Abstract
Purpose
We aimed to evaluate the clinical efficacy and short-term clinical outcomes of Kilt technique-based endovascular aneurysm repair (EVAR) with Seal® stent-grafts for abdominal aortic aneurysms (AAAs) with hostile neck anatomy (angle > 60°).
Materials and Methods
We retrospectively evaluated the pre-EVAR and follow-up computed tomography angiography findings of 24 patients (mean age 71 ± 11 years; age range 32–87 years; mean follow-up 50 ± 12 months) with hostile neck AAAs treated between 2010 and 2015. Serial change in aneurysmal neck angle was calculated using a standardized protocol. Relationships between clinical variables and outcomes were evaluated using univariate and multivariate Cox analyses and mixed-model regression. In addition, the Kaplan–Meier method was used to assess the cumulative rates of survival, endoleak, and reintervention.
Results
The primary technical success rate (success within 24 h after EVAR) was 100% (24/24). The survival rate was 96 ± 8% at 1 month, 6 months, 1 year, and 3 years, and 87 ± 18% at 5 years. Endoleaks occurred in three patients. Four reinterventions were performed in three patients; no surgical revisions were required. Causes of post-EVAR mortality included intracerebral hemorrhage at 14 days and rhabdomyolysis at 32 months. The most remarkable change after Kilt-based EVAR was an acute decrease in the neck angle, which was observed between the pre-EVAR and first follow-up visits (at 1 month) (P = 0.001).
Conclusion
Kilt-based EVAR with Seal® stent-grafts for AAAs with a severely angulated neck (angle > 60°) provided high technical success, low mortality, and low complication rates during short-term follow-up.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Typically, severe proximal neck angulation (> 60°) is considered to be a contraindication for endovascular aneurysm repair (EVAR) of abdominal aortic aneurysms (AAAs), regardless of the length and diameter of the aneurysmal neck, as high incidences of type Ia endoleaks and graft migration after EVAR have been observed [1,2,3,4,5]. Recently, advanced endovascular techniques have been developed to render such aneurysms more amenable to stent-graft sealing by adjusting the aneurysmal neck angulation [6, 7].
The most common prognostic factors for poor outcomes after EVAR include an angulated and short infrarenal aneurysmal neck, large neck diameter, large aneurysmal sac diameter, neck thrombus, and complex iliac artery anatomy [8,9,10]. Among these, an aneurysmal neck angulation > 60° has been reported as the most important determinant of outcomes after EVAR, with an increased rate of perioperative death and early surgical conversion [11].
The Kilt technique involves aortic-cuff stent-graft implantation prior to aortic main-body stent-graft implantation, allowing an increase in the length of the aneurysmal neck during EVAR, provided that the hostile neck length is sufficient to allow sealing of the device [6, 7]. From a technical perspective, this technique may have a superior performance in overcoming type Ia endoleaks, if an immediate migration of the main-body stent-graft and subsequent post-cuff stent-graft extension occurs during the conventional EVAR procedure. Since Jimenez et al. [6] reported the pre-cuff Kilt technique for infrarenal AAAs with angulated and dumbbell-shaped necks, no studies regarding the critical success factors of Kilt-based EVAR have been published in English. Therefore, in this retrospective study, we determined the clinical efficacy and short-term clinical outcomes of Kilt-based EVAR with Seal® stent-graft for AAAs with hostile neck anatomy (angle > 60°), which have not been previously described in the English literature.
Materials and Methods
The institutional review boards of all participating hospitals approved the study (IRB approval no.; 2016-02-001-002 at Kangdong Seong-Sim Hospital, Hallym University College of Medicine) and waived the requirement for informed consent given the study’s retrospective design.
Patients
All PACS images and electrical medical records of EVAR procedures using Seal® stent-grafts performed at nine university hospitals in Korea were reviewed, which were gathered by a clinical research coordinator at our institution. Of the 283 patients treated between 2010 and 2015, 24 patients (23 men; mean age 71 ± 11 years, median age 70 years; age range 32–87 years) with hostile AAA neck anatomy (angle > 60° and length < 10 mm or diameter > 28 mm) were treated with Kilt-based EVAR.
EVAR Procedure
Seal® aortic-cuff stent-grafts (S&G Biotech Inc., Seong-nam, Gyeonggi-do, Korea) were manufactured as a tubular aortic-cuff stent-graft, 20–34 cm in diameter and 6–10 cm in length, with a 3-cm uncovered proximal portion (Fig. 1A), or as a conical aortic-cuff stent-graft with a proximal portion in a similar configuration to that of the tubular aortic-cuff stent-graft, but with a shorter distal diameter (4 mm, which represents one standard size in the stent production size standards) (Fig. 1B).
The two configurations of the Seal® aortic-cuff stent-grafts. A Tubular aortic-cuff stent-graft, 20–34 mm in diameter and 6–10 cm in length, with a 3-cm uncovered proximal portion. The bare-metal stent has a non-interlocking diamond-shaped pattern, as well as six proximal barbs to provide better fixation to the aortic wall; the barbs have intentionally inward-facing tips (white arrows) to prevent direct injury to the aortic wall. B Conical aortic-cuff stent-graft with a proximal portion in the same configuration as that of the tubular aortic-cuff stent-graft, but with a distal diameter shorter by 4 mm (vertical suture line; black arrows)
The Seal® abdominal stent-graft includes three parts: (1) a 5-cm bifurcated main-body stent-graft (diameter 22–30 mm), which consists of a bifurcated Dacron graft over a nitinol-wire 3-stent frame, where the distal stent is attached to the ipsilateral 3-cm limb (short limb) and the contralateral 5-cm limb (long limb), and a 2-cm uncovered proximal stent that has two proximal barbs to prevent migration; and (2) two iliac-limb graft-stents. The Seal® abdominal stent-graft has a similar radial force and stent flexibility as that of other manufactures (e.g., Cook and Gore companies) and a two-step deployment mechanism, with deployment of the main-body stent-graft before the full expansion of the main body of the stent-graft [12].
After evaluating anatomical suitability for EVAR using computed tomography (CT), an initial anteroposterior abdominal aortography was performed to inspect the anatomy of the fusiform AAA, and hostile anatomy was characterized as a severely angulated proximal neck (Fig. 2A). During EVAR with the Kilt technique, the Seal® aortic-cuff stent-graft (26 mm × 8 cm) was advanced through a 260-cm stiff guide wire until the proximal bare portion of the cuff stent-graft covered the renal artery ostium (Fig. 2B). Subsequently, the bifurcated main-body stent-graft (26 mm × 5 cm) was advanced and deployed into the aortic-cuff stent-graft through a 260-cm stiff guide wire, with the proximal bare portion of the bifurcated main-body stent-graft overlapping the proximal portion of the aortic-cuff stent-graft (Fig. 2C). Next, the flared-type right and left iliac-limb graft-stents (12–22 mm × 120 mm; 12–16 mm × 120 mm, respectively) were advanced until the proximal gold markers of the ipsilateral and contralateral iliac-limb graft-stents were aligned with the gold marker at the bifurcation point of the main-body stent-graft. Completion abdominal aortography was performed to confirm the complete exclusion of the aneurysmal sac, as well as the absence of endoleaks and graft migration (Fig. 2D). If evidence of an endoleak was present on completion aortography, additional molding balloon angioplasty was performed to ensure complete modular overlap. The size criterion for the main-body stent-graft was based on pre-EVAR CTA findings, with 10–15% oversizing relative to the diameter of the aneurysmal neck. The size of the aortic-cuff stent-graft was selected based on the size of the main-body stent-graft, with 2–4-mm oversizing relative to the diameter of the main-body stent-graft to ensure complete sealing of the proximal AAA neck [6, 7].
Endovascular aneurysm repair using the Kilt technique. A Initial abdominal aortography shows a severely angulated neck (> 90°) and a fusiform aneurysmal sac of the abdominal aortic aneurysm. B The 26 mm × 8 cm Seal® aortic-cuff stent-graft is advanced over a 260-cm stiff guide wire until the proximal bare portion of the stent-graft covers the renal artery ostium. The stent-graft is subsequently deployed, and the procedure is referred to as the Kilt technique. C The 26 mm × 5 cm bifurcated main-body stent-graft is advanced into the aortic-cuff stent-graft over a 260-cm stiff guide wire, until the proximal bare portion of the bifurcated main-body stent-graft completely overlaps with the proximal uncovered portion of the cuff stent-graft. Thereafter, the flared-type right and left iliac-limb graft-stents (12–22 mm × 120 mm; 12–16 mm × 120 mm, respectively) were deployed at both common iliac arteries for the preservation of both hypogastric arteries. D Completion abdominal aortography performed after deploying the four-part stent-graft shows a completely excluded aneurysmal sac without endoleak or graft migration
Follow-Up
Follow-up visits involved clinical examination and CT angiography (CTA) and were performed at 1, 3, 6, and 12 months, and annually after discharge. The routine follow-up CTA protocol (1.5-mm thick slices, arterial phased scanning) covered the area from the suprarenal aorta to the ankle. Aneurysmal sac decrease, endoleaks, and stent-graft migration and occlusion were assessed.
Image Analysis
A standardized CT measurement protocol was used by the authors (Y.S. Jeon., Y.K. Cho and M. G. Song) to calculate the serial change in AAA neck angulation using orthogonal two-dimensional coronal CT images and three-dimensional (3D) volume-rendered images generated via post-acquisition software (iNtuition™; TeraRecon, Foster City, CA). Neck diameter and AAA neck length were measured in the axial view (1.5-mm slices) perpendicular to just below the most caudal renal artery; similarly, the proximal neck angle was measured as in Chaikof et al. [4]. The proximal neck of the AAA was defined as starting 1 mm below the distal renal artery; the distal neck of the AAA was defined as ending at a distal point, where the vessel diameter was increased by the aneurysmal sac [4]. We measured several 3D curves of the AAA neck on the embedded geometry (Virtual graft) of the 3D CT reconstructions (iNtuition™) to quantify the potential seal zone as follows; (1) neck center line length (mm); (2) neck inner curve length (mm); (3) neck outer curve length (mm); (4) actual seal zone centerline length (mm); (5) actual seal zone inner curve length (mm); and (6) actual seal zone outer curve length (mm) [13]. The potential seal zone was then modeled in the 3D reconstruction using Preview’s virtual graft feature to serve as a point of comparison to the more generally used proximal neck measurement. The virtual graft was defined to have the same lengths and diameters as the Seal® stent-graft and was placed just inferior to the distal renal artery at the start of the proximal neck. Using these end points for the modeled seal zone, the centerline length, inner curve, and outer curves were also measured [14].
Data Collection
The study outcomes were chosen based on the recommendations of the Ad Hoc Committee for Standardized Reporting Practices of the Society for Vascular Surgery/American Association for Vascular Surgery [15]. We focused on the technical success, clinical success, overall survival rate, all-cause mortality, aneurysm-related mortality, rates of freedom from endoleaks and reintervention, and device-related complications. Physical status was assessed according to the American Society of Anesthesiologists (ASA) guidelines [16]. We retrospectively evaluated the findings from the follow-up visits. Cause of death was determined based on death certificates, medical records, and autopsy reports (if available).
Definitions
Proximal AAA neck angulation was defined as the angle between the longitudinal axis of the proximal aortic neck and the longitudinal axis of the aneurysm lumen [4]. An AAA was considered to have hostile neck anatomy if at least one of the following criteria were fulfilled: angle > 60°, diameter > 28 mm, length < 10 mm, and circumferential neck thrombosis ≥ 50% [17]. Proximal neck dilatation was defined as an increase ≥ 3 mm or a change ≥ 10% in the neck diameter (inner-to-inner diameter of the stent-graft) relative to preoperative values. Stent-graft migration was defined as device displacement > 10 mm, or ≤ 10 mm if associated with symptoms requiring secondary intervention [17,18,19]. Satisfactory CT findings were defined as the absence of endoleaks, stent-graft complications (migration, kinking, stenosis, thrombosis), and aneurysm expansion.
Primary technical success was defined on an intent-to-treat basis and required the successful introduction and deployment of the device in the absence of surgical conversion, mortality, type I or III endoleaks, and graft-limb obstruction within 24 h after EVAR. If unplanned endovascular or surgical procedures were needed (< 30 days after EVAR), we assessed the secondary technical success [15]. Clinical success was also defined on an intent-to-treat basis and required the successful deployment of the endovascular device at the intended location without death as a result of aneurysm-related treatment, type I or III endoleaks, graft infection or thrombosis, aneurysm expansion (diameter increase ≥ 5 mm or volume increase ≥ 5%), aneurysm rupture, or conversion to open repair [15].
Statistical Analysis
Cox univariate and multivariate analyses were used to choose and performed to evaluate the effects of clinical variables and comorbidities on the clinical outcomes. Such variables included age; age > 70 years; hypertension; diabetes mellitus; cardiovascular disease; peripheral vascular disease; two or more comorbidities; ASA score > 4; angle, length, and diameter of the AAA neck; presence of type I or II endoleaks; and graft complications. Kaplan–Meier analysis was used to assess the cumulative rates of survival, endoleak, and reintervention. Mixed-model regression analysis was used to evaluate the clinical outcomes and longitudinal variables (angle, diameter, and length of the AAA neck). Categorical variables are reported as frequency and percentage, while continuous variables are reported as mean ± standard deviation. When the P value obtained in the Mauchly’s test of sphericity was < 0.05, the Greenhouse–Geisser correction was applied. P values < 0.05 were considered to indicate statistical significance. All statistical analyses were performed using SPSS software (version 11.0.1; SPSS Inc., Chicago, IL).
Results
Baseline characteristics of the 24 patients are provided in Table 1. The mean follow-up period was 50 ± 12 months (median 51 months; range 0.4–71 months). There was no case of circumferential neck thrombosis ≥ 50%. Data regarding hostile neck features are provided in Table 2 [20], and pre-EVAR neck parameter data are provided in Table 3. Six cases had both neck angulation > 90° and neck length < 10 mm.
The mean size of the aortic-cuff stent-graft and aortic main-body stent-graft was 27 ± 4 mm (median 26 mm; range 22–36 mm) and 28 ± 2 mm (median 28 mm; range 32–24 mm), respectively. Tubular Seal® aortic-cuff stent-grafts were used in 14 patients (mean diameter 25 ± 3 mm; median 26 mm; range 22–34 mm), and conical cuff stent-grafts were used in ten patients (mean diameter 29 ± 3 mm; median 27 mm; range 26–36 mm).
On completion angiography, no patient showed endoleak or graft migration. Femoral cut-down and percutaneous access were employed in 6 and 18 patients, respectively. The closure for percutaneous access was performed using the Perclose® device (Abbott, Abbott Park, IL). The duration of hospitalization was 6 ± 4 days (range 4–50 days) and 4 ± 3 days (range 2–10 days) for primary EVAR and reintervention, respectively.
Primary Technical Success
The primary technical success rate (i.e., within 24 h after EVAR) was 100% (24/24), and the secondary technical success rate (i.e., later than 24 h but within 30 days after EVAR) was 96% (23/24). The one case of secondary technical failure was due to a fatal intracerebral hemorrhage (ICH), detected one day after the procedure. The suspected etiology was prolonged anticoagulation, as the patient was treated with thoracic endovascular aneurysmal repair (TEVAR) using a Seal® thoracic stent-graft (30 mm × 10 cm) for a coincident mid-thoracic aorta aneurysm immediately after EVAR.
Primary Clinical Outcomes
Primary short-term clinical outcomes are provided in Table 4. Two patients (8%) were lost to follow-up because of death due to ICH within 14 days after EVAR and rhabdomyolysis at 32 months postoperatively; however, neither of these deaths counted toward aneurysm-related mortality.
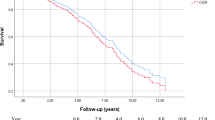
The survival rate was 96 ± 8% at 1 month, 6 months, 1 year, and 3 years, and 87 ± 18% at 5 years after EVAR (Fig. 3). The angle, length, and diameter of the neck on pre-EVAR CTA were not significantly associated with the incidence of endoleaks, graft migration rate, or clinical success rate (P values 0.18, 0.08, and 0.36, respectively).
Secondary Clinical Outcomes
Endoleaks
Endoleaks were detected in three patients (17%; 4 endoleaks) (Table 4). There were no primary type Ia endoleaks (< 30 days). Two secondary type Ib endoleaks were detected in one patient, with repeated endoleaks (at 6 days and 27 months after EVAR) due to a graft migration of the iliac-limb stent-graft, which resolved after two sessions of iliac graft-stent extension. Two type II endoleaks occurred at 10 days and 207 days in two patients due to collaterals from the lumbar arteries. The latter case was treated with coil embolization; the former case resolved spontaneously.
The rate of freedom from endoleaks was 96 ± 8% at 1 and 6 months, and 87 ± 13% at 1, 3 and, 5 years after EVAR (Fig. 4). The incidence of endoleak was significantly associated with aneurysm expansion and clinical failure (P = 0.02).
Reintervention
Four catheter-based reinterventions were performed in three patients (12%), including two repeat reinterventions in one patient. The causes of reintervention included type I and type II endoleaks (two type Ib endoleaks in one patient; one type II in one patient), and tight stenosis of the iliac artery, located distally to the unilateral iliac-limb graft-stent (one event in one patient). Reinterventions included two iliac-limb graft-stent extensions, one coil embolization, and one balloon angioplasty; all were successful. The rate of freedom from reintervention was 96 ± 8% at 1 month, 91 ± 11% at 6 months, and 87 ± 14% at 1, 3, and 5 years after EVAR (Fig. 5). Type I endoleak was significantly associated with reintervention (P = 0.003), whereas the neck angle (> 60°), neck diameter (≥ 28 mm), and neck length (< 10 mm) were not (all associations, P = 0.99).
Change in Proximal Neck Parameters
The changes in the neck and aneurysmal sac diameter after EVAR are provided in Table 3. The angle, length, and diameter of the AAA neck on pre-EVAR CTA were not significantly associated with technical or clinical success (P values 0.08, and 0.22, respectively).
Proximal neck dilatation (≥ 3 mm) was observed in six patients at the 1-year follow-up and in three patients at the 3-year follow-up. Among the latter three patients, two patients continued to show neck dilatation during the 1-year to 3-year follow-up period, and one patient showed late neck dilatation at 1 year after EVAR. The length of the aneurysmal neck became equal on both sides in 20 patients by the 1-year follow-up, whereas four patients did not show a significant change in neck length during the same period. Neck dilatation (≥ 3 mm) after EVAR was not significantly associated with endoleak occurrence or clinical failure (P values 0.5, and 0.37, respectively).
The neck angle changed substantially in 20 patients by the 1-year follow-up, most prominently between the pre-EVAR and first follow-up visits (mean interval 17 ± 15 days; median 18 days; range 0–45 days) (P = 0.001) (Fig. 6). Among them, progressive neck angle decrease was observed in three patients at 3 years after EVAR. However, there was no significant association between the change in neck diameter and that in neck length (P = 0.94). Finally, the stent-graft oversizing ratio was not significantly associated with the change in neck angle after EVAR (P = 0.09).
Kaplan–Meier curve describing the aneurysmal neck angle change in 24 patients who underwent Kilt-based endovascular aneurysm repair for an abdominal aortic aneurysm with a severely angulated neck at the first (mean ± SD; 66 ± 15 days), second (mean ± SD; 56 ± 25 day), and third follow-up with computed tomography (mean ± SD; 53 ± 29 days). SD standard deviation
Discussion
Within the field of EVAR for hostile neck AAA, the Kilt technique is more suitable and easier to perform than EVAR with a fenestrated and branched stent-graft, as the Kilt technique can effectively extend the length of the main-body fixation zone in the hostile neck and strengthen the support of the main-body stent-graft, resulting in enhanced stent-graft fixation and remodeling of the severely angulated aneurysmal neck [21,22,23]. According to the authors’ personal experience with Kilt-based EVAR, implantation of the aortic-cuff stent-graft into a severely angulated aneurysmal neck can be successfully achieved by providing support with a long vascular sheath and a stiffer guide wire. In the present series, there was no significant primary technical failure due to acute graft migration of the aortic-cuff or main-body stent-grafts during the procedure and perioperative period, which represents a very important finding. Our findings indicate that Kilt-based EVAR provides an excellent primary technical success rate (100%) and lower complication rate compared to that reported in previous studies [1,2,3].
The Seal® abdominal stent-graft has been available for EVAR in Korea since 2007, with good mid-term outcomes. Of the devices investigated to date, the Excluder and Zenith stents are reportedly associated with increased risk for short-term type Ia endoleaks and graft migration after EVAR for aneurysms with a severe neck angulation, while the Talent device is reportedly associated with increased risk for long-term type Ia endoleaks and secondary interventions [12].
It is meaningful that no cases of type Ia endoleak were observed in the present case series, despite a difficulty in the direct comparison between the Seal® device and other stents. This may be indicative of the excellent proximal fixation ability of Kilt-based EVAR via an adequate elongation and effectively decreased neck angulation of the proximal sealing zone after installation with Seal® aortic-cuff stent-graft, as well as enhanced suprarenal fixation power via the overlapping of two proximal fixations with the Seal® aortic-cuff stent-graft and aortic main-body stent-graft, preventing the acute migration of the Seal® aortic main-body stent-graft.
Although aortic dilatation (> 3 mm) was observed in six patients at one year and in three patients at 3 years after EVAR, there was no single incidence of a late type 1a endoleak. The lack of a late type 1a endoleak in the present study is likely due to the overlapping of the two radially strengthened aortic-cuffs and main-body stent-graft at the proximal neck. Stent-graft stiffness is recognized as another major risk factor for early type Ia endoleaks and is caused by small gaps forming between the severely angulated neck and the proximal stent-graft [2]. However, chronic aneurysmal neck dilatation is recognized as a major risk factor for late type Ia endoleaks after EVAR, which has been reported in 4.1 and 0.7% of patients with and without aortic dilatation, respectively [19]. In the present study, aortic dilatation (≥ 3 mm) was observed in nine patients altogether (six patients at 1 year and three patients at 3 years, which is similar to that in previous reports [19, 24], but was not associated with late type Ia endoleak. These findings may indicate that overlapping of the aortic-cuff and main-body stent-grafts at the proximal aneurysmal neck can be more effective in preventing type Ia endoleaks, and that overlapping of two radially strengthened stent-grafts do not have negative effects on aortic dilatation. Given that the present series had a high prevalence of more severe neck angulation on pre-EVAR CTA (50%; neck angle ≥ 90°), the primary technical success rate was excellent, which may be related to an increase in neck length via the pre-implanted aortic-cuff stent-graft and the enhanced anchoring effect of the overlapping stent-grafts, preventing acute distal migration during the procedure. Of the devices investigated for a hostile neck angle (> 60°), the Aorfix and Endurant endografts have reportedly similar technical success and survival rate in hostile and non-hostile neck groups during the short-term period. However, Aorfix endografts were reported to have a risk of graft migration at two years of follow-up [relative risk 4.33 (P = 0.002)] because of a significant neck dilatation (> 10%) in patients with oversized stent-grafts. In addition, Endurant endografts were reported to have a higher rate of type I endoleaks within 30 days in patients with hostile infrarenal neck angle (> 60°, P < 0.01) [25, 26].
The incidence of short-term graft complications was low in the present study. Right iliac-limb graft-stent migration recurred twice in one patient, which may have been related to a repeated severe kinking of the iliac-limb graft-stent caused by the complex iliac anatomy, corrected by repeat additional stent-graft installation. However, there were no other graft complications, such as graft occlusion or infection.
In the present study, the most remarkable aortic remodeling (processing to complete aortic anatomy normalization) observed after EVAR was a change in the aneurysmal neck angle, which occurred at the time of the 30-day follow-up. Aneurysmal neck angle change was more pronounced in the group with a more severe hostile angle (≥ 90°), suggesting that the Kilt technique aids in preventing acute migration and promoting early stability of the stent-graft by means of a remarkable decrease in neck angle.
The present study has several limitations worth noting (in addition to those described above). First, we retrospectively analyzed the outcomes of EVAR using one technique (Kilt technique) and one device, and did not directly compare the outcomes of several techniques (e.g., fenestrated stent-graft and Chimney techniques) and devices of other manufactures. Second, the follow-up intervals and CTA examination period varied within each center, and two patients were lost to follow-up, which may have influenced the results. Third, we could not exclude the potential effect of inter-observer variation in the CTA-based measurement of the aneurysmal neck parameters. Fourth, the results regarding technical/clinical success may reflect selection biases (both in terms of patients and centers) and precluded a direct comparison to observations from other studies. Fifth, studies with long-term follow-up are warranted to fully gauge the effects of device design and manufacturing, as well as a potential negative effect of double-layered metal stents in the renal arteries. Sixth, we were unable to determine the risk of type IV and V endoleaks due to the retrospective nature of this study.
Conclusion
Kilt-based EVAR with Seal® stent-grafts provided a high technical success rate and low mortality and complication rates during short-term follow-up for AAAs with a severely hostile neck. This technique appears to contribute to enhanced technical and clinical success rates even when the neck angle is more hostile (> 90°). Future studies with long-term follow-up should evaluate the durability and risk of late complications associated with this technique and device.
References
AbuRahma AF, Campbell J, Stone PA, et al. The correlation of aortic neck length to early and late outcomes in endovascular aneurysm repair patients. J Vasc Surg. 2009;50:738–48.
Hobo R, Kievit J, Leurs LJ, Buth J, EUROSTAR Collaborators. Influence of severe infrarenal aortic neck angulation on complications at the proximal neck following endovascular AAA repair: a EUROSTAR study. J Endovasc Ther. 2007;14:1–11.
Sternbergh WC 3rd, Carter G, York JW, Yoselevitz M, Money SR. Aortic neck angulation predicts adverse outcome with endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2002;35:482–6.
Chaikof EL, Fillinger MF, Matsumura JS, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1061–6.
Chisci E, Kristmundsson T, de Donato G, et al. The AAA with a challenging neck: outcome of open versus endovascular repair with standard and fenestrated stent-grafts. J Endovasc Ther. 2009;16:137–46.
Jimenez JC, Quinones-Baldrich WJ. Technical modifications for endovascular infrarenal AAA repair for the angulated and dumbbell-shaped neck: the precuff Kilt technique. Ann Vasc Surg. 2011;25:423–30.
Minion DJ, Yancey A, Patterson DE, Saha S, Endean ED. The endowedge and kilt techniques to achieve additional juxtarenal seal during deployment of the Gore Excluder endoprosthesis. Ann Vasc Surg. 2006;20:472–7.
Albertini JN, Perdikides T, Soong CV, et al. Endovascular repair of abdominal aortic aneurysms in patients with severe angulation of the proximal neck using a flexible stent-graft: European multicenter experience. J Cardiovasc Surg (Torino). 2006;47:245–50.
Peppelenbosch N, Buth J, Harris PL, et al. Diameter of abdominal aortic aneurysm and outcome of endovascular aneurysm repair: does size matter? A report from EUROSTAR. J Vasc Surg. 2004;39:288–97.
Greenberg R, Fairman R, Srivastava S, Criado F, Green R. Endovascular grafting in patients with short proximal necks: an analysis of short-term results. Cardiovasc Surg. 2000;8:350–4.
Boult M, Maddern G, Barnes M, Fitridge R. Factors affecting survival after endovascular aneurysm repair: results from a population based audit. Eur J Vasc Endovasc Surg. 2007;34:156–62.
Kim JH, Cho YK, Seo TS, et al. Clinical outcomes for endovascular repair of abdominal aortic aneurysm with the Seal stent graft. J Vasc Surg. 2016;64:1270–7.
Jordan WD, Ouriel K, Mehta M, et al. Outcome-based anatomic criteria for defining the hostile aortic neck. J Vasc Surg. 2015;61:1383–90.
Welborn MB, McDaniel HB, Johnson RC, et al. Clinical outcome of an extended proximal seal zone with the AFX endovascular aortic aneurysm system. J Vasc Surg. 2014;60:876–84.
Chaikof EL, Blankensteijn JD, Harris PL, et al. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–60.
Aronson WL, McAuliffe MS, Miller K. Variability in the American Society of Anesthesiologists Physical Status Classification Scale. AANA J. 2003;71:265–76.
Choke E, Munneke G, Morgan R, et al. Outcomes of endovascular abdominal aortic aneurysm repair in patients with hostile neck anatomy. Cardiovasc Intervent Radiol. 2006;29:975–80.
Leurs LJ, Stultiëns G, Kievit J. Buth J; EUROSTAR Collaborators. Adverse events at the aneurysmal neck identified at follow-up after endovascular abdominal aortic aneurysm repair: how do they correlate? Vascular. 2005;13:261–7.
Dillavou ED, Muluk S, Makaroun MS. Is neck dilatation after endovascular aneurysm repair graft dependent? Results of 4 US Phase II trials. Vasc Endovascular Surg. 2005;39:47–54.
Hobo R, Sybrandy JE, Harris PL, Buth J, EUROSTAR Collaborators. Endovascular repair of abdominal aortic aneurysms with concomitant common iliac artery aneurysm: outcome analysis of the EUROSTAR experience. J Endovasc Ther. 2008;15:12–22.
Greenberg RK, Sternbergh WC 3rd, Makaroun M, et al. Intermediate results of a United States multicenter trial of fenestrated endograft repair for juxtarenal abdominal aortic aneurysms. J Vasc Surg. 2009;50:730–7.
Ghouri MA, Dougherty KG, Krajcer Z. Technical tips for endovascular treatment of abdominal aortic aneurysms with challenging infrarenal neck anatomy using the excluder endoprosthesis. J Endovasc Ther. 2010;17:705–11.
Kolvenbach R, Pinter L, Cagiannos C, Veith FJ. Remodeling of the aortic neck with a balloon-expandable stent graft in patients with complicated neck morphology. Vascular. 2008;16:183–8.
Sampaio SM, Panneton JM, Mozes G, et al. Aortic neck dilation after endovascular abdominal aortic aneurysm repair: should oversizing be blamed? Ann Vasc Surg. 2006;20:338–45.
Malas MB, Jordan WD, Cooper MA, et al. Performance of the Aorfix endograft in severely angulated proximal necks in the PYTHAGORAS United States clinical trial. J Vasc Surg. 2015;62:1108–17.
Broos PP, Stokmans RA, van Sterkenburg SM, et al. Performance of the Endurant stent graft in challenging anatomy. J Vasc Surg. 2015;62:312–8.
Acknowledgements
This research was supported by the Central Medical Service Research Fund (Central Medical Service Inc., Seoul, Korea).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest to declare.
Ethical Standards
The study design was approved by our institutional review, and the requirement for informed consent was waived due to the study’s retrospective design.
Rights and permissions
About this article
Cite this article
Jeon, Y.S., Cho, Y.K., Song, M.G. et al. Clinical Outcomes of Endovascular Aneurysm Repair with the Kilt Technique for Abdominal Aortic Aneurysms with Hostile Aneurysm Neck Anatomy: A Korean Multicenter Retrospective Study. Cardiovasc Intervent Radiol 41, 554–563 (2018). https://doi.org/10.1007/s00270-017-1867-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00270-017-1867-y