Abstract
Background
Multimodality therapy has been used in the management of gastric cancer associated with locoregional spread. However, the accurate clinical staging still remains to be established. The neutrophil/lymphocyte ratio (NLR) in the peripheral blood is reported to be an easily assessable prognostic factor in cancer patients. We evaluated the predictive significance of the NLR and other serological parameters in patients with wall-penetrating gastric cancer.
Methods
Two hundred sixty-two patients who underwent gastric cancer surgery between 2002 and 2005 were identified retrospectively. Wall penetration was defined as wall invasion deeper than the muscularis propria (≥T2). Blood data were collected from routinely performed blood examinations before treatment and were analyzed with respect to T stage, nodal status, and histological features. A high NLR was defined as less than 3.2 based on ROC curve analysis, and the predictive value of a high NLR for T4 cancer was evaluated.
Results
Elevated levels of NLR (P = 0.004) and C-reactive protein (CRP) (P = 0.017) and the decrease in lymphocyte count (Lym, P = 0.032) and serum hemoglobin (Hb, P < 0.001) were correlated with the T stage, but there was no meaningful correlation with either positive nodal status or histological differentiation. With respect to the predictive value for stage T4, an elevated NLR (OR = 2.206, 95% CI = 1.187–4.100; P = 0.012), decrease of Hb (OR = 1.875, 95% CI = 1.005–3.500; P = 0.048), and poorly differentiated histology (OR = 3.134, 95% CI = 1.593–6.167; P = 0.001) were identified as independent predictive factors.
Conclusion
Our findings suggest that the preoperative values of the NLR may be reliable for predicting T4 disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the second leading cause of cancer-related death worldwide. Annually, around 930,000 cases are diagnosed and approximately 700,000 deaths occur [1]. In Japan, the 5-year survival rate of gastric cancer patients has reached nearly 60%, attributable in part to early detection via screening [2, 3]. Though the treatment for patients with locoregional spread is still a challenge, the outcomes have lately improved with the introduction of multimodality therapies [4–6]. In particular, neoadjuvant therapies are in the limelight, with verification of their efficacy and safety under way. Therefore, precise clinical staging is considered crucial for appropriate treatment. At present, the outcome prediction is based predominantly on the TNM classification of the cancer [7]. Novel developments in imaging modalities have improved the diagnostic accuracy of the clinical stage. With respect to T staging, multidetector-row computed tomography (MDCT) with accuracy rates of 76–93% has been demonstrated to be a superior tool to endoscopic ultrasound (EUS) [8–11]. However, treatment plans occasionally need to be altered at laparotomy, indicating the superior effectiveness of staging laparoscopy [12].
Recently, the contribution of host inflammatory reactions to cancer development has been reported [13]. In particular, tumor growth-promoting infiltration by inflammatory cells and adjacent tissue remodeling are influenced by paracrine and autocrine loops of chemokine and cytokines [14]. Though the detailed mechanism still remains unclear, the addition of inflammatory-based optional factors to the TNM classification would be expected to help in the management. Indeed, several studies have investigated the role of the immune system in the growth progression or cessation of gastric cancers, and hematological and biochemical parameters have been suggested as simply assessable indices of the systemic pathophysiologic status [15–22]. In this study we evaluated whether preoperative peripheral blood parameters are useful in predicting locoregional extension in gastric cancer patients.
Patients and methods
Patients
The cases of 872 patients with gastric cancer who had undergone surgery at the National Cancer Center Hospital East between January 2002 and December 2005 were retrospectively reviewed from the database. Of these, 106 patients with esophagogastric junctional cancer, which was defined as cancer with its epicenter within 5 cm of the junction and extending into the esophagus, were not included. In addition, 43 patients who had received neoadjuvant chemotherapy and 57 patients with stage IV cancer, including peritoneal seeding, positive peritoneal cytology, para-aortic lymph node metastases, and liver metastases were excluded. Wall-penetrating gastric cancer was defined as cancer with deeper wall invasion than the submucosa (≥stage T2); thus, 404 patients with T1 disease were excluded. Therefore, a total of 262 patients with wall-penetrating tumors were investigated. The demographic data of the patients were recorded.
Disease staging
The staging was conducted according to the International Union Against Cancer (UICC) TNM classification (7th edition) [23]. The T stage and nodal status were determined based on histopathological evaluation. The accuracy rate of preoperative diagnosis for T4 stage was 50.3%. The histological features of the resected specimens were also evaluated. Papillary and tubular adenocarcinomas were categorized as well differentiated, whereas poorly differentiated adenocarcinoma and signet ring cell carcinoma were classified into the poorly differentiated category.
Peripheral blood parameters
Routine venous blood samples were collected from the patients for preoperative determination of blood cell counts, laboratory parameters, and tumor markers. Samples from patients with evidence of infectious conditions were excluded. Blood samples were collected in ethylenediamine tetraacetic acid (EDTA)-containing tubes for blood cell counting, in tubes containing coagulation accelerators for determination of laboratory parameters, and in 3.13% sodium citrate-containing tubes for analysis of the coagulation profile. Blood cell counts and the coagulation profile were measured using an XE-2100™ Automated Hematology System and an CA-1500 System automated blood coagulation analyzer (Sysmex Co., Kobe, Japan), respectively. The laboratory parameters and serum levels of carcinoembryonic antigen (CEA) were estimated using a Automatic Analyzer model 7700 series (Hitachi High Technologies Co., Ltd., Tokyo, Japan). Serum levels of the carbohydrate antigen (CA) 19-9 were determined using a Full Random Access Immunoassay LUMIPULSE® system (Fujirebio, Inc., Tokyo, Japan). Hematologic values, including the white blood cell count (WBC), neutrophil count (Neu), lymphocyte count (Lym), monocyte count (Mono), and platelet count (Plt), and the laboratory data, including the serum hemoglobin (Hb) and albumin (Alb), prothrombin time (PT), C-reactive protein (CRP), activated partial thromboplastin time (APTT), and serum CEA and CA19-9 were retrospectively identified. Then, the neutrophil/lymphocyte ratio (NLR) was calculated by dividing the neutrophil count by the lymphocyte count.
Statistics
Data are presented as medians and ranges. The preoperative hematological and biochemical parameters were compared in relation to the T stage, nodal status, and histological features using the Kruskal–Wallis test and the Mann–Whitney U test. Receiver operating characteristic (ROC) curves were constructed in order to calculate the sensitivity, specificity, positive predictive value, and negative predictive value for T4 disease. Cutoff values for the NLR with the best combination of predictive values were chosen. First, the χ2 and Fisher’s exact tests were used for univariate comparison of each of the hematological parameters of interest. Variables found to be significant on univariate analysis were subjected to multivariate analysis by multiple logistic regression analysis. The significance level was set at P < 0.05. All statistical analyses were performed using SPSS II for Windows (SPSS Japan, Tokyo, Japan).
Results
Demographic characteristics
The median age of the 262 patients enrolled in the study at diagnosis was 64 years, all the patients were Asian, 180 (68.7%) were male, and 82 (31.3%) were female. The demographics and tumor-related factors are summarized in Table 1. The depth of invasion of the primary lesion was assessed as stage T4 in 61 (23.3%) patients. The number of patients with positive nodal status was 158 (60.3%). All of the patients underwent curative resection with systematic D2 lymph node dissection. In all, 41 (15.6%), 116 (44.3%), and 105 (40.1%) patients were classified as having stage I, stage II and stage III disease, respectively. The type of surgical intervention used was total gastrectomy in 84 (32.1%) patients, distal and proximal gastrectomy in 169 (64.5%) and 8 (3.1%) patients, respectively, and pancreatoduodenectomy in 1 (0.4%) patient. The histological features are given in Table 1. Of the 262 patients, 115 (43.9%) were classified into the well-differentiated tumor category and 143 (54.6%) into the poorly differentiated tumor category. Mucinous carcinoma was diagnosed in 4 (1.5%) patients. The median follow-up period of the surviving cases was 54.5 months, and 36 (13.7%) patients died within 5 years of surgery. The overall cumulative 3-year (3Y-OS) and 5-year (5Y-OS) survival rates were 87.8 and 82.9%, respectively.
Relationship between the peripheral blood parameters and tumor-related factors
In all 262 patients, the median levels of the all serum parameters were within the normal ranges. The data for each T stage were analyzed and are presented in Table 2. An increase in the NLR (P = 0.004) and CRP (P = 0.017) and a decrease in the Lym (P = 0.032), Hb (P < 0.001), and CEA (P = 0.029) in a T stage-dependent manner were observed. The data were also compared in relation to the nodal status and histological features of the tumors (data not shown). Patients with nodal positivity showed only decreased serum Hb and Alb levels compared to the patients with node-negative status. Poorly differentiated tumors were associated with decreased Mono and CEA.
Predictive significance of parameters for T4 tumor stage
The peripheral hematological parameters showed a greater degree of correlation with the T stage than with the nodal status or histological features. Thus, the predictive value of the parameters for T4 disease, which was defined as the presence of serosal invasion according to the UICC TNM classification [23], was assessed.
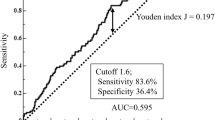
The ROC curves for the continuous variables of NLR constructed for predicting T4 disease are shown in Fig. 1. The area under the curve (AUC) was recorded as 0.605 (95% confidence interval [CI], 0.525–0.684) for the NLR. The sensitivity, specificity, positive predictive value, and negative predictive value of NLR >3.2 (n = 103) for predicting T4 disease were 55.7, 65.7, 33.0, and 83.0%, respectively. We defined high NLR according to the cutoff values mentioned above, which yielded the best combination of predictive values.
Next, the predictive value of the NLR was compared with those of the other parameters. Univariate and multivariate logistic regression analyses to determine the predictive factors for T4 disease were performed, as shown in Table 3. Significant differences in the odds ratio (OR) were observed for differential levels of Hb (OR = 2.020, 95% CI = 1.126–3.624; P = 0.018), NLR (OR = 2.036, 95% CI = 1.139–3.639; P = 0.026), CA19-9 (OR = 2.175, 95% CI = 1.020–4.640; P = 0.044), and poorly differentiated histology (OR = 3.061, 95% CI = 1.602–5.848; P = 0.001). According to multiple logistic regression analysis, Hb (OR = 1.875, 95% CI = 1.005–3.500; P = 0.048), NLR (OR = 2.206, 95% CI = 1.187–4.100; P = 0.012), and poorly differentiated histology (OR = 3.134, 95% CI = 1.593–6.167; P = 0.001) were identified as independent predictive factors for T4 disease.
Discussion
Blood samples were easily assessable and reliable factors for preoperative prediction. In particular, NLR has been reported to be a prognostic factor in gastric cancer patients [15, 17, 20–22]. We analyzed the relationship between NLR and tumor-related factors in patients with wall-penetrating gastric cancer, for whom we sometimes need to consider neoadjuvant therapies. A large phase III trial demonstrated the efficacy of neoadjuvant chemotherapy in similar patients who were estimated to be stage II or higher with neither distant metastases nor locally advanced inoperable disease [5]. In the present study, NLR was correlated with T stage rather than with the nodal status or the histological features. The result suggested that the tissue damage and remodeling around invasive tumors had an effect on the systemic inflammatory response. The prognostic value of NLR in gastric cancer might depend on the T stage.
Neutrophils represent early acute inflammation and migrate to the affected sites to neutralize and eliminate potentially injurious stimuli [24]. Increased neutrophil counts have been observed in patients with gastric cancer [25]. Likewise, the production of tumor-promoting inflammatory chemokines and cytokines has been shown to trigger the recruitment of myeloid cells to most tumors related to inflammation, and gastric cancer cells overexpress C-X-C motif chemokine ligand 8 (CXCL8, known as IL-8), which induces migration of the chemokine receptor CXCR1 (also known as IL-8 receptor α) expressed on neutrophils across the tumor site [25]. Recruited neutrophils, along with the tumor-associated macrophages (TAM), have been shown to be a major source of matrix metalloproteinase 9 (MMP9) in various murine tumor models [26]. The prior partial degradation of the extracellular matrix by MMPs allows cell infiltration into the tissue [27]; in addition, vascular endothelial growth factor A (VEGF-A), derived from TAM, mediates endothelial cell mitogenesis and vascular permeability [28]. The present study demonstrated a T-stage-dependent increase in the neutrophil count in the peripheral blood (Table 2), reflecting recruitment of neutrophils from the bone marrow to the tumor site; however, no statistically significant differences were observed.
The lymphocytopenia was presumably a part of an immune-tolerated microenvironment around the tumor and has been suggested as an independent prognostic factor in several cancers [18]. Interestingly, a significant decrease in the lymphocyte count was also observed in a T-stage-dependent manner (Table 2). This result indicated the possibility that neutrophil-induced tissue damage and remodeling around the tumor site contributed to the establishment of the host’s adaptive immunity.
T4 disease has newly been defined in the 7th edition of the UICC TNM classification as a tumor perforating the serosa or invading adjacent structures [23], and includes remodeling through all the gastric layers. In cases with T4 disease, the neutrophil counts reached their peak, while the lymphocyte counts reached their nadir, yielding the maximum NLR (Table 2). An earlier study also reported a consistent T-stage-dependent increase in NLR in gastric cancer [20]. Furthermore, a high NLR, defined as >3.2 in the present study, was an independent predictor of T4 disease according to multiple logistic regression analysis, as was a decrease in the Hb and poorly differentiated tumor histology. Anemia presumably reflected the blood loss at the tumor site. CA19-9 was correlated with the T stage but was not an independent predictive factor for T4 disease. The histological differentiation grade was the most reliable risk factor, but the histology of biopsy samples obtained through endoscopic examination did not always match that of the corresponding resected specimens. Although serum CRP and Alb have been previously suggested as inflammation-based prognostic factors in advanced cancers [16, 29, 30], both were not meaningful predictive factors for T4 disease. Therefore, the high baseline of NLR was suggested as a valuable predictive factor for T4 disease, and its predictive value was superior to serum tumor markers.
The clinical diagnostic accuracy rate for T4 disease was 50.3% in the present study. This low accuracy rate was attributable to the conventional axial CT images without gastric water filling or radiological examinations. Actually, it was known that the clinical TNM staging of gastric cancer before treatment was not in accord with pathological staging in a substantial number of cases. Recently, the accuracy of preoperative staging of gastric cancer was improved by MDCT with multiplanar reconstruction (MPR) images. The diagnostic accuracy of MDCT for T4 disease was reported to be up to 93% [8–11], and a prospective study to confirm the reproducibility in a larger sample is awaited. Though the NLR seems to add valuable preoperative information to clinical TNM staging, the utility of NLR still requires the comparison with novel imaging modalities like MDCT before the introduction into clinical practice.
In conclusion, we found NLR as an independent predictive factor for T4 disease to be superior to other serum factors. The clinical utility of NLR still needs to be confirmed with prospective analysis.
References
Jemal A, Siegel R, Ward E et al (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Noguchi Y, Yoshikawa T, Tsuburaya A et al (2000) Is gastric carcinoma different between Japan and the United States? Cancer 89:2237–2246
Maruyama K, Okabayashi K, Kinoshita T (1987) Progress in gastric cancer surgery in Japan and its limits of radicality. World J Surg 11:418–425. doi:10.1007/BF01655804
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Cunningham D, Allum WH, Stenning SP et al (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Macdonald JS, Smalley SR, Benedetti J et al (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725–730
Brennan MF (2005) Current status of surgery for gastric cancer: a review. Gastric Cancer 8:64–70
Chen CY, Hsu JS, Wu DC et al (2007) Gastric cancer: preoperative local staging with 3D multi-detector row CT—correlation with surgical and histopathologic results. Radiology 242:472–482
Habermann CR, Weiss F, Riecken R et al (2004) Preoperative staging of gastric adenocarcinoma: comparison of helical CT and endoscopic US. Radiology 230:465–471
Hwang SW, Lee DH, Lee SH et al (2010) Preoperative staging of gastric cancer by endoscopic ultrasonography and multidetector-row computed tomography. J Gastroenterol Hepatol 25:512–518
Kumano S, Murakami T, Kim T et al (2005) T staging of gastric cancer: role of multi-detector row CT. Radiology 237:961–966
de Graaf GW, Ayantunde AA, Parsons SL et al (2007) The role of staging laparoscopy in oesophagogastric cancers. Eur J Surg Oncol 33:988–992
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Mantovani A, Allavena P, Sica A et al (2008) Cancer-related inflammation. Nature 454:436–444
Aliustaoglu M, Bilici A, Ustaalioglu BB et al (2010) The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol 27(4):1060–1065
Crumley AB, McMillan DC, McKernan M et al (2006) An elevated C-reactive protein concentration, prior to surgery, predicts poor cancer-specific survival in patients undergoing resection for gastro-oesophageal cancer. Br J Cancer 94:1568–1571
Mohri Y, Tanaka K, Ohi M et al (2010) Prognostic significance of host- and tumor-related factors in patients with gastric cancer. World J Surg 34:285–290. doi:10.1007/s00268-009-0302-1
Ray-Coquard I, Cropet C, Van Glabbeke M et al (2009) Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res 69:5383–5391
Satomi A, Murakami S, Ishida K et al (1995) Significance of increased neutrophils in patients with advanced colorectal cancer. Acta Oncol 34:69–73
Shimada H, Takiguchi N, Kainuma O et al (2010) High preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancer. Gastric Cancer 13:170–176
Ubukata H, Motohashi G, Tabuchi T et al (2010) Evaluations of interferon-gamma/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol 102(7):742–747
Yamanaka T, Matsumoto S, Teramukai S et al (2007) The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology 73:215–220
Sobin LH, Wittekind C (eds) (2010) TMN Classification of Malignant Tumours (UICC). Wiley-Blackwell, Chichester, UK
Serhan CN, Brain SD, Buckley CD et al (2007) Resolution of inflammation: state of the art, definitions and terms. FASEB J 21:325–332
Eck M, Schmausser B, Scheller K et al (2003) Pleiotropic effects of CXC chemokines in gastric carcinoma: differences in CXCL8 and CXCL1 expression between diffuse and intestinal types of gastric carcinoma. Clin Exp Immunol 134:508–515
Coussens LM, Tinkle CL, Hanahan D et al (2000) MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 103:481–490
Kido S, Kitadai Y, Hattori N et al (2001) Interleukin 8 and vascular endothelial growth factor—prognostic factors in human gastric carcinomas? Eur J Cancer 37:1482–1487
Yancopoulos GD, Davis S, Gale NW et al (2000) Vascular-specific growth factors and blood vessel formation. Nature 407:242–248
Crumley AB, Stuart RC, McKernan M et al (2008) Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG-ps) in patients receiving palliative chemotherapy for gastroesophageal cancer. J Gastroenterol Hepatol 23:e325–e329
McMillan DC (2008) An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer. Proc Nutr Soc 67:257–262
Disclosure
The authors have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aizawa, M., Gotohda, N., Takahashi, S. et al. Predictive Value of Baseline Neutrophil/Lymphocyte Ratio for T4 Disease in Wall-Penetrating Gastric Cancer. World J Surg 35, 2717–2722 (2011). https://doi.org/10.1007/s00268-011-1269-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-011-1269-2