Abstract
Background
The association between iodine levels and the risk of papillary thyroid cancer (PTC) has been suggested, but not definitively established. This study is to compare the iodine status of a group of patients with PTC (with and without BRAF V600E) with that of a healthy population cohort.
Methods
A cohort of patients scheduled for thyroidectomy was enrolled, along with a community-based health-screening cohort with no known history of thyroid disease. Median urinary iodine (UI) levels, creatinine-adjusted median UI levels, and food frequency questionnaire (FFQ) scores (mean ± SD) were compared. In a subgroup analysis, these values were compared between BRAF V600E-positive and BRAF V600E-negative patients in the PTC group.
Results
The PTC group consisted of 210 patients, and the control group consisted of 90 healthy individuals. Among the 191 PTC patients whose BRAF V600E mutational status was reported, 169 (88.5%) were revealed positive for the mutation. The median UI levels were significantly higher in the PTC group (786.0 μg/l) than the control group (112.0 μg/l; p < 0.001), as was the case with creatinine-adjusted median UI levels (884.6 μg/g creatinine versus 182.0 μg/g creatinine; p < 0.001) and FFQ scores (66.2 ± 17.5, range 13–114 versus 54.6 ± 21.5, range 16–134; p < 0.001). No significant differences were seen in the subgroup analysis between BRAF V600E-positive and BRAF V600E-negative patients.
Conclusions
Our results indicate that iodine status differs significantly between patients with PTC and healthy controls, suggesting that iodine may be involved in the occurrence of PTC, although the association between iodine levels and BRAF mutational status did not reach statistical significance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of papillary thyroid carcinoma (PTC) shows one of the highest increase rates of all cancers [1]. Many researchers attribute this rapid increase to the advancement of diagnostic technology, asserting that increasing incidence without increasing mortality is indicative of over-diagnosis [2]. However, as new evidence reveals that PTC increase could be associated with higher mortality, the contribution of external factors to the incidence of PTC should be addressed [1, 3]. Many putative carcinogenic factors may contribute to the PTC occurrence, including irradiation at a young age [4,5,6,7,8], exposure to iodine and nitrate, environmental pollutants, and obesity [1, 9,10,11,12,13].
The effects of the level of iodine intake on the prevalence of thyroid disease have been reported. Iodine deficiency is associated with the prevalence of endemic goiter [14]. By contrast, excessive intake of several high-iodine food contents demonstrates the association with increased incidence of PTC [10, 12, 15, 16]. The results of a study carried out in an iodine-rich region of China demonstrated a higher prevalence of the BRAF V600E mutation in areas with high iodine concentrations in the drinking water than in areas with normal iodine concentration in water [15].
The South Korean diet is rich in iodine, [17] as seaweed and other seafood are frequently consumed. The incidence of thyroid cancer is very high in South Korea. Moreover, the prevalence of the oncogenic BRAF V600E mutation is 79% in South Korea, as opposed to the 49% worldwide [18]. However, the correlation between PTC/BRAF V600E mutation and iodine status in South Korea has not yet been determined. Therefore, we compared the iodine status in patients with PTC and in healthy controls, and also between patients with PTC with and without the BRAF V600E mutation in this study.
Materials and methods
Study population
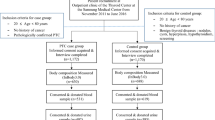
Approval of the institutional review board (B-1602-336-307) was obtained, and patients with PTC who were scheduled for thyroidectomy between March and December 2015 were consecutively recruited to participate in a prospectively collected biobank at our institution situated in Seongnam-si, Gyeonggi-do, South Korea. Patients underwent surgery, and diagnosis was confirmed by histopathological examination of excised tissue specimens. Patients with a written informed consent on biobank collection and whose pathologic report indicated PTC after thyroidectomy were included in the study. Exclusion criteria were previous head and neck irradiation history, administration of iodine-containing medications such as amiodarone, and contrast-enhanced CT work-up 3 weeks within operation day (to eliminate the effect of iodine present in contrast agents).
For the control group, individuals were recruited from a health-screening program of a community-based cohort situated on Ganghwa region; the program involves biannual blood tests and food frequency questionnaires (FFQs). Among the 161 regional inhabitants, individuals with a history of amiodarone administration, head and neck irradiation, or thyroid disease, including malignancy, were excluded from the study.
The study complied with the Declaration of Helsinki in its latest version.
Data acquisition
Information on to the PTC group, including age, sex, operational records, and postoperative pathological reports, was obtained from the hospital’s electronic medical records. The pathological reports included information on histological diagnosis, tumor size, number of tumors, the presence of extrathyroidal extension (ETE), lymph node metastasis, lymphatic invasion, vascular invasion, and thyroiditis. BRAF V600E mutational status was determined by direct sequencing analysis by PyroMarkQ24 MDx polymerase chain reaction amplification sequencing (Qiagen, Hilden, Germany) [19].
The acute (< 48 h) iodine status of a population is best represented by the median urinary iodine (UI) level—around 95% of iodine is known to be excreted within 48 h—[20, 21]. Preoperative midnight fasting urine was obtained on the morning of operation for the PTC group. Urine samples were frozen at −20 °C and sent to a certified laboratory for analysis. The median UI levels were acquired using methods based on the Sandell–Kolthoff reaction, with the AU5800 device (Beckman Coulter, Brea, California, USA), and Autolab iodine (IVD-LAB, Seoul, Korea) as indicator. Urinary creatinine levels were determined by the Jaffe method with the AU5800 device and OSR6178 reagent (Beckman Coulter). The levels of median UI and median UI adjusted by urinary creatinine (to calibrate variation in the daily urine volume) were determined. Midnight fasting urine samples were collected from the control group individuals on the morning of a health examination and assayed in the laboratory in the same manner as the PTC group samples. To facilitate comparisons with other references, an equation to convert creatinine-adjusted median UI into 24 h urinary iodine excretion (µg per day), 24 h urinary iodine (µg per day) = e {e 0.35935 × ln (iodine: creatinine ratio [µg/g])0.379026} [22] was applied.
As 95% of iodine is excreted from the body through urine, the median UI level was not considered adequate in reflecting the long-term iodine status of the participants. Therefore, FFQ scores were also acquired from both the PTC and control groups to semi-quantitatively assess chronic iodine status of the preceding year. Also, by obtaining dietary data of the past year, it was possible to circumvent issues of reverse causation as past diet is unlikely to be influenced by diagnosis of cancer. In order to overcome the limitations of previous epidemiologic studies which investigated the correlation between consumption of one or two high-iodinated food products and thyroid cancer, we created a list, based on a pre-existing questionnaire [23], of 14 frequently consumed food items were chosen on the basis of high iodine content [24]: egg, preserved squid with salt, clam, oyster, seaweed, sea tangle, milk, yogurt, ice cream, cheese, and four types of kimchi. A designated interviewer thoroughly explained to all patients how to fill in the questionnaire with any remaining ambiguities answered on the first postoperative day for the cases and on the day of health exam for the controls. The recall time was within 1 year. The FFQ score was calculated by multiplying the frequency (on a scale from 1 to 9) and amount (on a scale from 1 to 3) of intake during the past year for each food item and adding the value of all 14 scores to give a possible FFQ score range of 1–378. All dietary data acquisition from participant education to score calculation was done by a single research assistant in both groups.
Statistical analyses
All data were analyzed with the SPSS 20.0 statistical software package (SPSS Inc., Chicago, IL, USA). Shapiro–Wilk test was conducted to demonstrate the normality of distribution for all variables, after which Mann–Whitney U test, Mood’s median tests [25], or Student’s t tests was applied accordingly to each variables. A Pearson’s correlational study was performed to assess if there were any significant correlation between acute (urinary) and chronic (dietary) iodine analyses between the PTC and control groups. A p-value < 0.05 was considered statistically significant.
Results
Clinicopathological demographics
The PTC group contained 210 patients who were scheduled for thyroidectomy, and the control group contained 90 healthy individuals from the Ganghwa community cohort. Normality of distribution was demonstrated only in FFQ scores (p = 0.391). Therefore, median values (median UI, creatinine-adjusted median UI) were dealt the median test, other non-parametric variables with Mann–Whitney U tests, nominal variables with χ 2 tests, and FFQ scores with Student’s t tests. Significant differences were seen in both mean age (p < 0.001) and male: female ratio (p = 0.001) in the PTC group versus the control group (Table 1). The pathological features of the tumors in the PTC group are also summarized in Table 1.
Within the PTC group, BRAF V600E mutational status was evaluated in 191 patients (91.0%), of whom 169 (88.5%) were BRAF V600E-positive and 22 (11.5%) were BRAF V600E-negative. The mean age and male: female ratio did not differ between the groups (Table 2). No differences were observed in mean tumor size, multiplicity, ETE, lymph node metastasis, lymphatic invasion, or vascular invasion between BRAF V600E-positive and BRAF V600E-negative groups. However, the proportion of patients with thyroiditis was lower in the BRAF V600E-positive group (24.9%) than in the BRAF V600E-negative group (59.1%; p = 0.002; Table 2).
Median UI levels (PTC group versus control group)
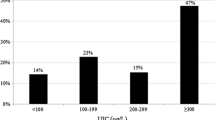
The median UI level in the PTC group was 786 μg/l (lowest value: 10 μg/l, 1st interquartile value (IQ): 385.8 μg/l, 3rd IQ: 1967.8 μg/l, and highest value: 22,564 μg/l). The median UI level in the control group was 111.5 μg/l (lowest value: 19 μg/l, 1st IQ: 54.3 μg/l, 3rd IQ: 251.8 μg/l, and highest value: 7569 μg/l). The median test demonstrated that a statistically significant difference was present between the median UI levels in the two groups (p < 0.001; Fig. 1).
In the PTC group, the median UI adjusted by urinary creatinine was 885 μg/g creatinine (lowest value: 8 μg/g creatinine, 1st IQ: 427.6 μg/g creatinine, 3rd IQ: 2018.9 μg/g creatinine, and highest value: 36,909 μg/g creatinine). The creatinine-adjusted median UI in the control group was 181.7 μg/g creatinine (lowest value: 29.9 μg/g creatinine, 1st IQ: 103.5 μg/g creatinine, 3rd IQ: 391.1 μg/g creatinine, and highest value: 8656.2 μg/g creatinine). A significant difference was present between the creatinine-adjusted median UI levels in the two groups (p < 0.001; Fig. 1). Based on the creatinine-adjusted median UI to 24 h urinary iodine excretion conversion equation, the PTC and control group excreted 2167 and 362 µg of iodine per day, respectively.
FFQ scores (PTC group versus control group)
In the PTC group, the mean FFQ scores of 66.2 ± 17.5 (range 13–114) in the PTC group and 54.6 ± 21.5 (range 16–134) in the control group were significantly different (p < 0.001; Fig. 1). The correlation between median UI/creatinine-adjusted median UI and FFQ scores for both PTC (p = 0.201/p = 0.374) and control (p = 0.114/p = 0.218) group did not retain statistical significance.
BRAFV600E-positive versus BRAFV600E-negative patients
In the subgroup analysis of patients with PTC, the median UI levels were higher in the BRAF V600E-positive group (884 μg/l and 954 µg/g creatinine) than in the BRAF V600E-negative group (792.9 µg/l and 948 µg/g creatinine), although the differences were not statistically significant (p = 0.841 for both comparisons, Table 2). Similarly, there was no difference (p = 0.746, Table 2) between mean FFQ scores in the BRAF V600E-positive group [65.7 ± 17.2 (range 13–114)] and BRAF V600E-negative group [66.8 ± 19.3 (range 24–92)]. After adjusting for clinicopathological characteristics such as patient age, sex, tumor size, ETE, metastatic lymph nodes, lymphatic invasion, and the presence of thyroiditis, the difference still did not achieve statistical significance (data not shown).
Discussion
Considering the large amount of iodine intake, the increasing incidence of PTC and the high prevalence of the BRAF V600E mutation in South Korea when compared with that of other countries [18] investigating the iodine status of Korean patients with PTC—including those with BRAF V600E mutation—is important. The present study demonstrated significantly higher levels of median UI and creatinine-adjusted median UI in patients with PTC, compared with healthy controls, indicating a difference in acute iodine status. Moreover, higher FFQ scores also indicated higher chronic iodine status in the PTC group compared with the controls.
Iodine is indispensable, not only as an essential trace element, but also as a substrate for thyroid hormone synthesis. Iodine is also deeply involved in normal human development early in life and also in homeostasis of cellular metabolism. Iodine status is intricately related to thyroid function, and both deficient and excessive iodine intake are detrimental. A “U-shaped curve”-like relationship exists between iodine status and thyroid disease [26,27,28]. Deficient iodine intake leads to endemic goiter, whereas excessive intake results in iodine-induced hyperthyroidism and autoimmune thyroid diseases. Although epidemiological evidence suggests that excessive iodine intake is related to the occurrence of PTC [12, 16, 29, 30], the role of iodine in this process has not been firmly established.
South Korea is well known for its high level of iodine intake. A study of 11 female Korean patients with thyroid cancer revealed that mean urinary iodine levels were 6180 ± 4770 µg/l [31] and the mean value in our PTC patient group was 2323 ± 4015 µg/l. According to the equation converting creatinine-adjusted median UI into 24 h urinary iodine excretion, the PTC and control group excreted 2167 and 362 µg of iodine per day in our study. In previous reports of iodine excretion in the Korean population, average values ranged from 479 µg per day to 3520 µg per day [32, 33]. Elsewhere, the reported average iodine intake is 138–353 µg per day in the USA [34], 45 µg per day in Germany [35], and 226 µg per day and 163 µg per day for women and men, respectively, in the UK [36].
The results of epidemiological studies suggest a positive correlation between iodine status and thyroid cancer occurrence [10, 12, 15, 16, 26, 30, 37]. Notably, in a prospective cohort study from Japan that followed 52,679 individuals for a mean follow-up period of 14.5 years, higher consumption of seaweed (which accounts for 80% of Japanese iodine intake) was associated with increased incidence of thyroid cancer (hazard ratio: 1.71; 95% confidence interval: 1.01–2.90; p = 0.04) [29]. Moreover, experimental evidence demonstrates that tumor cell growth and migration peaked around iodine concentrations of 1.0 × 10−3 mmol/l and were inhibited at higher and lower concentrations. The iodine concentration in the human thyroid gland ranges from 1.0 × 10−6 mmol/l to 1.0 × 10−5 mmol/l, suggesting that high in vivo concentrations (nearing 1.0 × 10−3 mmol/l) may play a role in thyroid cancer growth and migration [38]. As Japan and China are also countries with high iodine intake, these results may have similar implications for the association of iodine to PTC in Korea [15, 29].
BRAF V600E mutations in PTCs alter the expression of genes that are involved in iodine metabolism [39, 40]. The expression of NIS mRNA (the gene for the sodium/iodide co-transporter) and AIT (apical iodide transporter) is lower in PTCs with the BRAF V600E mutation than in those with wild-type BRAF V600E [39]. Epidemiological evidence demonstrates high prevalence of the BRAF V600E mutation in iodine-rich areas such as China [15]. Moreover, the prevalence of BRAF V600E in Korea has increased from 62.2 to 73.7% in the past 20 years [18, 41], whereas the global prevalence is ~ 45% (range 27.3–87.1%) [41].
In the medical literature, median UI level is a well-accepted, easily obtainable, and cost-efficient method for determining iodine status. Individual urinary iodine concentration varies even within a day, although these variations can be evened out when measuring the levels of whole populations [20]. The precision of the median UI measurement becomes more accurate as the population size increases. The median UI represents iodine status with precision ranges of ± 30, ± 20, and ± 10% with group sizes of 14, 31, and 122 individuals, respectively [42]. According to this result, the precision ranges of median UI measurements in the PTC, control, BRAF V600E-positive, and BRAF V600E-negative groups were ± 10, ± 20, ± 20, and ± 30%, respectively, in the present study. In clinical practice, the urinary iodine level is adjusted by the value of urinary creatinine, to calibrate variation in urinary concentration, especially when measuring the iodine status after a low-iodine diet before radioiodine ablation therapy [43]. It is also useful when converting to 24 h urinary iodine excretion by the aforementioned formula [22].
Concerning dietary results, previous epidemiological studies on thyroid cancer occurrence and food questionnaire results suggest a positive correlation between high-iodinated food consumption and thyroid cancer [44,45,46], and our results support this conclusion by demonstrating higher FFQ scores in the PTC compared to the control group (p < 0.001). The lack of correlation between the urinary and dietary results for both patient and control groups shows that acute and chronic iodine status do not necessarily correlate together and further emphasize the importance of analyzing both of them separately.
The relatively large difference of the urinary compared to the dietary study results between the PTC and control group may be explained in two ways; the unlikely chance of iodine from residual contrast agents remaining in the blood stream for more than 3 weeks, and cancer patients trying to intake more nutritious food—including iodine—after the knowledge of cancer and impending operation. However, as both urinary and dietary analyses suggest higher iodine for PTC patients, such trend should be considered valid.
The levels of median UI and creatinine-adjusted median UI in the BRAF V600E-positive group were higher than those in the BRAF V600E-negative group, although the difference was not statistically significant. Even after adjusting for the clinicopathological characteristics, such as patient age and sex, and tumor size, ETE, metastatic lymph nodes, lymphatic invasion, and the presence of thyroiditis, the difference was not statistically significant, possibly because of the small number of patients in the mutation-negative group. This implies that further studies should include a larger BRAF V600E-negative group to strengthen the statistical power and to reduce the precision range of urinary iodine results in this comparison.
Moreover, the retrospective nature of the patient selection process and the small number of enrolled cases rendered case and control group matching impossible, which is reflected in the demographics of Table 1. Also, it led to the study being underpowered to perform appropriate multivariable analyses, and therefore, the conclusion may be susceptible to type II error. Ideally, both groups should come from the same population to reveal a causal relationship. It also resulted in a lack of protocol to minimize the effect of recall bias for the control group dietary analysis—as they would be less desperate to accurately remember their diet—and of change in dietary patterns immediately after cancer diagnosis. Finally, the low number of total participants prevented us from matching the case to control. Further reducing the number by matching would widen the precision range of the median UI value, and therefore, we chose not to match the groups. Our results, therefore, may only allude to the possibility of iodine association to PTC.
At last, to ensure that the case and control groups accurately reflect the median UI level of the Korean population, it is necessary to externally validate the results with samples from a heterogeneous group.
Our data demonstrate a difference in iodine status between the PTC group and the healthy cohort. These data are consistent with previous epidemiological and experimental studies that suggest a correlation between high iodine levels and the occurrence of thyroid cancer. Data concerning BRAF V600E mutation status failed to reach statistical significance, which underscores the importance of additional studies with more patients to confirm the clinical implications of these associations. The results presented herein would be supported by additional studies to ascertain the causal relationship between iodine status and PTC in a prospective, case–control matched manner with more participants.
References
Pellegriti G, Frasca F, Regalbuto C et al (2013) Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol 2013:965212
Ahn HS, Kim HJ, Welch HG (2014) Korea’s thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med 371:1765–1767
Choi YM, Kim TY, Jang EK et al (2014) Standardized thyroid cancer mortality in Korea between 1985 and 2010. Endocrinol Metab (Seoul) 29:530–535
Williams D (2008) Radiation carcinogenesis: lessons from Chernobyl. Oncogene 27(Suppl 2):S9–18
Pearce MS, Salotti JA, Little MP et al (2012) Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380:499–505
Mazonakis M, Tzedakis A, Damilakis J et al (2007) Thyroid dose from common head and neck CT examinations in children: is there an excess risk for thyroid cancer induction? Eur Radiol 17:1352–1357
Ron E, Lubin JH, Shore RE et al (1995) Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res 141:259–277
Richardson DB (2009) Exposure to ionizing radiation in adulthood and thyroid cancer incidence. Epidemiology 20:181–187
Pellegriti G, De Vathaire F, Scollo C et al (2009) Papillary thyroid cancer incidence in the volcanic area of Sicily. J Natl Cancer Inst 101:1575–1583
Feldt-Rasmussen U (2001) Iodine and cancer. Thyroid 11:483–486
Dijkstra B, Prichard RS, Lee A et al (2007) Changing patterns of thyroid carcinoma. Ir J Med Sci 176:87–90
Harach HR, Escalante DA, Onativia A et al (1985) Thyroid carcinoma and thyroiditis in an endemic goitre region before and after iodine prophylaxis. Acta Endocrinol (Copenh) 108:55–60
Hwang Y, Lee KE, Park YJ et al (2016) Annual average changes in adult obesity as a risk factor for papillary thyroid cancer: a large-scale case–control study. Medicine (Baltimore) 95:e2893
WHO/UNICEF/ICCIDD (2007) Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. World Health Organization: http://whqlibdoc.who.int/publications/2007/9789241595827_eng.pdf. Accessed 9789241595820 August 9789241592013)
Guan H, Ji M, Bao R et al (2009) Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J Clin Endocrinol Metab 94:1612–1617
Wang F, Wang Y, Wang L et al (2014) Strong association of high urinary iodine with thyroid nodule and papillary thyroid cancer. Tumour Biol 35:11375–11379
Choi J, Kim HS, Hong DJ et al (2012) Urinary iodine and sodium status of urban Korean subjects: a pilot study. Clin Biochem 45:596–598
Hong AR, Lim JA, Kim TH et al (2014) The frequency and clinical implications of the BRAF(V600E) mutation in papillary thyroid cancer patients in Korea over the past two decades. Endocrinol Metab (Seoul) 29:505–513
Viertler C, Groelz D, Gundisch S et al (2012) A new technology for stabilization of biomolecules in tissues for combined histological and molecular analyses. J Mol Diagn JMD 14:458–466
WHO (2013) Urinary iodine concentrations for determining iodine status in populations. World Health Organizations, Geneva
Zimmermann MB, Andersson M (2012) Assessment of iodine nutrition in populations: past, present, and future. Nutr Rev 70:553–570
Kim HK, Lee SY, Lee JI et al (2010) Usefulness of iodine/creatinine ratio from spot-urine samples to evaluate the effectiveness of low-iodine diet preparation for radioiodine therapy. Clin Endocrinol 73:114–118
Lee KE, Park YJ, Cho B et al (2015) Protocol of a thyroid cancer longitudinal study (T-CALOS): a prospective, clinical and epidemiological study in Korea. BMJ Open 5:e007234
Moon SJ, Kim JY, Chung YJ et al (1998) The iodine content in common Korean foods. Korean Nutr Soc 31:7
Mood AM (1954) On the asymptotic efficiency of certain nonparametric two-sample tests. Ann Math Stat 25:8
Laurberg P, Bulow Pedersen I, Knudsen N et al (2001) Environmental iodine intake affects the type of nonmalignant thyroid disease. Thyroid 11:457–469
Todd CH, Allain T, Gomo ZA et al (1995) Increase in thyrotoxicosis associated with iodine supplements in Zimbabwe. Lancet 346:1563–1564
Stanbury JB, Ermans AE, Bourdoux P et al (1998) Iodine-induced hyperthyroidism: occurrence and epidemiology. Thyroid 8:83–100
Michikawa T, Inoue M, Shimazu T et al (2012) Seaweed consumption and the risk of thyroid cancer in women: the Japan Public Health Center-based prospective study. Eur J Cancer Prev 21:254–260
Pagano L, Caputo M, Sama MT et al (2012) Clinical-pathological changes in differentiated thyroid cancer (DTC) over time (1997–2010): data from the University Hospital “Maggiore della Carita” in Novara. Endocrine 42:382–390
Kim JY, Kim KR (2000) Dietary iodine intake and urinary iodine excretion in patients with thyroid diseases. Yonsei Med J 41:22–28
Kim H, Lee H, Park K et al (1985) A study on the urinary iodide excretion in normal subjects and patients with thyroid disease. Korean J Intern Med 29:625–631
Kim JY, Moon SJ, Kim KR et al (1998) Dietary iodine intake and urinary iodine excretion in normal Korean adults. Yonsei Med J 39:355–362
Murray CW, Egan SK, Kim H et al (2008) US Food and Drug Administration’s Total Diet Study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol 18:571–580
Scientific Committee on Food (2002) Opinion of the scientific committee on food on the tolerable upper intake level of iodine, European Commission. Health & Consumer Protection Directorate-General, Brussels
Expert Group on Vitamins and Minerals (2003) Safe upper levels for vitamins and minerals. Food Standards Agency, London
Sehestedt T, Knudsen N, Perrild H et al (2006) Iodine intake and incidence of thyroid cancer in Denmark. Clin Endocrinol 65:229–233
Xiang J, Wang X, Wang Z et al (2015) Effect of different iodine concentrations on well-differentiated thyroid cancer cell behavior and its inner mechanism. Cell Biochem Biophys 71:299–305
Durante C, Puxeddu E, Ferretti E et al (2007) BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab 92:2840–2843
Fuziwara CS, Kimura ET (2014) High iodine blocks a Notch/miR-19 loop activated by the BRAF(V600E) oncoprotein and restores the response to TGFbeta in thyroid follicular cells. Thyroid 24:453–462
Song YS, Lim JA, Park YJ (2015) Mutation profile of well-differentiated thyroid cancer in asians. Endocrinol Metab (Seoul) 30:252–262
Andersen S, Karmisholt J, Pedersen KM et al (2008) Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr 99:813–818
Lim CY, Kim JY, Yoon MJ et al (2015) Effect of a low iodine diet vs. restricted iodine diet on postsurgical preparation for radioiodine ablation therapy in thyroid carcinoma patients. Yonsei Med J 56:1021–1027
Cho YA, Kim J (2015) Dietary factors affecting thyroid cancer risk: a meta-analysis. Nutr Cancer 67:811–817
Bosetti C, Kolonel L, Negri E et al (2001) A pooled analysis of case–control studies of thyroid cancer. VI. Fish and shellfish consumption. Cancer Causes Control CCC 12:375–382
Liu ZT, Lin AH (2014) Dietary factors and thyroid cancer risk: a meta-analysis of observational studies. Nutr Cancer 66:1165–1178
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors involved in this study have actual or potential competing conflict of interests to declare.
Rights and permissions
About this article
Cite this article
Lee, JH., Song, RY., Yi, J.W. et al. Case–Control Study of Papillary Thyroid Carcinoma on Urinary and Dietary Iodine Status in South Korea. World J Surg 42, 1424–1431 (2018). https://doi.org/10.1007/s00268-017-4287-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-4287-x