Abstract
Importance
Retrorectal tumors are rare lesions that comprise a multitude of histologic types. Reports are limited to small single-institution case series, and recommendations on the ideal surgical approaches are lacking.
Objective
The purpose of the study was to provide a comprehensive review of the epidemiology, pathologic subtypes, surgical approaches, and clinical outcomes of retrorectal tumors.
Evidence Review
We conducted a review of the literature using PubMed and searched the reference lists of published studies.
Results
A total of 341 studies comprising 1708 patients were included. Overall, 68 % of patients were female. The mean age was 44.6 ± 13.7 years. Of all patients, 1194 (70 %) had benign lesions, and 514 patients (30 %) had malignant tumors. Congenital tumors (60.5 %) were the most frequent histologic type. Other pathologic types were neurogenic tumors (14.8 %), osseous tumors (3.1 %), inflammatory tumors (2.6 %), and miscellaneous tumors (19.1 %). Biopsy was performed in 27 % of the patients. Of these patients, incorrect diagnoses occurred in 44 %. An anterior surgical approach (AA) was performed in 299 patients (35 %); a posterior approach (PA) was performed in 443 (52 %), and a combined approach (CA) was performed in 119 patients (14 %). The mean length of stay (LOS) of PA was 7 ± 5 days compared to 8 ± 7 days for AA and 11 ± 7 days for CA (p < 0.05). The overall morbidity rate was 13.2 %: 19.3 % associated with anterior approach, 7.2 % associated with posterior approach, and 24.7 % after a combined approach (p < 0.05). Overall postoperative recurrence rate was 21.6 %; 6.7 % after an anterior approach, 26.6 % after a posterior approach, and 28.6 % after a combined approach (p < 0.05). A minimally invasive approach (MIS) was employed in 83 patients. MIS provided shorter hospital stays than open surgery (4 ± 2 vs. 9 ± 7 days; p < 0.05). Differences in complication rate were 19.8 % in MIS and 12.2 % in open surgery and not statistically significant.
Conclusions and Relevance
Retrorectal tumors are most commonly benign in etiology, of a congenital nature, and have a female predominance. Complete surgical resection is the cornerstone of retrorectal tumor management. A minimal access surgery approach, when feasible, appears to be a safe option for the management of retrorectal tumors, with shorter operative time and length of stay.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Retrorectal tumors are rare tumors with a wide range of histology and are found within the pararectal space, which is defined as the potential space between the mesorectum and pelvic wall [1]. The wide range of histological variance may, in fact, be due to the presence of multiple embryologic remnants and miscellaneous tissue types in the presacral and retrorectal spaces [2]. They range from benign cysts to malignant masses that can invade the surrounding pelvic structures [2]. The variety and heterogeneity of retrorectal tumors have resulted in confusion over their classification and management [3–9].
The presentation, diagnosis, and treatment of retrorectal tumors can perplex clinicians. These tumors are generally asymptomatic or present with vague symptoms leading to misdiagnosis [10]. Once a diagnosis is made, surgical resection may be difficult given the anatomical location of the tumor. However, complete surgical resection is recommended in all cases including benign tumors given the potential for development of symptoms and malignancy [2, 11–15]. The surgical approach for these tumors may depend on various factors, such as their size and location. Several surgical approaches have been proposed for resection of retrorectal tumors. The described approaches include the abdominal (anterior), the perineal (posterior), and the abdominoperineal (combined anterior and posterior) approaches [3, 10, 13, 16]. Choosing a suitable approach is key to a successful operation and may help provide optimal exposure, minimize damage, and reduce complications. Laparoscopic approach has been successfully employed in recent years in a few cases.
The rarity of these tumors and the lack of availability of literature pose challenges for surgeons and to date, only small case series exist in literature. Given the variability of pathology, symptoms, and treatment options, we present a comprehensive review of literature describing the etiology of retrorectal tumors, diagnosis paradigm and treatment options for these tumors. This literature review aims to provide the 21st century surgeon a guide to the diagnosis and treatment of these rare lesions.
Methods
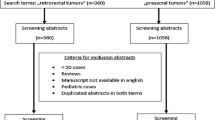
A PubMed search using the terms “pararectal tumor,” “presacral,” and “retrorectal” was utilized to identify the reported cases of retrorectal, non-gynecologic tumors, as well as reviews describing similar cases was conducted. A flowchart of the selection process according to the statement on Preferred Reporting Items for Systematic Reviews and Meta-analyses is presented in Fig. 1. The target population consisted primarily of adults (≥14 years old), with histologically confirmed retrorectal tumor diagnoses. Only English-language articles were assessed. Case reports, review articles, and case series were included, irrespective of size. Patients with non-primary tumors and pediatric patients were excluded. Based on available literature [7], pathology was divided into the following five categories: congenital, inflammatory, neurogenic, osseous, and miscellaneous.
The data were analyzed with the statistical program SPSS® (SPSS Inc., Chicago, IL, USA) version 17, for Windows®. The t test and chi-square test were used to assess statistical significance and p < 0.05 was considered statistically significant.
Results
A total of 341 studies with 1708 patients were identified. The majority of patients were female (68 %). Age ranged from 14 to 91 years, with mean age of 44.6 ± 13.7 years. Table 1 depicts the overall incidence, the incidence of malignancy, and the pathologic diagnosis from large single-institution case series as reported in the literature [3–7, 9, 15–34].
Classification
In accordance with the Uhlig and Johnson classification [7], the primary tumors reported in the literature were classified as congenital, neurogenic, osseous, inflammatory, or miscellaneous (Table 2). Of the patients reviewed, 1194 (70 %) had benign tumors, while 514 patients (30 %) had malignant tumors. Malignancy occurred in 44.3 % of the men and 20.5 % of the women (p < 0.05). Congenital tumors (60.4 %) were the most frequent histologic type, followed by miscellaneous tumors (19.1 %), neurogenic tumors (14.8 %), osseous tumors (3.1 %), and inflammatory tumors (2.6 %).
Congenital lesions
Congenital tumors were identified in 1033 patients (60.5 %). The mean age was 43.7 ± 13.5 years. A female predominance (74 %) was found in patients with congenital tumors; 767 patients (74 %) had benign tumors, and tailgut cyst was the most prevalent lesion (45 % of benign congenital lesions). The remaining 265 (16 %) had malignant tumors. Chordoma was the most common malignant congenital tumor (64 %). Malignant degeneration occurred in 65 patients and included 60 tailgut cysts, 3 dermoid cysts, 1 epidermoid cyst, and 1 duplication cyst.
Patients with malignant tumors were significantly older than those with benign tumors (53.9 ± 11.5 vs. 40.1 ± 12.2 years; p < 0.05). The presence of a congenital malignant tumor was more common in males (p < 0.05) (Table 3). Malignant tumors had a higher rate of postoperative complications (21 vs. 7 %; p < 0.05). Furthermore, malignant tumors had a higher rate of recurrence than benign tumors (46.2 vs. 11.4 %; p < 0.05) (Table 3).
Neurogenic lesions
Neurogenic tumors were identified in 252 patients (14.8 %). The mean age of these patients was 42.8 ± 12.8 years. The majority of neurogenic tumors were benign (89 %, n = 224). Neurilemmomas were the most common benign neurogenic tumors (86 %, n = 160) (Table 3). Neurofibrosarcoma (n = 7) were the most common malignant neurogenic tumors. Malignant tumors had a higher recurrence rate compared to benign neurogenic tumors (42 vs. 6.7 %; p < 0.05) (Table 3).
Osseous lesions
Fifty-three patients (3.1 %) had osseous tumors, with a mean age was 37.9 ± 12.7 years. Of these patients, 58 % were male and 37 patients (70 %) had malignant tumors. Giant cell tumors were the most common benign osseous tumors (63 %), and Ewing tumors were the most common malignant osseous tumor (43 %). Increasing age was associated with more malignant tumors compared to benign osseous lesions (41.6 ± 14.0 vs. 32.2 ± 8.4 years, p < 0.05). However, there was no difference in postoperative complications and recurrence.
Inflammatory lesions
Inflammatory tumors were present 2.6 % of reported cases (n = 45). All of these tumors were benign: 31 abscesses, 4 granulomas, 4 fibrosis, 1 cyst hydatid, 1 inflammatory cyst, and 4 unknown cysts. The mean age was 44.9 ± 9.8 years. A male predominance (56 %) was found in the patients with an inflammatory lesion (56 vs. 44 %, p > 0.05).
Miscellaneous lesions
There were 326 patients (19.1 %) who had miscellaneous tumors. Mean age was 50.9 ± 14.4 years old and 62 % of the patients were female. There were no differences in age between benign and malignant groups. Malignant tumors had greater recurrence rates than benign tumors (53.4 vs. 11.5 %; p < 0.05). Leiomyoma was the most frequent benign histologic type (35 %), followed by a fibroma (8.5 %). The most frequent malignant miscellaneous tumors were metastatic tumors (13 %).
Clinical presentation
Of the patients reviewed, 20.6 % were asymptomatic at the diagnosis time. If present, the most common symptoms were lower back pain (14.8 %), abdominal pain (10.0 %), constipation (8.8 %), urinary symptom (5.3 %), pelvic pain (5.0 %), abscess/fistula (4.3 %), asymptomatic mass (4.2 %), leg pain (2.9 %), hip pain (1.4 %), tenesmus (0.8 %), rectal bleeding (0.8 %), dyspareunia (0.3 %), and headache (0.5 %). At the physical examination, 1554 patients (91 %) had a palpable mass in the presacral region. In most cases, other diagnostic evaluations were conducted, including sigmoidoscopy (36.5 %), ultrasonography (39.7 %), computed tomography (CT; 80.7 %), and magnetic resonance imaging (MRI) (69.5 %). Biopsy was performed in 27 % of the patients. Of these patients, incorrect diagnoses occurred in approximately half (44 %).
Surgical approach and outcomes
The operative approaches are listed into Table 4. The posterior approach was performed in 443 patients (51.5 %), anterior approach in 299 patients (34.7 %), and a combined approach in 119 patients (13.8 %). Adjacent organs (sacrum, coccyx, or rectum) were resected in 35 % of patients. The combined resection rate was significantly higher using a combined approach (81 %), compared with that of the anterior or posterior approach (12 vs. 33 %, respectively; p < 0.05). The overall mean postoperative hospital stay was 8 ± 6 days, with 8 ± 7 days after an anterior approach; 7 ± 5 days after a posterior approach; and 11 ± 7 days after a combined approach (p < 0.05).
The overall postoperative complication rate was 13.2 %. Posterior approach had an associated morbidity of 7.2 %, anterior approach 19.3 % and combined approach 24.7 % (p < 0.05). Postoperative complications included post-operative bleeding (28 %), neurogenic bladder (23 %), neurologic complication (18 %), wound infection (15 %), rectal injury (5 %), ureter injury (3 %), leakage (3 %), constipation (3 %) and meningitis (2 %). The overall postoperative recurrence rate was 21.6 %, with 6.7 % after an anterior approach, 26.6 % after a posterior approach, and 28.6 % after a combined approach (p < 0.05).
Minimal invasive approach
Laparoscopy was performed in 83 patients (Table 4). Laparoscopy was associated with shorter length of stay compared to open surgery (4 ± 2 vs. 9 ± 7 days; p < 0.05). Laparoscopic surgery had lower recurrence rates than open surgery (0 vs. 21.2 %; p < 0.05). Complication rate was similar between the two groups (19.8 % laparoscopy vs. 12.2 % open, p = 0.44). The operative time was shorter in laparoscopic surgery (148 ± 74 vs. 175 ± 126 min; p < 0.05). Six percent of all laparoscopic cases involved malignant tumors (n = 5), including one tailgut cyst with malignant transformation, one GIST, one colloid sarcoma, one Ewing tumor, and one chondrosarcoma [35–38].
Robotic surgery was applied in five benign and two malignant minimally invasive cases (malignant neurofibroma, GIST). No recurrence or operative death was reported [39–41].
Discussion
Retrorectal tumors are heterogeneous group of tumors given that they arise from the pararectal space, which contains multiple embryologic remnants derived from various tissues; retrorectal tumors are rare tumors and thereby no consensus has been made in the literature regarding specific diagnosis, treatment, and surgical approach. To date, despite a multitude of smaller reviews and case reports, the optimal surgical approach is still in question. Our series of 1708 patients is the largest review of these entities ever conducted. We show that these tumors have a female predominance, and are most commonly benign in etiology and of a congenital classification. The most common presentation is that of perianal and low back pain, and these tumors can often be palpated on physical exam. Diagnostic methods such as sigmoidoscopy, ultrasonography, CT and MRI can be used to aid in diagnosis. Malignant lesions are more common in men and are associated with higher complication rates after resection, as well as higher recurrence rates. According to our results, the posterior approach emerges as the preferred method of operation with the lowest morbidity rate. Combined anterior/posterior approach had the highest recurrence and complication rate. However, a minimally invasive method of treatment specifically laparoscopy and robotic-assisted laparoscopy approach is feasible and was associated with significant shorter length of stay and possible lower recurrence rate, although it was mostly employed in cases of benign tumors.
Incidence
The incidence of retrorectal tumors in the general population is unknown. In medical literature, various reports have indicated the potential incidence of retrorectal tumors. At the Mayo Clinic, Whittaker et al. collected 22 cases from 1922 to 1936 and found the incidence to be only 1 out of approximately 40,000 registrations [9]. Jao et al. reported 120 cases of retrorectal tumors during a 19-year period, suggesting that approximately 6.3 patients would be diagnosed with retrorectal tumors on a yearly basis [5]. However, reports are generally from referral centers and thus do not represent the true prevalence of these tumors; therefore the above reported statistics may be much higher than that of the general population [2]. Uhlig and Johnson reported 63 adult cases over 30 years and demonstrated that, on average, 2 patients per year were diagnosed with retrorectal tumors in a standard major metropolitan area [7]. Our review demonstrates that retrorectal tumors are rare entities and in fact, Hobson et al. reported that a surgeon practicing outside the setting of a major referral center can expect to see, on average, at least 1 patient with a presacral tumor during the course of a typical career [2].
Pathological classification
As these tumors tend to have heterogeneity with respect to their histology, various classification systems have been proposed to categorize them [8]. In our analysis, we adhered to the classification proposed in 1975 by Uhlig and Johnson, distinguishing among congenital, inflammatory, neurogenic, osseous, and miscellaneous tumors [7].
The other important consideration for categorizing a retrorectal mass is its malignant nature [42]. Overall, 21–50 % of presacral tumors are malignant or contain areas of malignant change [5, 7, 15, 20]. The most common malignant lesion is the chordoma. Usually, malignant lesions are solid with signs of local invasion and bone destruction. Lev-Chelouche et al. divided retrorectal tumors into 4 groups according to the lesions’ pathology: benign congenital, malignant congenital, benign acquired, and malignant acquired [20]. In the medical literature we reviewed, 1194 (70 %) had benign tumors, and 514 patients (30 %) had malignant tumors.
Retrorectal congenital tumors are more common in women (74 %), while the other disease groups had a similar distribution between the two genders. It is noteworthy, however, that malignant tumors occur more frequently in men [3, 5, 10]. Stewart et al. reported 20 cases of presacral tumors over 20 years and claimed malignancy occurred in 50 % of adults and was more common in men (62 %) than in women (38 %) [4]. In our review, 32 % of the patients were male, and malignancy occurred in 44.2 % of the men and 20.5 % of the women. A malignant diagnosis increased the risk of recurrences in congenital (46.2 vs. 11.4 %; p < 0.05), neurogenic (42 vs. 6.7 %; p < 0.05), and miscellaneous tumors (53.4 vs. 11.5 %; p < 0.05). Patients with malignant congenital tumors had also a higher risk to develop postoperative complications (21 vs. 7 %; p < 0.05). In an attempt to guide the therapeutic approach, it has been noted that solid tumors have a higher probability of malignancy than cystic lesions; however, cystic masses may still develop into malignant lesions [5, 15].
Diagnosis
Due to their retroperitoneal location, retrorectal tumors often grow to a significant size so to become symptomatic due to their mass effect on surrounding organs. Lower back pain and abdominal pain are undoubtedly the most common symptoms, and are more frequently associated with infectious or malignant etiology [4, 5]. Glasgow et al. reported that pain is present in 71 % of patients with malignancies versus 22 % of patients with benign lesions [15]. Retrorectal tumors can frequently present with infection, and patients whose tumors are infected might also experience pain. An infection within the tumor may present as an abscess, a draining sinus, or a fistula tract. Therefore, these tumors may be initially misdiagnosed as fistulas, perirectal abscesses, or pilonidal diseases. Singer et al. reported that patients underwent an average of 4.1 surgical procedures before the correct diagnosis of a retrorectal lesion was made [10]. Thus, it is important to have a high index of suspicion of a retrorectal tumor for the successful diagnosis and treatment of this condition [7, 15].
Since symptoms associated with retrorectal tumors may be vague and non-specific, the most important aspect for the diagnostic process is an accurate physical assessment, which facilitates the identification of an appropriate surgical method. A careful rectal examination is essential to ascertain the diagnosis in >90 % of the patients [2, 5]. Most lesions are soft, compressible, and easily missed if the physician does not maintain a high index of suspicion [2, 5]. Although small lesions may not be detected by sigmoidoscopy, flexible sigmoidoscopy can determine the involvement of the rectal mucosa and confirm the level of proximal extension of the tumor [2]. Transrectal ultrasonography (TRUS), combined with proctoscopy, has a sensitivity of 100 % and provides information on the size, consistency of the mass, and evidence of local invasion [15].
Moving onto imaging techniques, CT and MRI are widely used to confirm the diagnosis of a retrorectal tumor for preoperative surgical planning [2]. A CT scan of the pelvis can identify small tumors, distinguish a cystic lesion from a solid lesion, and reveal sacral involvement or invasion of adjacent structures. [2, 5]. MRI is particularly useful in delineating soft tissue planes and evaluating the presence or absence of bony invasion and nerve involvement. Nonetheless, Glasgow et al. reported the accuracy of an MRI and a CT scan for a specific histologic retrorectal tumor type was only 28 and 18 %, respectively [15]. They advocated that it is not advisable to avoid resection based solely on noninvasive studies [15].
Biopsy of a retrorectal mass is controversial, because it can lead to contamination or tumor spread [2, 15]. Jao et al. reported their findings for patients who had biopsies prior to tumor resection, and they advocated that preoperative biopsies may elicit tumor spread, abscesses, fecal fistulas, or meningitis, so biopsies should not be performed if the tumors are potentially resectable [2, 5, 43, 44]. A biopsy should be performed only if the lesion appears to be unresectable and if a tissue diagnosis is required to guide adjuvant therapy. In our series, 27 % of the patient of the patients underwent biopsy to confirm the diagnosis. Of these patients, incorrect diagnoses occurred in 44 %. An accurate pathological diagnosis with a preoperative biopsy is often very difficult and the biopsy may lead to a misdiagnosis. Also, the histological type of retrorectal tumor does not influence the choice of surgical approach. Therefore, a biopsy prior to an operation is usually not recommended.
Treatment paradigm and surgical approaches
Once the diagnosis is established, surgical resection is the best therapeutic option, even in asymptomatic patients, as many lesions may contain malignant elements, possess the potential for growth or malignancy, or cause complications, such as infection [2]. Complete surgical resection is the cornerstone in the management of retrorectal tumors. En bloc excision permits confirmation of the diagnosis and eliminates the risk of complications such as infection, compression, potential for recurrence, and malignant degeneration [5, 12, 20].
The narrow space of the pelvis and the anatomical complexity of the retrorectal region can be challenging with respect to the surgical treatment of these tumors. The extent of surgery is determined by the characteristics of the tumor. Benign retrorectal tumors require complete gross resection, whereas malignant tumors will require radical resection, including en bloc resection of adjacent organs [42].
In certain circumstances where there is significant adjacent organ invasion, or severe adhesion to normal organ, combined resection including the normal adjacent organs may be necessary. Invasion of the rectum requires a rectal resection, and sacrococcygeal invasion requires coccygectomy or sacrectomy. In these complicated cases, a multidisciplinary team consisting of a radiologist, colorectal, urological, plastic, orthopedic, and neuro-surgeons may be necessary.
The surgical treatment of retrorectal tumors may be associated with an increased risk of complications such as presacral bleeding and rectal and nerve injuries. Jao et al. reported that the rate of postoperative complication after resection of a retrorectal tumor was 45 % [5]. In our literature review, postoperative complications included neurogenic bladder (15 %), wound infection (11 %), dysesthesia (7 %), fecal incontinence (7 %), massive bleeding (4 %), retrorectal abscess (3 %), and fecal fistula (1 %) [5]. Glasgow et al. reported 7 major postoperative complications in their 34 cases of retrorectal tumors, which included the following: transected ureter (n = 1), extensive soft-tissue infection (n = 1), reoperation for bleeding (n = 2), and impotency (n = 2) [15]. They mentioned that complications tended to occur more frequently in patients with malignant tumors; however, the choice of operative approach did not influence the occurrence of a postoperative complication [15].
Three major surgical approaches have been proposed for the resection of retrorectal tumors: the anterior (abdominal) approach, the posterior (perineal, trans-sacral, and parasacrococcygeal) approach, and the combined anterior and posterior pelvic approach. The appropriate technique depends on the lesion’s location, size, as well as the tumor’s relationship to the adjacent structures [20].
Anterior approach
Surgical management of tumors above the S3 level is best managed with an anterior (open or laparoscopic) or combined approach. Also, lesions which are potentially malignant are amenable to this approach, as it provides direct visualization of the pelvic side walls and pelvic viscera. The anterior approach has traditionally been the preferred method for tumors with the inferior most portion extending above the S4 level and if the sacrum is not involved [2, 42, 45]. A particular advantage of the anterior approach is that it allows the surgeon to have excellent exposure to major pelvic structures, such as the iliac vessels and ureters [3].
Posterior approach
In contrast to the anterior approach, the posterior approach is preferred for small, benign tumors that do not extend above the S4 level. When the superior border of the tumor is palpable and mobile on distal examination, the posterior approach should be considered. Also, this approach is preferred in cases of nerve involvement, given the improved visualization of nerves via a posterior approach [2]. However, the major disadvantages of the posterior approach are the risk of major intraoperative pelvic hemorrhage and potential injury to the lateral pelvic nerves [12]. A larger tumor, or a tumor in an intermediate location, may require a combined approach [2, 3]. Because of the potential for progression of these tumors deep into the pelvis and as identification of the planes between the tumor and the surrounding tissues becomes more difficult, patients may have to be repositioned for the perineal phase of the procedure.
Combined approach
The benefits of the combined approach include improved visualization of vital structures such as the ureters, vessels, pelvic nerves, and rectum by means of the anterior approach as well as enhanced exposure of the nerve roots provided by the posterior approach. If large tumors or infected masses involve the rectum or presacral fascia, the tumors often obscure normal surgical planes and may cause significant adhesions to the adjacent tissue. A combined approach is suggested in these situations [2, 3, 28].
Minimally invasive approach
The location of these tumors pose a challenge given that working in a narrow, deep space can be difficult and may increase the risk of damage to the pelvic vessels and nerves. In recent years, minimally invasive surgical techniques have been increasingly utilized to excise retrorectal tumors (Table 5).
In our review, we report 83 cases of laparoscopic excisions or combined laparoscopic and perineal surgical approaches for retrorectal tumors [27, 35–41, 46–79]. These reports demonstrated the safety of laparoscopy for treating retrorectal tumors. Laparoscopy offers the advantage of enhanced visualization of pelvic structures and facilitates precise dissection of the tumor from adjacent structures. There are no absolute indications to choose a laparoscopic access over laparotomy, and it still remains unclear whether open surgery or laparoscopy provides the best long-term results. In our review, there was no operative mortality or recurrences of tumor during the follow-up period (mean of 30 months); however, it is noteworthy that only 6 % of laparoscopic cases were for malignant cases (one tailgut cyst with malignant transformation, one GIST, one sarcoma, one Ewing tumor, one chondrosarcoma) [35–38]. Based on that total number of laparoscopic cases, we can conclude that laparoscopy is a safe and feasible method for retrorectal tumors; however, long-term studies with more are needed in order to define an advantage for both benign and malignant cases.
In the context of minimally invasive surgery, robotic surgery has several potential advantages compared to laparoscopic surgery, particularly within the narrow confines of the pelvis (three-dimensional view, superior dexterity, multi-articulated instruments). To date, to the best of our knowledge, only seven robotic cases have been described [39–41], and only in two cases the robotic technique was applied to malignant tumors (one malignant neurofibroma, one GIST). Based on previous studies on robotic resection of rectal tumors [80, 81], and the absence of recurrence or operative death in the robotic cases listed in our review [40, 41], we can hypothesize a similar outcome for patients who underwent robotic and laparoscopic retrorectal tumors resection. However, larger robotic retrorectal case-series are needed in order to draw a definitive conclusion.
Limitations
The limitations of this study include lack of large-scale studies. Because of the rarity of these tumors, the majority of published studies were case reports or case series. A small number of reviews have been described. Despite the limitation of this review, this is the largest and most comprehensive review in the literature and the first to compare surgical outcomes to date.
Conclusion
The most common retrorectal tumors are benign congenital tumors. Diagnosis of these lesions is accomplished via a thorough physical examination in conjunction with radiological imaging, ultrasonography, and ultrasound endoscopy. Biopsy, in most instances, can be avoided since it leads to an inaccurate diagnosis and may cause tumor seeding and other complications. The recommended treatment for retrorectal tumors is complete surgical excision given their malignant potential, their propensity to cause local complications, and their potential for recurrence. Minimal invasive surgery appears to be associated with shorter length of stay, shorter operative time, and possible lower recurrence rates. Robotic approach appears to be a safe and feasible option for the management of retrorectal tumors but conclusion is difficult due to the small number of cases available thus far.
References
Mirilas P, Skandalakis JE (2010) Surgical anatomy of the retroperitoneal spaces. Part II: the architecture of the retroperitoneal space. Am Surg 76:33–42
Hobson KG, Ghaemmaghami V, Roe JP et al (2005) Tumors of the retrorectal space. Dis Colon Rectum 48:1964–1974
Bohm B, Milsom JW, Fazio VW et al (1993) Our approach to the management of congenital presacral tumors in adults. Int J Colorectal Dis 8:134–138
Stewart RJ, Humphreys WG, Parks TG (1986) The presentation and management of presacral tumours. Br J Surg 73:153–155
Jao SW, Beart RW Jr, Spencer RJ et al (1985) Retrorectal tumors. Mayo Clinic experience, 1960–1979. Dis Colon Rectum 28:644–652
Cody HS 3rd, Marcove RC, Quan SH (1981) Malignant retrorectal tumors: 28 years’ experience at Memorial Sloan-Kettering Cancer Center. Dis Colon Rectum 24:501–506
Uhlig BE, Johnson RL (1975) Presacral tumors and cysts in adults. Dis Colon Rectum 18:581–589
Lovelady SB, Dockerty MB (1949) Extragenital pelvic tumors in women. Am J Obstet Gynecol 58:215–236
Whittaker LD, Pemberton JD (1938) Tumors ventral to the sacrum. Ann Surg 107:96–106
Singer MA, Cintron JR, Martz JE et al (2003) Retrorectal cyst: a rare tumor frequently misdiagnosed. J Am Coll Surg 196:880–886
Bullard Dunn K (2010) Retrorectal tumors. Surg Clin North Am 90:163–171 Table of Contents
Ghosh J, Eglinton T, Frizelle FA et al (2007) Presacral tumours in adults. Surgeon 5:31–38
Buchs N, Taylor S, Roche B (2007) The posterior approach for low retrorectal tumors in adults. Int J Colorectal Dis 22:381–385
Glasgow SC, Dietz DW (2006) Retrorectal tumors. Clin Colon Rectal Surg 19:61–68
Glasgow SC, Birnbaum EH, Lowney JK et al (2005) Retrorectal tumors: a diagnostic and therapeutic challenge. Dis Colon Rectum 48:1581–1587
Localio SA, Eng K, Ranson JH (1980) Abdominosacral approach for retrorectal tumors. Ann Surg 191:555–560
McColl I (1963) The classification of presacral cysts and tumours. Proc R Soc Med 56:797–798
Vorobyov GI, Odaryuk TS, Kapuller LL, Shelygin YA, Kornyak BS (1992) Surgical treatment of benign, myomatous rectal tumors. Dis Colon Rectum 35(4):328–331
Wang JY, Hsu CH, Changchien CR et al (1995) Presacral tumor: a review of forty-five cases. Am Surg 61:310–315
Lev-Chelouche D, Gutman M, Goldman G et al (2003) Presacral tumors: a practical classification and treatment of a unique and heterogeneous group of diseases. Surgery 133:473–478
Grandjean JP, Mantion GA, Guinier D et al (2008) Vestigial retrorectal cystic tumors in adults: a review of 30 cases. Gastroenterol Clin Biol 32:769–778
Woodfield JC, Chalmers AG, Phillips N et al (2008) Algorithms for the surgical management of retrorectal tumours. Br J Surg 95:214–221
Pappalardo G, Frattaroli FM, Casciani E et al (2009) Retrorectal tumors: the choice of surgical approach based on a new classification. Am Surg 75:240–248
Mathis KL, Dozois EJ, Grewal MS, Metzger P, Larson DW, Devine RM (2010) Malignant risk and surgical outcomes of presacral tailgut cysts. Br J Surg 97(4):575–579
Yang BL, Gu YF, Shao WJ, Chen HJ, Sun GD, Jin HY, Zhu X (2010) Retrorectal tumors in adults: magnetic resonance imaging findings. World J Gastroenterol 16(46):5822–5829
Gao XH, Zhang W, Fu CG et al (2011) Local recurrence after intended curative excision of presacral lesions: causes and preventions. World J Surg 35:2134–2142. doi:10.1007/s00268-011-1155-y
Li GD, Chen K, Fu D et al (2011) Surgical strategy for presacral tumors: analysis of 33 cases. Chin Med J (Engl) 124:4086–4091
Lin C, Jin K, Lan H et al (2011) Surgical management of retrorectal tumors: a retrospective study of a 9-year experience in a single institution. Onco Targets Ther 4:203
Bosca A, Pous S, Artes MJ et al (2012) Tumours of the retrorectal space: management and outcome of a heterogeneous group of diseases. Colorectal Dis 14:1–1418
Macafee DA, Sagar PM, El-Khoury T et al (2012) Retrorectal tumours: optimization of surgical approach and outcome. Colorectal Dis 14:1411–1417
Chereau N, Lefevre JH, Meurette G, Mourra N, Shields C, Parc Y, Tiret E (2013) Surgical resection of retrorectal tumours in adults: long-term results in 47 patients. Colorectal Dis 15(8):e476–e482
Messick CA, Hull T, Rosselli G, Kiran RP (2013) Lesions originating within the retrorectal space: a diverse group requiring individualized evaluation and surgery. J Gastrointest Surg 17(12):2143–2152
Sagar AJ, Tan WS, Codd R, Fong SS, Sagar PM (2014) Surgical strategies in the management of recurrent retrorectal tumours. Tech Coloproctol 18(11):1023–1027
Simpson PJ, Wise KB, Merchea A, Cheville JC, Moir C, Larson DW, Dozois EJ (2014) Surgical outcomes in adults with benign and malignant sacrococcygeal teratoma: a single-institution experience of 26 cases. Dis Colon Rectum 57(7):851–857
La Rosa S, Boni L, Finzi G, Vigetti D, Papanikolaou N, Tenconi SM, Dionigi G, Clerici M, Garancini S, Capella C (2010) Ghrelin-producing well-differentiated neuroendocrine tumor (carcinoid) of tailgut cyst. Morphological, immunohistochemical, ultrastructural, and RT-PCR study of a case and review of the literature. Endocr Pathol 21(3):190–198
Takahashi R, Nagayama S, Mori Y, Isoda H, Yoshizawa A, Manabe T, Sakai Y (2010) A large cystic gastrointestinal stromal tumor of the rectum in the retrorectal space. Int J Clin Oncol 15(6):601–607
Duclos J, Maggiori L, Zappa M, Ferron M, Panis Y (2014) Laparoscopic resection of retrorectal tumors: a feasibility study in 12 consecutive patients. Surg Endosc 28(4):1223–1229
Possover M, Uehlinger K, Ulrich Exner G (2014) Laparoscopic assisted resection of a ilio-sacral chondrosarcoma: a single case report. Int J Surg Case Rep 5(7):381–384
Morelli L, Tassinari D, Rosati CM et al (2012) Robot-assisted excision of a huge pararectal dermoid cyst via a totally transabdominal route. J Minim Invasive Gynecol 19:772–774
Oh JK, Yang MS, Yoon do H, Rha KH, Kim KN, Yi S, Ha Y (2014) Robotic resection of huge presacral tumors: case series and comparison with an open resection. J Spinal Disord Tech 27(4):E151–E154
Ploumidis A, Mottrie A, Spinoit AF, Gan M, Ficarra V, Andrianne R (2014) Robot-assisted excision of a pararectal gastrointestinal stromal tumor in a patient with previous ileal neobladder. Case Rep Urol 2014:632852
Dozois EJJD, Dozois RR (2007) Presacral tumors. In: Wolff BG, Fleshman JW, Beck DE et al (eds) The ASCRS textbook of colon and rectal surgery. Springer, New York, pp 501–514
Cho BC, Kim NK, Lim BJ, Kang SO, Sohn JH, Roh JK, Choi ST, Kim SA, Park SE (2005) A carcinoembryonic antigen-secreting adenocarcinoma arising in tailgut cyst: clinical implications of carcinoembryonic antigen. Yonsei Med J 46(4):555–561
Schwarz RE, Lyda M, Lew M, Paz IB (2000) A carcinoembryonic antigen-secreting adenocarcinoma arising within a retrorectal tailgut cyst: clinicopathological considerations. Am J Gastroenterol 95(5):1344–1347
Neale JA (2011) Retrorectal tumors. Clin Colon Rectal Surg 24:149–160
Sharpe LA, Van Oppen DJ (1995) Laparoscopic removal of a benign pelvic retroperitoneal dermoid cyst. J Am Assoc Gynecol Laparosc 2:223–226
Melvin WS (1996) Laparoscopic resection of a pelvic schwannoma. Surg Laparosc Endosc 6(6):489–491
Salameh JR, Votanopoulos KI, Hilal RE et al (2002) Rectal duplication cyst in an adult: the laparoscopic approach. J Laparoendosc Adv Surg Tech A 12:453–456
Kohler C, Kuhne-Heid R, Klemm P et al (2003) Resection of presacral ganglioneurofibroma by laparoscopy. Surg Endosc 17:1499
Bax NM, van der Zee DC (2004) The laparoscopic approach to sacrococcygeal teratomas. Surg Endosc 18:128–130
Konstantinidis K, Theodoropoulos GE, Sambalis G et al (2005) Laparoscopic resection of presacral schwannomas. Surg Laparosc Endosc Percutan Tech 15:302–304
Sriganeshan V, Alexis JB (2006) A 37-year-old woman with a presacral mass. Tailgut cyst (retrorectal cystic hamartoma). Arch Pathol Lab Med 130:e77–e78
Yang CC, Chen HC, Chen CM (2007) Endoscopic resection of a presacral schwannoma. Case report. J Neurosurg Spine 7:86–89
Sharma D, Nandini R, Goel D et al (2008) Retrorectal dermoid cyst in an adult. ANZ J Surg 78:408
Palanivelu C, Rangarajan M, Senthilkumar R et al (2008) Laparoscopic and perineal excision of an infected “dumb-bell” shaped retrorectal epidermoid cyst. J Laparoendosc Adv Surg Tech A 18:88–92
Gunkova P, Martinek L, Dostalik J et al (2008) Laparoscopic approach to retrorectal cyst. World J Gastroenterol 14:6581–6583
Cho SH, Hong SC, Lee JH et al (2008) Total laparoscopic resection of primary large retroperitoneal teratoma resembling an ovarian tumor in an adult. J Minim Invasive Gynecol 15:384–386
Chen Y, Xu H, Li Y et al (2008) Laparoscopic resection of presacral teratomas. J Minim Invasive Gynecol 15:649–651
Al-Khattabi M, Chouillard E, Louboutin A et al (2010) Giant pararectal epidermoid tumor mimicking ovarian cyst: combined laparoscopic and perineal surgical approach. J Minim Invasive Gynecol 17:113–115
Champney MS, Ehteshami M, Scales FL (2010) Laparoscopic resection of a presacral ganglioneuroma. Am Surg 76(4):E1–E2
Lu NH, Tseng MJ (2010) Laparoscopic management of tailgut cyst: case report and review of literature. J Minim Invasive Gynecol 17:802–804
Rao M, Sagar P, Duff S et al (2010) Laparoscopic excision of a retrorectal schwannoma. Tech Coloproctol 14:353–355
Witherspoon P, Armitage J, Gatt M et al (2010) Laparoscopic excision of retrorectal schwannoma. Dis Colon Rectum 53:101–103
Asuquo SE, Nguyen SQ, Scordi-Bello I, Divino CM (2011) Laparoscopic management of presacral myelolipoma. J Soc Laparoendosc Surg 15(3):406–408
Kye BH, Kim HJ, Cho HM, Chin HM, Kim JG (2011) Clinicopathological features of retrorectal tumors in adults: 9 years of experience in a single institution. J Korean Surg Soc 81(2):122–127
Leppard WM, Adams DB, Morgan KA (2011) Tailgut cysts: what is the best surgical approach? Am Surg 77(8):E160–E161
Lim SW, Huh JW, Kim YJ et al (2011) Laparoscopy-assisted resection of tailgut cysts: report of a case. Case Rep Gastroenterol 5:22–27
Marinello FG, Targarona EM, Luppi CR et al (2011) Laparoscopic approach to retrorectal tumors: review of the literature and report of 4 cases. Surg Laparosc Endosc Percutan Tech 21:10–13
Tsutsui A, Nakamura T, Mitomi H, Onozato W, Sato T, Ozawa H, Naito M, Ikeda A, Ihara A, Watanabe M (2011) Successful laparoscopic resection of a sacrococcygeal teratoma in an adult: report of a case. Surg Today 41(4):572–575
Paul PG, Pravinkumar T, Sheetal B (2012) Sacrococcygeal neurofibroma: rare cause for chronic pelvic pain. J Minim Invasive Gynecol 19:517–520
Jones M, Khosa J (2013) Presacral tumours: a rare case of a dermoid cyst in a paediatric patient. BMJ Case Rep 15:2013
Nedelcu M, Andreica A, Skalli M, Pirlet I, Guillon F, Nocca D, Fabre JM (2013) Laparoscopic approach for retrorectal tumors. Surg Endosc 27(11):4177–4183
Szyllo K, Lesnik N (2013) Sacrococcygeal teratoma—case report and review of the literature. Am J Case Rep 14:1–5
Fong SS, Codd R, Sagar PM (2014) Laparoscopic excision of retrorectal tumours. Colorectal Dis 16(11):O400–O403
Gascon Hove M, Pueyo Rabanal A, Cea Soriano M, Galindo Jara P, Garijo Álvarez JA (2014) Single-port access surgery resection of a presacral schwannoma. Tech Coloproctol 18(4):407–408
Imboden S, Al-Fana A, Kuhn A, Mueller MD (2014) Pandora’s box and retrorectal tumors in laparoscopy: a case report and review of the literature. Int J Surg Case Rep 5(10):706–709
Kassir R, Kaczmarek D (2014) A late-recognized Currarino syndrome in an adult revealed by an anal fistula. Int J Surg Case Rep 5(5):240–242
Kneist W, Kauff DW, Lang H (2014) Laparoscopic neuromapping in pelvic surgery: scopes of application. Surg Innov 21(2):213–220
Zhou JL, Wu B, Xiao Y, Lin GL, Wang WZ, Zhang GN, Qiu HZ (2014) A laparoscopic approach to benign retrorectal tumors. Tech Coloproctol 18(9):825–833
Young MT, Menon G, Feldmann TF, Mills S, Carmichael J, Stamos MJ, Pigazzi A (2014) Laparoscopic versus robotic-assisted rectal surgery: a comparison of postoperative outcomes. Am Surg 80(10):1059–1063
Lee SH, Lim S, Kim JH, Lee KY (2015) Robotic versus conventional laparoscopic surgery for rectal cancer: systematic review and meta-analysis. Ann Surg Treat Res 89(4):190–201. doi:10.4174/astr.2015.89.4.190
Disclosure
None.
Funding
Funding for this study was provided by departmental support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Seong Kyu Baek and Grace Soon Hwang denotes co-first authorship.
Rights and permissions
About this article
Cite this article
Baek, S.K., Hwang, G.S., Vinci, A. et al. Retrorectal Tumors: A Comprehensive Literature Review. World J Surg 40, 2001–2015 (2016). https://doi.org/10.1007/s00268-016-3501-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-016-3501-6