Abstract
Background
Intraoperative cholangiography (IOC), even though is an important tool in biliary surgery, it is still a matter of debate when used as a routine procedure, this supported in the surgical and legal safety for the patient and the surgeon. We do not have knowledge of the real expositional risk of the surgeon to ionizing radiation (IR) during the cholangiography procedure, because many surgeons do not use protection and dosimeters, so we cannot determine occupational radiation exposure.
Study design
A prospective cohort study was conducted to assess the radiation exposure of a group of surgeons performing laparoscopic cholecystectomy, regardless of the type of surgery (elective or urgent). A descriptive, bivariate analysis was made, with a linear simulation model for prediction. We evaluate the frequency of use of protection-established devices, number of images per surgery, and frequency of IOC. The radiation received was measured by dosimeters at different distances.
Results
A total of 597 IOC were made in the evaluated period. Mean number of IOC per surgeon was five monthly, with an average of two images per surgery. 60% of surgeons did not use protection devices during IOC. The surgeon radiation received was 0.147 millisieverts (mSv) at 1 m, 0.039 mSv at 1.6 m, and 0.007 mSv at 2.5 m.
Conclusions
The volume, quality, and sufficiency of protection, coupled with the distance to the X-ray generator, are the major determinants to define the exposure to IR. We can predict the annual ionizing radiation according to the volume of the accomplished procedures. Although exposure doses are really low and make this a safe procedure, continuous exposure can lead to serious illnesses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since Mirizzi described intraoperative cholangiography (IOC) in 1931 [1, 2], it has contributed to the effectiveness and safety of open or minimally invasive cholecystectomy [3]. Although it has been useful in clinical decision making, IOC has not been routinely incorporated into this surgery in most institutions worldwide arguing the following 4 reasons: the low probability of choledocholithiasis in the general population (<10%) [4]; logistical requirements for its implementation with prolonged surgical times [5]; its associated learning curve; the possible bias associated with interpreting images, and radiation exposure to which the surgeons and operation room personnel are exposed; and as a result, in harmful cumulative ionizing radiation (IR) [6]. On the other hand, IOC is useful in decision making, helps addressing complex cases, and identifies overlooked injuries, and it is a great tool to provide further explanations in a medical–legal context [7]. These pros have led to consider it as a tool for routine use in cholecystectomy because it helps surgeons and institutions to legally prove the reasons for specific intraoperative decisions [8] and improves the surgeon’s skills in this type of surgeries [8]. Whatever the indication is, one of the most important concerns regarding its use is exposure to IR during the procedure. This concern led The International Commission on Radiological Protection (ICRP) in 1928, to establish the protection of workers from exposure to IR in the practice of medicine as its first recommendation [9, 10].
Few studies have assessed the effect of exposure to IR in the surgical area; some have determined its effect on patients, showing that the levels of IR exposure are safe and do not represent a contraindication to its implementation. Nowadays, increasing procedures where IR is used, studies have shown that radiological protection measures are often ignored or used incorrectly [11, 12]. The purpose of this research was to establish the levels of exposure to radiation during IOC and evaluating the estimated cumulative radiation in surgeons performing gallbladder and bile duct surgeries.
Method
Study design
A prospective cohort study was conducted to assess the radiation exposure of a group of surgeons performing laparoscopic cholecystectomy (LC) and routine IOC (an institutional policy of the department of general surgery), regardless of the type of surgery (elective or urgent) between May 2016 and the date of the obtainment of the sample size. The surgeon in charge performed all IOCs through the selective cannulation of the cystic duct. A Perifix®Standard Catheter (B. Braun) was used, instilling 10 ml of nonionic iodinated contrast (Optiray® 320 × 20 ml) diluted 50%. Portable digital X-ray equipment (General Electric Optima®) was used, and this portable device refers to the digital real-time radiography taking and processing equipment that generates less exposure to IR compared to the continuous pulse of the C-arc of fluoroscopy producing a lower and more predictable exposure on the right upper abdominal quadrant, with a kilovoltage based on the thickness of the patient (between 70 and 80 kVp) and an exposure time between 40 and 50 mAs. The dispersed IR emitted during IOC was measured (instantaneous dose) by several personal dosimeters (MYDOSE mini PDM-227 electronic personal dosimeter) [13] at different distances from the portable X-ray equipment (1 m, 1.6 m, and 2.5 m) according to the position of the surgeon and the length of the catheter. To interpret our results in simulation analyses, three a priori categories were determined based on the volume of IOCs performed by the surgeon: low volume, medium volume, and high volume. The other independent predictors incorporated into the model were interpreted as stochastic variables to determine the behavior according to the volume of procedures. We collected data on the frequency of IOC, the use of established protection devices, the number of surgery images, and the number of gallbladder and bile duct surgeries performed by each surgeon at our institution.
Statistical analysis
Measurements of frequency, central tendency, and dispersion were calculated for continuous variables. Absolute values and proportions were used for categorical data. The assumption of normality in the variables of interest was evaluated using the nonparametric Shapiro–Wilk test. Values of p < 0.05 were considered as significant for all hypothesis tests. For each estimator, 95% confidence intervals were calculated. The data were analyzed using Stata 15.0. The following deterministic generalized linear model was constructed to relate the stochastic structure of the data as a function of the independent predictors (i.e., the number of images per IOC [X1], the monthly average of images taken by the surgeon [X2], the dispersion of IR based on the distance between the surgeon and the X-ray unit [X3], and the proportion of surgeons using protective devices [X4]) related to the IR exposure dose (dependent variable):
Using bootstrapping, we obtained estimators for the IR exposure doses during IOC that were applied to the generalized linear model. The clinical research ethics committee of our institution approved the research protocol, considering the procedures to have minimal risk. The researchers have no conflicts of interest to declare.
Results
Between May 2016 and April 2017, 597 LCs were performed under routine IOCs, establishing an average of five procedures per surgeon per month, with an average of two images per surgery. Sixty percent of these procedures were performed without using protective devices during cholangiography. The equivalent dose received by the surgeon was measured by three dosimeters with exposure results of 0.147, 0.039, and 0.007 mSv at 1.0, 1.6, and 2.5 m from the emitting equipment, respectively. Most surgeons performing these procedures were men (79.6%) with an average age of 50 years and an average professional experience of 20 years (Table 1).
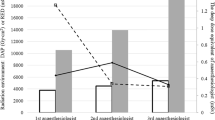
The results of the simulation according to the variability in the selected predictors are shown by a graphical model of additive linear relationships, where the last node represents the estimated amount of cumulative radiation with a time horizon of 12 months (Fig. 1). The cumulative dose of IR was calculated, considering the number of images per IOC, the average procedures per surgeon per year, the degree of dispersion according to the distance, and the percentage of protection to calculate exposure expressed in mSv per year. Surgeons were classified into three categories, determined by the volume of IOC procedures: high volume (10 procedures per month), medium volume (five procedures per month), and low volume (one procedure per month). The calculated IR exposure values were between 0.033 and 1.41 mSv per year for the low-volume group, between 0.9 and 7 mSv per year for the medium-volume group, and between 0.3 and 14.1 mSv per year for the high-volume group. Volume, quality, sufficiency of protection, and distance to the X-ray generator were the major determinants of the effect of IR exposure; however, protection was the most important variable.
Discussion
This study objectively measured the levels of IR exposure generated by IOC during LC to surgeons and their protective actions against the exposure itself. The use of IOC is controversial, although it has unquestionable medical and legal utility in situations where positive findings are discovered. It is frequently performed in some general hospitals, but few studies have evaluated the IR exposure of the surgical group and they do not take into consideration the cumulative dose which is directly related to the frequency of the procedure and has relevant clinical importance [14, 15].
IR generates free radicals and can produce injuries that directly or indirectly alter DNA, causing mutations, cell apoptosis, or necrosis. IR is part of our lives, and we are constantly exposed to it. As a starting point, we should consider an average environmental exposure of 2.4 mSv/year; this rate is higher in areas such as Brazil, India, and Iran, where it can reach 5–15 mSv/year. The ICRP recommends a maximum occupational radiation dosage of 20 mSv/year for workers and 100 mSv over five consecutive years [15]. The health effects of IR are classified into two categories based on the time when they appear. Short-term effects (from weeks to 1 year, also called deterministic) are those that are visible, documented, and confirmed in a relatively short time. These include tissue reactions such as skin erythema, hair loss, cataracts (confirming that the eyes are the most radio-sensitive tissues, needing a cumulative yearly dosage of 500 mSv to cause cataracts), temporary infertility with a dose of 100 mSv in a single exposure, and definitely sterility with cumulative doses of 400 mSv per year, circulatory disease, and others occurring among workers who perform interventional procedures. Long-term effects can take from years to decades to manifest (these so-called stochastic effects are only estimated) and include cancer and genetic defects [16].
Several studies have suggested that the risk of developing cancer increases by 5% for each mSv received, with a mean latency of 10–20 years after exposure. These calculations were estimated based on studies of Nagasaki and Hiroshima survivors [15]. Some preexisting autoimmune and connective tissue diseases predispose patients to develop severe random skin lesions after exposure to IR [12, 17]. However, no studies exist to compare our results in a surgical scenario using cholangiography to determine potential risk levels. Exposure to IR can be limited by considering certain generalities about exposure beforehand including the following [15]:
Radiation is inversely proportional to the square distance between the X-ray tube and the person.
Both the type of equipment and the technique used have an influence; when oblique X-ray projections are used, IR over the medical team is increased.
The use of pulsed fluoroscopy decreases exposure. However, taking individual images causes even less exposure from portable digital X-ray equipment.
The X-ray beam must be collimated to limit the size of the radiation field to the area of interest. This procedure reduces the amount of irradiated tissue of the patient, decreases the dispersion of the IR, and results in better image quality. Dispersion increases linearly with the area of the radiation field. Field light operation must be verified before each procedure.
When starting a case, the image receiver should be placed over the area of interest, with the collimators almost closed. The collimators should be opened gradually until the desired field of vision is obtained.
The number of images should be limited to those necessary for diagnosis or to document the results.
Specific recommendations for health workers [18]:
Use a lead or equivalent material apron, with a minimum of 2 mmPb.
Use a lead or equivalent material collar, with a minimum of 2 mmPb.
Use goggles with lead or equivalent material glass. Eye protectors can also be attached to workers’ goggles, and full-face shields also function as splash guards. Lead goggles (minimum of 2 mmPb) must have side-shields to reduce lateral radiation.
Receive training and adhere to the radiological protection culture.
Include the use of lead devices on the patient safety checklist.
Use the personal dosimeter inside protective garments.
Although the above recommendations are mandatory, clinical professionals using X-rays outside the radiology department lack or have inadequate training [18]. Exposure to IR during IOC, even in a low-volume scenario, is mathematically interpreted as low risk; however, not using IR protection is neither safe nor adequate and cannot be considered an acceptable surgical practice. The benefits of many procedures using IR are well established, having been accepted by the medical community and society at large. When a procedure involving IR is medically justified, benefits are identifiable and often measurable. On the other hand, the risk of adverse consequences is often difficult to estimate and quantify.
All radiological studies must be based on the principles of justification, protection, and limited dose (the ALARA principle: as low as reasonably achievable). For exposed patients and health workers, exposure duration, distance, and shielding are also key aspects to be analyzed. Our study analyzed the most conservative scenario, assuming the number of images taken during each IOC was one per surgery (Fig. 1); however, each professional could perform their own simulations based on the data provided by our model. If a surgeon performs one LC per month and takes two images per cholangiography, 1 m away from the equipment, and uses protection measures 90% of the time, then their cumulative exposure level will be 1.4112 mSv per year. The strength of the present study lies in the method that objectively measures the IR to which the surgeons are exposed to implement a standard procedure that was the product of an institutional culture facilitating its execution and reducing unnecessary imaging that might otherwise increase the cumulative doses of IR. Despite that, non-protection is a daily practice in some institutions this study is part of education strategies that seek to raise awareness of the dangers of IR and educating about adequate protection and predicting individual IR level is a very important goal for every surgeon.
Future research should consider these recommendations and investigate the specific health effects of surgeons exposed to low-, medium-, or high-volume practices. Such studies should aim to determine not only the exposure level but also the adequacy of the protective equipment [18]. Our study has limitations worth considering among them, the variability in the emission of IR across the different machines available on the market. Moreover, we did not evaluate the effect of free-of-exposure or interprocedural time, which is a factor that helps to decrease the risk of exposure.
Conclusions
The radiation dose of IOC is low (% of the allowed limit), making it a safe practice. However, control and protective measures should be increased to reduce or adjust the radiological risk for both patients and medical teams, especially in situations of high-volume surgery and proximity to the X-ray machine. In this way, a recommendation should be made to encourage routine IOC if these conditions are met. It is our institutional policy to recommend the protection against IR, and we present an easy application and interpretation tool enabling surgeons to estimate the cumulative levels of exposure to IR based on their own standards of practice. Training schools must include training programs in radiological protection that should address theoretical knowledge first and then the application of this successful clinical practice. Performing either selective or routine IOC brings safety for the patient, the doctor, and the institution. Hence, the importance of considering our experience.
Abbreviations
- IOC:
-
Intraoperative cholangiography
- IR:
-
Ionizing radiation
- LC:
-
Laparoscopic cholecystectomy
- ICRP:
-
International Commission on Radiological Protection
References
Mirrizi P (1937) Operative cholangiography. Surg Gynecol Obstet 65:702–710
Mirizzi PL, Quiroza LC (1931) La exploracion de las vias biliares principales en el curso de la operacion. Proc Third Argent Cong Surg 1:694
Metcalfe MS, Ong T, Bruening MH, Iswariah H, Wemyss-Holden SA, Maddern GJ (2004) Is laparoscopic intraoperative cholangiogram a matter of routine? Am J Surg 187(4):475–481
Maple JT, Ben-Menachem T, Anderson MA, Appalaneni V, Banerjee S, Cash BD et al (2010) The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc 71(1):1–9
Ding G-Q, Cai W, Qin M-F (2015) Is intraoperative cholangiography necessary during laparoscopic cholecystectomy for cholelithiasis? World J Gastroenterol 21(7):2147–2151
Hughes MC, Jarrin GT, Bucina BA (2015) Increasing the safety of cholecystectomy in the rural setting with mandatory intraoperative cholangiogram. J Am Coll Surg 221(4):S71
Strasberg SM (2005) Biliary injury in laparoscopic surgery: part 1. Processes used in determination of standard of care in misidentification injuries. J Am Coll Surg 201(4):598–603
Strasberg SM (2005) Biliary injury in laparoscopic surgery: part 2. Changing the culture of cholecystectomy. J Am Coll Surg 201(4):604–611
Radiological IC on, Protection I (2007) The 2007 recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 37(2–4):1–332
Van Dijk AH, de Reuver PR, Besselink MG, van Laarhoven KJ, Harrison EM, Wigmore SJ et al (2017) Assessment of available evidence in the management of gallbladder and bile duct stones: a systematic review of international guidelines. HPB 19(4):297–309
Buddingh KT, Weersma RK, Savenije RAJ, van Dam GM, Nieuwenhuijs VB (2011) Lower rate of major bile duct injury and increased intraoperative management of common bile duct stones after implementation of routine intraoperative cholangiography. J Am Coll Surg 213(2):267–274
Sirinek KR, Schwesinger WH (2015) Has intraoperative cholangiography during laparoscopic cholecystectomy become obsolete in the era of preoperative endoscopic retrograde and magnetic resonance cholangiopancreatography? J Am Coll Surg 220(4):522–528
Southern Scientific. Electronic personal dosimeter, MYDOSE mini PDM-227—Southern Scientific [Internet]. https://www.southernscientific.co.uk/products-by-manufacturer/aloka/mydose-mini-pdm-227?source=1206. Accessed 22 Jan 2019
Rehani MM, Ciraj-Bjelac O, Vano E, Miller DL, Walsh S, Giordano BD et al (2010) ICRP Publication 117. Radiological protection in fluoroscopically guided procedures performed outside the imaging department. Ann ICRP 40(6):1–102
Karthikesalingam A, Markar SR, Weerakkody R, Walsh SR, Carroll N, Praseedom RK (2009) Radiation exposure during laparoscopic cholecystectomy with routine intraoperative cholangiography. Surg Endosc 23(8):1845–1848
UNCEARS: United Nations of Scientific Committee on the Effects of Atomic Radiation. UNSCEAR 2008 report—vol I: sources [Internet]. http://www.unscear.org/unscear/publications/2008_1.html. Accessed 3 March 2019
Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ (2010) Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology 254(2):326–341
Miller DL, Vano E, Bartal G, Balter S, Dixon R, Padovani R et al (2010) Occupational radiation protection in interventional radiology: a joint guideline of the Cardiovascular and Interventional Radiology Society of Europe and the Society of Interventional Radiology. J Vasc Interv Radiol 21(5):607–615
Funding
We have not received financial support for the preparation of this study. The research and ethics committee of the institution reviewed and approved the development of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barrios, A., Vega, N., Martínez, J. et al. Cumulative Exposure to Ionizing Radiation Among Surgeons During Intraoperative Cholangiography. World J Surg 44, 63–68 (2020). https://doi.org/10.1007/s00268-019-05170-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-019-05170-0