters of the angulated group at baseline, 1 month, 3–6 months, and ≥1 year were 59.89 ± 9.23, 57.47 ± 9.93, 53.87 ± 11.40, and 50.2 ± 15.42 mm, while the mean aneurysmal sac diameters of non-angulated group were 58.65 ± 10.71, 55.60 ± 10.49, 53.68 ± 11.92, and 51.70 ± 17.98 mm, respectively. No intergroup difference in diameter was seen at any of the checkpoints (p > 0.05); however, a slight sac regression tendency was evident (Fig. 4).
Aneurysm neck angulation changes before and after endovascular aneurysm repair (EVAR). The angulated and non-angulated groups demonstrated significant angulation reductions immediately after EVAR that remained consistent during the follow-up period. The angulated group had a greater average degree of straightening than the non-angulated group
Aneurysm neck angulation changes before and after endovascular aneurysm repair with device-specific evaluation. Recoiling was noted in patients in the angulated group using the Endurant device (En), whereas straightening was consistent in patients in the angulated group in whom the Zenith device (Ze) was used. No difference was seen in late configuration between the two groups
The endoleaks, complications, and re-intervention rates are presented in Tables 3 and 4. The overall incidence of endoleaks was 30.6, 29.4, and 30.6 % at 1 month, 6 months, and 1 year, respectively. At these time points, the incidence of type I endoleak was 1 of 34 patients, 0 of 22, and 0 of 16 in the angulated group and 3 of 38, 1 of 29, and 0 of 20 in the non-angulated group, respectively. We encountered 5 cases of type I endoleak (1 in the angulated and 4 in the non-angulated groups), of which 4 cases were detected within 1 month and 1 within 6 months. Endotension was also documented in 2 cases (2.8 %), 1 in each group. Complications occurred in 7 patients of the angulated group and 2 patients of the non-angulated group. The re-intervention rates were 20.6 % for the angulated group and 13.2 % for the non-angulated group. No statistically significant intergroup differences were observed in endoleaks, complications, or re-intervention rates (p > 0.05).
Re-intervention procedures were used to manage type I endoleaks in 3 cases by aortic extensions, 1 case in the angulated group and 2 cases in the non-angulated group (the other 2 cases of minimal endoleaks resolved spontaneously during follow-up); type II endoleaks in 2 cases by embolization; endotension in 2 cases; and graft stenosis/occlusion in 5 cases. The 2 cases of limb occlusion in the non-angulated group were managed with femoral–femoral bypass. Four re-intervention procedures were performed within 1 month after EVAR for iliac limb stenosis/occlusion. Two cases of endotension were diagnosed during long-term follow-up and re-linings were performed thereafter. No patient experienced technical or clinical failure or EVAR-related death.
Discussion
Technically, the suitability of EVAR is usually based on the manufacturer’s IFU, which requires that certain criteria be respected for better outcomes. In this study, the IFU of each specific stent graft was taken into consideration whenever EVAR was indicated. As shown in Table 1, because the patients in the angulated group had relatively favorable aneurysmal neck anatomy with adequate neck length and proper neck diameter, therefore, they were candidates for EVAR after multidisciplinary discussions. The management was similar for 4 patients with short neck in the non-angulated group.
In our series, both groups demonstrated significant and consistent post-procedural angulation decreases, meaning that EVAR has a straightening effect regardless of aneurysm neck angulation degree. Moreover, the angulated group showed a 22.45 % higher mean degree of straightening than the non-angulated group, indicating that the more angulated the neck is before the procedure, the more straightening it demonstrates thereafter. An angulated aneurysm neck could be immediately straightened by the introduction of a guidewire, delivery system, and/or stent graft due to various factors such as neck anatomy and stent graft and wire design and configuration; however, the stent’s radial force retains the unique and consistent impact of the aortic angle over time [6, 12, 13].
The angulated proximal neck leads to challenges in adequate material implantation, accurate deployment, and proper fixation [14]. The introduction of newly designed devices recently improved EVAR technical success and clinical outcomes, thus widening its indications, particularly in cases of challenging proximal neck anatomy. In this study, we compared the changes in neck angulation in the subgroup of patients in whom the Zenith and Endurant, the latest generation of stent grafts specifically designed to treat more challenging neck morphology, were used. Table 2 shows the significant decrease in angulation and lack of difference in late configuration between the two devices in the non-angulated group. Similar findings were obtained for the angulated group. Interestingly, recoil was observed in the angulated group with the Endurant device during follow-up, whereas the Zenith device showed a consistent straightening ability (Figs. 5, 6).
A 73-year-old man with a severely angulated aneurysm neck (A). Endovascular aneurysm repair was performed using an Endurant device. A post-procedural computed tomography angiogram image shows a significant decrease in angulation after 1 week (B). However, recoil was observed after 6 months (C) and 1 year (D)
Recently published data regarding the use of the Endurant to treat patients with a hostile neck demonstrated that this device is highly conformable and technically feasible, and provides acceptable results [15–22]. However, to our knowledge, this is the first study to describe recoil of the Endurant device during follow-up. This device consists of an M-shaped nitinol stent attached to a multifilament polyester graft, a highly conformable but kink-resistant main body, a suprarenal fixation, and a tip-capture delivery system. These components increase control over deployment, enhance proximal positioning, and provide potentially greater flexibility and migration resistance [15, 16]. On the other hand, the Zenith is a self-expanding stainless steel Z-stent that consists of an endoskeleton in the sealing portion of the first ring and the distal seal of the iliac limbs as well as an exoskeleton. Thus, the Zenith is more rigid and stronger and stretches the angulation for a longer period of time [8, 23]. Therefore, the Endurant was presumed to have better flexibility, whereas the Zenith showed a better straightening ability. We believe that these factors are potential hypotheses for explaining the late recoil seen in patients in the angulated group treated with the Endurant device.
We noted a slight sac regression tendency during follow-up despite no significant intergroup difference at any time point. A similar trend in neck and sac remodeling was also described in another study [24]. However, our study findings imply a relatively comparable homogeneity of sample sizes since we found no statistically significant difference in patient number, aneurysm sac diameter, or neck morphology except for neck angulation between the two groups (Table 1). Multiple stepwise regression analysis results including all variables also indicated that preoperative neck angulation was the only predictor of an early postoperative angle change (p < 0.001), whereas sac diameter was the predictor of late angle configuration (p = 0.006).
In this study, the incidences of endoleak were in keeping with those of previous reports [10, 13, 17, 25, 26]. There was no intergroup difference in any endoleak type, including type I, during follow-up (p > 0.05). However, a meta-analysis revealed a significant increase in 30-day type I endoleaks and late type I endoleaks in patients with a hostile neck [27]. We presume that a reasonable explanation for this mismatch is that the angulated groups had relatively favorable neck anatomy (length and diameter), which could be responsible for the similarity in the endoleak rates between the two groups.
The rate of complications and re-interventions was not statistically different. In the 30-day period, we noted 4 cases of limb occlusion. These events occurred in older patients (>80 years) with underlying iliac artery risk factors such as tortuous, calcification, and stenosis. One patient had an aortoiliac aneurysm. The iliac arteries of Asian people are shorter and smaller than those of Caucasians. These factors would complicate access, impair fixation, facilitate kinking, and result in a higher incidence of access- and device-related complications [28–30].
Our study has several limitations. This was a retrospective analysis of a relatively small sample size. We also used 4 different types of stent grafts with different profiles and configurations based on physician preference. These were potential confounders that created selection bias.
In conclusion, EVAR is applicable to cases of severely angulated neck anatomy and provides consistent neck straightening over long-term follow-up. Recoil was observed in the angulated group treated with the Endurant device during follow-up, and further investigations are necessary to clarify its late configuration.
References
Stather PW, Sidloff DA, Rhema IA, Choke E, Bown MJ, Sayers RD. A review of current reporting of abdominal aortic aneurysm mortality and prevalence in the literature. Eur J Vasc Endovasc Surg. 2014;47(3):240–2.
Walker TG, Kalva SP, Yeddula K, et al. Clinical practice guidelines for endovascular abdominal aortic aneurysm repair: written by the Standards of Practice Committee for the Society of Interventional Radiology and endorsed by the Cardiovascular and Interventional Radiological Society of Europe and the Canadian Interventional Radiology Association. J Vasc Interv Radiol: JVIR. 2010;21(11):1632–55.
Aburahma AF, Campbell JE, Mousa AY, et al. Clinical outcomes for hostile versus favorable aortic neck anatomy in endovascular aortic aneurysm repair using modular devices. J Vasc Surg. 2011;54(1):13–21.
Stather PW, Sayers RD, Cheah A, Wild JB, Bown MJ, Choke E. Outcomes of endovascular aneurysm repair in patients with hostile neck anatomy. Eur J Vasc Endovasc Surg. 2012;44(6):556–61.
Hobo R, Kievit J, Leurs LJ, Buth J. Influence of severe infrarenal aortic neck angulation on complications at the proximal neck following endovascular AAA repair: a EUROSTAR study. J Endovasc Ther. 2007;14(1):1–11.
Hoshina K, Akai T, Takayama T, et al. Outcomes and morphologic changes after endovascular repair for abdominal aortic aneurysms with a severely angulated neck. Circ J. 2013;77(8):1996–2002.
Hoshina K, Hashimoto T, Kato M, Ohkubo N, Shigematsu K, Miyata T. Feasibility of endovascular abdominal aortic aneurysm repair outside of the instructions for use and morphological changes at 3 years after the procedure. Ann Vasc Dis. 2014;7(1):34–9.
Arko FR 3rd, Murphy EH, Boyes C, et al. Current status of endovascular aneurysm repair: 20 years of learning. Semin Vasc Surg. 2012;25(3):131–5.
Antoniou GA, Georgiadis GS, Antoniou SA, Kuhan G, Murray D. A meta-analysis of outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile and friendly neck anatomy. J Vasc Surg. 2013;57(2):527–38.
Igari K, Kudo T, Toyofuku T, Jibiki M, Inoue Y. Outcomes following endovascular abdominal aortic aneurysm repair both within and outside of the instructions for use. Ann Thorac Cardiovasc Surg. 2014;20(1):61–6.
de Vries JP. The proximal neck: the remaining barrier to a complete EVAR world. Semin Vasc Surg. 2012;25(4):182–6.
van Keulen JW, Moll FL, Arts J, Vonken EJ, van Herwaarden JA. Aortic neck angulations decrease during and after endovascular aneurysm repair. J Endovasc Ther. 2010;17(5):594–8.
Lee HK, Chung SY, Kim JK, Yoo SH, Choi SJ. Changes in suprarenal and infrarenal aortic angles after endovascular aneurysm repair. Ann Surg Treat Res. 2014;87(4):197–202.
Molony DS, Kavanagh EG, Madhavan P, Walsh MT, McGloughlin TM. A computational study of the magnitude and direction of migration forces in patient-specific abdominal aortic aneurysm stent-grafts. Eur J Vasc Endovasc Surg. 2010;40(3):332–9.
Bastos Goncalves F, de Vries JP, van Keulen JW, et al. Severe proximal aneurysm neck angulation: early results using the Endurant stentgraft system. Eur J Vasc Endovasc Surg. 2011;41(2):193–200.
Matsagkas M, Kouvelos G, Peroulis M, et al. Standard endovascular treatment of abdominal aortic aneurysms in patients with very short proximal necks using the Endurant stent graft. J Vasc Surg. 2015;61(1):9–15.
Zandvoort HJ, Goncalves FB, Verhagen HJ, et al. Results of endovascular repair of infrarenal aortic aneurysms using the Endurant stent graft. J Vasc Surg. 2014;59(5):1195–202.
Setacci F, Sirignano P, de Donato G, et al. AAA with a challenging neck: early outcomes using the Endurant stent-graft system. Eur J Vasc Endovasc Surg. 2012;44(3):274–9.
Bisdas T, Weiss K, Eisenack M, Austermann M, Torsello G, Donas KP. Durability of the Endurant stent graft in patients undergoing endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2014;60(5):1125–31.
Stokmans RA, Teijink JA, Forbes TL, et al. Early results from the ENGAGE registry: real-world performance of the Endurant Stent Graft for endovascular AAA repair in 1262 patients. Eur J Vasc Endovasc Surg. 2012;44(4):369–75.
Torsello G, Troisi N, Donas KP, Austermann M. Evaluation of the Endurant stent graft under instructions for use vs off-label conditions for endovascular aortic aneurysm repair. J Vasc Surg. 2011;54(2):300–6.
Bastos Goncalves F, Hoeks SE, Teijink JA, et al. Risk factors for proximal neck complications after endovascular aneurysm repair using the endurant stent graft. Eur J Vasc Endovasc Surg. 2015;49(2):156–62.
Ishibashi H, Ishiguchi T, Ohta T, et al. Remodeling of proximal neck angulation after endovascular aneurysm repair. J Vasc Surg. 2012;56(5):1201–5.
Tsilimparis N, Dayama A, Ricotta JJ 2nd. Remodeling of aortic aneurysm and aortic neck on follow-up after endovascular repair with suprarenal fixation. J Vasc Surg. 2015;61(1):28–34.
Mertens J, Houthoofd S, Daenens K, et al. Long-term results after endovascular abdominal aortic aneurysm repair using the Cook Zenith endograft. J Vasc Surg. 2011;54(1):48.e2–.
Rutherford RB. Open versus endovascular stent graft repair for abdominal aortic aneurysms: an historical view. Semin Vasc Surg. 2012;25(1):39–48.
Stather PW, Wild JB, Sayers RD, Bown MJ, Choke E. Endovascular aortic aneurysm repair in patients with hostile neck anatomy. J Endovasc Ther. 2013;20(5):623–37.
Iwakoshi S, Ichihashi S, Higashiura W, et al. A decade of outcomes and predictors of sac enlargement after endovascular abdominal aortic aneurysm repair using zenith endografts in a Japanese population. J Vasc Interv Radiol: JVIR. 2014;25(5):694–701.
Wyss TR, Dick F, Brown LC, Greenhalgh RM. The influence of thrombus, calcification, angulation, and tortuosity of attachment sites on the time to the first graft-related complication after endovascular aneurysm repair. J Vasc Surg. 2011;54(4):965–71.
Park KH, Lim C, Lee JH, Yoo JS. Suitability of endovascular repair with current stent grafts for abdominal aortic aneurysm in Korean patients. J Korean Med Sci. 2011;26(8):1047–51.
Acknowledgments
This study was supported by Inha University Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Statement of Informed Consent
Inform consent was obtained from all individuals participants included in the study.
Rights and permissions
About this article
Cite this article
Le, T.B., Moon, M.H., Jeon, Y.S. et al. Evaluation of Aneurysm Neck Angle Change After Endovascular Aneurysm Repair Clinical Investigations. Cardiovasc Intervent Radiol 39, 668–675 (2016). https://doi.org/10.1007/s00270-015-1260-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-015-1260-7