Abstract
This study demonstrates a strong association of high urinary iodine with thyroid nodules and papillary thyroid cancer as well as aggressive cancer features, suggesting that high urinary iodine is a risk factor for thyroid cancer. The risk of high iodine intake for thyroid cancer has been suggested but not established. The objective of the study was to evaluate the relationship between urine iodine levels and thyroid nodule and thyroid cancer. We preoperatively tested fasting urine iodine in 154 thyroid nodule patients and correlated the results with pathological diagnoses and compared with 306 subjects as normal control. The median urine iodine (MUI) was 331.33 μg/L in patients with benign thyroid nodules versus 466.23 μg/L in patients with papillary thyroid cancer (PTC) (P = 0.003), both of which were in the excessive iodine state and higher than the MUI of 174.30 μg/L in the control group (P < 0.001), which was in the sufficient iodine state. Excessive iodine state (MUI > 300 μg/L) was seen in 62.75 % of patients with benign thyroid nodules and 66.99 % of patients with PTC, both of which were significantly higher than the iodine excessive rate of 19.93 % in the control group (P < 0.001). Moreover, MUI in patients with PTC with lymph node metastasis was significantly higher than that of PTC patients without lymph node metastasis (P < 0.001). Urine iodine of thyroid cancer patients with stage III and IV disease was significantly higher than that of patients with stage I and II diseases (P < 0.001). Multivariable analyses showed that, like sand calcification of thyroid nodule and TSH, urine iodine was an independent risk factor for PTC. These data demonstrate a significant association between high urinary iodine and benign and malignant thyroid nodules and PTC aggressiveness, supporting high urinary iodine as a risk factor for thyroid malignancy. Further studies are warranted to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thyroid nodule is a very common endocrine disease, seen in up to 5–10 % of adult people on physical palpation and up to 50–70 % of people on ultrasonography, with a malignancy risk of about 5–10 % [1–3]. Accompanying the increased diagnosis of thyroid nodules, the incidence of thyroid cancer has been rapidly rising worldwide in recent years [4–6]. The most common type of thyroid cancer is papillary thyroid cancer (PTC), followed by follicular thyroid cancer (FTC), which respectively account for 85–90 % and 10–15 % of all thyroid cancers [4, 6]. Like other human malignancies, thyroid cancer is a genetics-driven disease, but environmental factors may also play a role in its development. Among the potential environmental risk factors is the high iodine intake, which has been suggested to be likely a risk factor particularly for PTC [7, 8]. The issue on the relationship between high iodine intake and PTC has received considerable attention, as it has important public health implications, including a potential impact on the practice of universal salt iodization (USI) in many countries. This may particularly affect the way on how to define the standard allowance of iodine nutrition supplements and the safe upper limit of urine iodine concentration, which is currently 300 μg/L. However, the role of iodine in thyroid cancer risk has not been established. Most of the data on this issue have come from epidemiology population studies; case–control studies to look at the effect of iodine on thyroid cancer risk in individual patients are sparse. To further investigate iodine as a potential risk factor for thyroid cancer, in the present study, we examined preoperatively urine iodine in a large cohort of patients with thyroid nodules who were scheduled for thyroidectomy and analyzed postoperatively its relationship with the pathological diagnoses of the thyroid nodules and, in the malignant cases, the pathological characteristics of the cancer, such as the status of lymph node metastases and tumor stages.
Materials and methods
Subjects
With the approval by our institutional ethics committee and informed written patient consent, we preoperatively recruited thyroid nodule patients with normal thyroid functions into this study who were scheduled for thyroidectomy because of suspicion for thyroid cancer and nodular goiter at the General Surgery Department of the Affiliated Hospital of Qingdao University School of Medicine from June 2010 to June 2011. Follicular thyroid cancer patients were not included in the final analysis of the study due to too a small number of cases. All the patients selected in this study, whose B-ultrasound results showed thyroid cancer or nodular goiter, chose surgery as their treatment. Thyroid cancer was suspected when B-ultrasound results showed the following: hypoechoic lesion, rich blood supply in the nodules (normal TSH), irregular border and absent halo, microcalcification, the presence of needle-like calcification and clusters of calcification, and abnormal ultrasonic results of the cervical lymph node. The cases in the benign nodule group were also surgery patients because of nodule-related oppression symptoms or progressive growth of nodules. The pathology results of some patients suspected of thyroid cancer turned out to be benign after the operation, so we grouped the cases by the pathology results finally. The final analyses included a total of 154 patients with definitive postoperative pathological diagnoses, including 51 benign thyroid nodule patients (10 men and 41 women, age 48.3 ± 11.5 years) and 103 PTC patients (27 men and 76 women, age 50.5 ± 10.32 years). We also recruited a control group of 306 normal people (74 men and 232 women, age 49.84 ± 11.23 years) from a Qingdao Epidemiological Survey program who had normal thyroid functions and had no thyroid nodules on thyroid ultrasonography. All of the subjects had lived in Qingdao for more than 10 years, without receiving iodine-containing agents or medications, such as amiodarone/diodone, or imaging study-related contrast 6 months before the surgery. They also had not taken antithyroid drugs or thyroid hormones 3 months before the surgery and had no history of irradiation to the head and neck and abnormal liver and kidney functions.
Data acquisition
Basic information of the patients (e.g., age, sex, and disease history) and data on preoperative thyroid B-Doppler ultrasonography as well as postsurgical pathological findings were all collected from the standard medical records of the patients. Pathological information included histological diagnoses, tumor sizes, characteristics of thyroid cancer (e.g., the status of lymph node metastasis and extrathyroidal extension), and tumor stages.
Laboratory test
Preoperative serum TSH, triiodothyronine (FT3), thyroxine (FT4), thyroglobulin antibody (TgAb), and thyroperoxidase antibody (TPOAb) were tested using chemiluminescence detection kits from Roche at the Core Endocrine Laboratory of our hospital following the manufacturer’s instructions. Fasting urine was collected from the study subjects after they had similar food as usual and stayed in Qingdao at least for 1 week before collection. Study subjects were instructed to avoid high-iodine diet and excessively drink fluids on the urine collection days. Two 5-mL morning urine specimens were collected on two consecutive days; they were mixed before measurement. The urine specimens were sealed in polyethylene tubes to prevent evaporation until use. The urine samples of patients were collected before operation. The patients did not take FNA or any other instrumentation. Urinary iodine was measured by As-Ce catalytic spectrophotometry. In this assay process, iodine catalyzes the arsenic-cerium oxidation-reduction reaction in which yellow Ce4+ is reduced to colorless Ce3+, and iodine concentration is calculated from the absorbance of remaining Ce4+ [9].
Statistical analyses
Data were analyzed using the SPSS 17.0 statistic software. The urine iodine data were analyzed using Mann–Whitney U test, the percentage data were compared by chi-square test, and multiple variable analyses were performed by logistic regression analysis. P values ≤0.05 were considered statistically significant.
Results
High urine iodine levels in patients with thyroid nodules and thyroid cancer
As summarized in Table 1, there were no statistically significant differences of FT3 and FT4 in the patients with malignant or benign thyroid nodules than that in the control group (P > 0.05). The TSH in patients with thyroid cancer was significantly higher than that in patients with benign thyroid nodules and in the control group (P < 0.01). The median urine iodine (MUI) was 331.33 μg/L in patients with benign thyroid nodules and 466.23 μg/L in patients with thyroid cancer, both of which were in iodine-excessive state. The MUI was 174.3 μg/L in the control group, which was in iodine-sufficient state. There, MUI was significantly higher in the patients with malignant or benign thyroid nodules than that in the control group (P < 0.001). The MUI in patients with thyroid cancer was significantly higher than that in patients with benign thyroid nodules (P = 0.003), and the maximum urine iodine in patients with thyroid cancer was significantly higher than that in patients with benign thyroid nodules and that in the control group (P < 0.001) (Table 1).
High rates of iodine-excessive states in patients with benign and malignant thyroid nodules
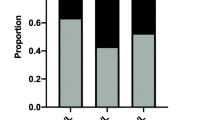
Human iodine nutritional status is classified into several states by leading authoritative international organizations, such as the World Health Organization based on MUI levels [10]: iodine-deficient state with MUI < 100 μg/L, iodine-sufficient state with MUI of 100–199 μg/L, iodine-super-sufficient state with MUI of 200–300 μg/L, and iodine-excessive state with MUI > 300 > g/L. According to these criteria on iodine nutritional states, we divided the iodine nutritional states in each patient group in our study into four groups corresponding to the four levels of MUI and analyzed their occurrence rates in different patient groups. As summarized in Table 2, iodine-excessive state (MUI > 300 μg/L) was seen in 62.75 % of patients with benign thyroid nodules and 66.99 % of patients with thyroid cancer, which were both significantly higher (P < 0.001) than the 19.93 % of the iodine-excessive state in the control group. In contrast, the rates of iodine-deficient or sufficient states in the control group were both higher than those in the patients with benign thyroid nodules or thyroid cancer.
Association of high urine iodine with aggressive pathological features of papillary thyroid cancer and its independent prediction for thyroid cancer
We found a significantly higher MUI in the 27 PTC patients who had lymph node metastasis than that in the 76 patients without lymph node metastasis, which was 1,584.62 μg/L in the former versus 315.61 μg/L in the latter (P < 0.001). We also examined the relationship of urine iodine levels with the sizes of metastasized lymph nodes of PTC patients who had lymph node metastasis and found no difference. Specifically, MUI in the 73 PTC patients with metastasized lymph nodes ≥1 cm was 829.43 μg/L versus 801.75 μg/L in the 30 PTC patients with lymph nodes <1 cm (P = 0.115). The MUI level was associated with high disease stages of PTC. Specifically, we found a significantly higher MUI in the 67 PTC patients with stage III or IV disease than that in 36 patients with stage I or II disease, which was 1,258.82 μg/L in the former versus 298.62 μg/L in the latter (P < 0.001).
Interestingly, we also found an association of high MUI with coarse or sand calcification of PTC detected on preoperative ultrasonography (Table 3).
On logistic regression multivariable analysis adjusting for TSH, FT3, FT4, TgAb, TPOAb, calcification type, patient age and sex, and thyroid nodule size, we found urine iodine to be an independent risk factor predicting thyroid cancer with an odds ratio of 5.39 (95 % CI 3.22–4.24, P < 0.001) (Table 4). Sand calcification and TSH were also two independent risk factors for PTC, but TgAb was not.
Discussion
As an essential trace element, iodine, by functioning as a substrate for thyroid hormone synthesis, plays a crucial role in normal development and growth of early human life and cellular and molecular metabolism throughout human life. Appropriate iodine is essential to maintain normal physiological functions. Insufficient iodine intake can cause hypothyroidism and related diseases. On the other hand, excessive iodine intake can also cause thyroid diseases, such as thyroiditis and thyroid dysfunction. It has been well established that iodine intake and development of thyroid diseases exhibit a U-shaped curvilinear relationship, illustrating that excessive and insufficient intakes of iodine both are unhealthy [11].
The relationship of high iodine take with thyroid cancer has also been suggested in epidemiology studies in recent years but has not been definitively established. By conducting the present study of a large series of individual patients with matched urine iodine and thyroid tumor histology diagnoses and pathological characteristics, we demonstrate a significant association of high MUI with PTC and its aggressiveness. By multivariable analysis, we also showed that high urine iodine was an independent predictor for thyroid malignancy. Interestingly, the present study also showed an association of high iodine intake with the development of even benign thyroid nodules. The data thus provide further evidence to support high iodine intake as a risk factor for thyroid tumorigenesis, particularly the development of thyroid cancer.
Almost 80 to 90 % of iodine comes from our daily diets. Living in Qingdao, a coastal city in China, residents here take rich iodine from sea foods, which leads to a high level of urine iodine. The MUI in 2000 and 2004 in coastal areas in China, including Qingdao, was 289.52 and 356.06 μg/L, respectively, which was much higher than that in interior regions of China [12, 13]. This explains the naturally commonly high MUI found in the patients in the present study.
Iodine nutritional status is typically categorized at four states based on MUI: <100 μg/L—iodine-insufficient state, 100–199 μg/L—iodine-sufficient state, 200–300 μg/L—iodine-super-sufficient state, and >300 μg/L—iodine-excessive state [10]. Most of the thyroid cancer patients in our study had MUI levels that fell in the iodine-excessive range. As one of the countries that suffered from iodine deficiency disorders (IDDs) in the past, China implemented a USI program to eliminate IDDs in 1996, which significantly increased the iodine intake of Chinese people and their MUI (165–330–306 μg/L, 1995–1997–1999). The implementation of USI, however, seemed to have been associated with an increase in the incidence of thyroid diseases, including thyroid cancer. For example, a 2009 study reported that the incidence of thyroid cancer has been tripled in the prior 10 years, especially in the coastal cities in China [14]. The histological types of thyroid cancer have also been shifted more toward PTC, apparently with more aggressiveness [15, 16]. The association of high iodine intake with increased PTC predominance has also been found in other epidemiology studies. For example, after USI in Australia, the incidence of PTC was increased from 3.07/100,000 to 7.8/100,000 [17]. The incidence of PTC in iodine-sufficient areas was higher than that in iodine-insufficient areas, and high iodine intake was associated with increased incidence of PTC and correspondingly a decrease in the incidence of FTC [15]. A 5-year prospective epidemiological study from China found that the incidence of thyroid cancer was 19.37/100,000 in communities with high drinking water iodine (MUI 633.5–650.9 μg/L), which is solely from PTC, and this incidence was significantly higher than that in iodine-deficient communities (4–6/100,000) [18]. All these epidemiology data from China and other countries are consistent with our finding of the association of high MUI with PTC in the present study.
The pathogenesis of high iodine level related thyroid nodules is explained by the Wolff-Chaikoff effect [19]: Too much iodine intake can lead to autoregulation of the thyroid, which may suppress NIS and reduce the iodine transferred into thyroid cells; then, the cellular iodine level will drop and reduction of FT3 and FT4 synthesis further; at last, the high TSH excretion will stimulate the epithelium proliferation of thyroid follicular cells, which lead to the goiter. However, most people will adapt the high-level iodine without goiter, named escape of the Wolff-Chaikoff effect. Although our body can maintain normal hormone metabolism in high-iodine-level environments by adaption, due to the escape, too much iodine will lead to pectin retention and inhibition of deiodinase of protein; finally, expanded follicle lumens result in the goiter [20].
Due to the concern that excessive iodine intake may accelerate the development of thyroid diseases, including thyroid cancer, China has modified the regulations on the implementation of salt iodination and the supplementation of dietary iodine several times from 1996 to 2000, with the upper limit being decreased from 60 to 35 mg/kg per day. The Chinese Ministry of Health published national food safety standards for iodine content of edible salt in September, 2011, which decreased the limit further from 35 to 20–30 mg/kg, leaving some room for individual adjustment according to actual circumstances. Our results in the present study seem to favor this practice.
In summary, our data show that high urine iodine is a risk factor for thyroid tumorigenesis, particularly the development of PTC and its aggressiveness. These data are consistent with the association of high iodine intake with thyroid cancer risk revealed in epidemiology studies. Our data provide further evidence supporting the concept that it is important to achieve adequate iodine intake at the right level to meet the normal nutritional need of the human body while at the same time avoiding either insufficient or excessive supplement of iodine.
References
Cibas ES. Fine-needle aspiration in the work-up of thyroid nodules [J]. Otolaryngol Clin North Am. 2010;43(2):257–71.
Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328:553–9.
Guth S, Theune U, Aberle J, et al. Very high prevalence of thyroid nodules detected by high frequency (13 MHz) ultrasound examination. Eur J Clin Invest. 2009;39:699–706.
Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995. Cancer. 1998;83:2638–48.
Leenhardt L, Grosclaude P, Cherie-Challine L Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee Thyroid 2004; 14, 1056–1060
Howlader N, Noone AM, Krapcho M et al. SEER Cancer Statistics review, 1975–2009 (Vintage 2009 populations), National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site, April 2012. 2012.
Lind P, Langsteger W, Molnar M, et al. Epidemiology of thyroid diseases in iodine sufficiency. Thyroid. 1998;8(12):1179–83.
Gomez Segovia I, Gallowitsch HJ, Kresnik E, et al. Descriptive epidemiology of thyroid carcinoma in Carinthia, Austria: 1984–2001. Histopathologic features and tumor classification of 734 cases under elevated general iodination of table salt since 1990: population-based age-stratified analysis on thyroid carcinoma incidence. Thyroid. 2004;14(4):277–86.
MOH, Ministry of Health, P.R. China. WS /T 107–2006 Method for determination of urinary iodine by As-Ce catalytic spectrophotometry[S]. People’s Medical Publishing House, 2006.
WHO/UNICEF/ICCIDD. Assessment of the iodine deficiency disorders and monitoring their elimination [S].Geneva: WHO, 2001
Laurberg P, Bulow Pedersen I, Knudsen N, et al. Environmental iodine intake affects the type of nonmalignant thyroid disease [J]. Thyroid. 2001;11(5):457–69.
Zhao SH, Wang L, Wang YG, et al. Influence of iodine nutrition status and susceptible HLA alleles on the pathogenesis of Graves’ disease and Hashimoto’s thyroiditis in Shandong coastal area [J]. Chin J Endocrinol and Metab. 2002;18(6):462–3.
Zhao SH, Wang YG, Yan SL, et al. The effect of iodine nutrition status on anti-thyroid medicine for Graves’ disease [J]. Acta Acadmiae Medicinae Qingdao Universitatis. 2004;40(3):195–6.
Chen JE, Song LJ. New epidemic characteristics of thyroid cancer [J]. Chin J Clin Med. 2009;16(5):812–3.
Dal Maso L, Bosetti C, La Vecchia C, et al. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors [J]. Cancer Causes Control. 2009;20(1):75–86.
Knobel M, Medeiros-Neto G. Relevance of iodine intake as a reputed predisposing factor for thyroid cancer [J]. Arq Bras Endocrinol Metabol. 2007;51(5):701–12.
Bacher-stier C, Riccabona G, Totsch M, et al. Incidence and clinical characteristics of thyroid carcinoma after iodine prophylaxis an endemic goiter country [J]. Thyroid. 1997;7:733–41.
Hx G, Li CY, Teng WP, et al. High iodine intake is a risk factor of post-partum thyroiditis: result of a survey from Shenyang, China [J]. J Endocrinol Invest. 2005;28(10):876–81.
Eng PH, Cardona GR, Previti MC. Regulation of the thyroid iodide symporter by iodide in FRTL-5 cells [J]. Eur J Endocrinol. 2001;144(2):139–44.
Wu L, Yu JC, Kang WM, et al. Iodine nutritional status and thyroid diseases.[J]. Acta Acad Med Sin. 2013;35(4):363–8.
Conflict of interest
None
Funding
Shandong medical and health research projects, China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Fang Wang and Yangang Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, F., Wang, Y., Wang, L. et al. Strong association of high urinary iodine with thyroid nodule and papillary thyroid cancer. Tumor Biol. 35, 11375–11379 (2014). https://doi.org/10.1007/s13277-014-2397-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2397-8