Abstract
We aimed to investigate the prognostic significance of neutrophil, lymphocyte, platelet, mean platelet value (MPV), platelet-lymphocyte ratio (PLR) and neutrophil–lymphocyte ratio (NLR) in patients with locally advanced gastric cancer (LAGC). One hundred sixty-eight patients with LAGC who had been followed-up between 2004 and 2008 were included in present study. The results of hematological (platelet, lymphocyte, neutrophil and MPV) and biochemical (uric acid and LDH) parameters were evaluated before treatment. NLR was divided into two groups as <2.56 and ≥2.57 and PLR was also divided into two groups as ≤160 and >160. Platelet counts and lymphocyte counts were also divided into two groups; ≤300.000/mm3 and >300.000/mm3, and <1,500/mm3 and ≥1,500/mm3, respectively. Results were evaluated with Kaplan–Meier and Long-rank tests. The mean age of patients at diagnosis was 60.1 ± 12.1 and 114 of patients (67.8%) were male. For 168 patients, 48 months overall survival (OS) rate was 45.2% and the median OS was 39 months (range 33–44). In patients whose PLR was less than 160 (n = 54), the median OS was 45 months (range 38–52) and also for cases whose PRL was greater than 160 (n = 114), the median OS was 27 months (range 22–32) (p = 0.006). While for fifty patients whose lymphocyte counts were less than 1,500, the median OS was 27 months (range 21–33), in cases with high lymphocyte counts (≥1,500) (n = 118), it was 41 months (range 35–48) (p = 0.03). The median OS was 41 (range 34–48) and 30 (range 23–37) months in two platelets groups, respectively (p = 0.24). However, in the patients whose NLR was less than 2.56 (n = 107), median OS was better than with cases whose NLR was greater than or equal to 2.56 (42 vs. 27 months). Routine peripheral blood counts may be useful prognostic factor for evaluating the accuracy of risk stratification in patients with radically resected gastric cancer Our results need to be confirmed by study including larger sample size in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Assessment of the inflammatory response to the tumor may be easier and more-cost effective in clinical practice. Therefore, the role of immune system on disease cessation or progression has been recently investigated, and some hematological parameters including leukocyte counts have been shown as both a diagnostic and prognostic factors in various types of malignancies [1–5]. Furthermore, the neutrophil to lymphocyte ratio (NLR) has been documented as a simple index of systemic inflammatory response in critically ill patients with malignancy [6–9]. Similary, preoperative platelet to lymphocyte ratio (PLR) has been also suggested as an independent significant prognostic indicator in pancreatic cancer [10].
Gastric cancer is one of the most common cancer and the leading cause of cancer death and mortality in both sexes [11]. Altered immune response to gastric cancer has been previously reported [12, 13]. Both NLR and thrombocytosis have seperately investigated as a prognostic factors in gastric cancer [7, 9, 14]. In this study, we aimed to assess the prognostic values of pretreatment lymphocyte, neutrophil, platelet counts, mean platelet volume (MPV), NLR and PLR in patients with gastric cancer.
Materials and methods
Between 2004 and 2008, a total of 168 patients with locally-advanced gastric cancer who followed-up in the Department of Medical Oncology outpatient clinics, were included in the study. Patients’ hematological parameters (neutrophil, lymphocyte, thrombocyte, MPV were measured before treatment. The exclusion criteria were history of blood transfusion within the last two months, active bleeding, bleeding diathesis, hyper- or hypothyroidism, infections, disseminated intravascular coagulation, heparin treatment or connective tissue disease.
Venous blood samples taken from patients admitted to the oncology outpatient clinic for adjuvant chemotherapy, and were collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes. Blood counts were analyzed on a Sysmex hemocounter before the initiation of chemotherapy. The results of these pretreatment blood counts were retrospectively evaluated. Patients were staged according to the American Joint Committee on Cancer (AJCC) Staging Manuel. Demographical data of the patients were recorded and survival data were extracted from patients’ charts. The results of pretreatment hematologic tests consisting of neutrophil, lymphocyte and platelet counts and MPV and pretreatment clinical biochemical tests, including serum total and direct bilirubin, urea, creatinine, albumin and lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) enzyme activities were also evaluated.
NLR
NLR was calculated as neutrophil count divided by lymphocyte count. The calculated values were defined at two categories as <2.56 and ≥2.56. [2].
PLR
PLR was calculated as platelet count divided by lymphocyte count. The calculated values were divided into two categories as ≤160 or >160 [3].
Lymphocyte, and platelet counts
Lymphocyte counts were divided into two categories as less than 1,500/mm3 and equal or greater than 1,500/mm3. Similarly platelet counts defined as less than or equal to 300,000/mm3 and greater than 300,000/mm3 [2, 4, 5].
Statistical analysis
Frequency, percent ratio, arithmetic mean, standard deviation, 95% confidence intervals and mean values were used for the description of the variables. The Kolmogorov–Smirnov test was used to test the fitness of the continuous variables to normal distribution. Variables following a standard normal distribution were compared by the Student’s t-test and Mann–Whitney-U test was used for non-parametric independent group comparison. Survival analyses were performed by the Kaplan–Meier test. Overall survival (OS) was described as the time from diagnosis to the date of the patient’s death or last known contact. The patient data available permitted the calculation of 12 months survival rates thus the overall survivals of the cases were defined for a period of one year. Survival rates of the subgroups were compared by the Log-Rank test. P-values less than or equal to 0.05 were considered to be statistically significant.
Results
Of the 168 patients in the study, 114 were males (67.8%) and 54 (32.2%) were females. The mean age of the patients at the time of diagnosis was 60.1 ± 12.1 years (ranges 28–83 years). For all patients, 48 months overall survival (OS) rate was 45.2% and the median OS time was 39 months (range 33–44 months). The pretreatment serological and hematological data of the patients are summarized in Table 1.
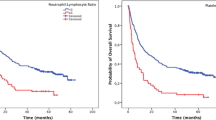
The median OS time was significantly higher for patients whose PLR was less than or equal to 160 (n = 54, 32%), than the patients whose PRL was greater than 160 (n = 114,68%) [45 months (95% CI; 38–52) vs. 27 months (95% CI; 22–32), p = 0.006, Fig. 1.].
A total of 50 patients (30%) had a lymphocyte counts less than 1,500/mm3, while others (70%) had lymphocyte counts were greater than or equal to 1,500/mm3. The median OS interval was significantly higher in patients with lymphocyte counts which were greater than or equal to 1,500/mm3 than those with <1,500/mm3 [41 months (95% CI; 35–48) vs. 27 months (95% CI; 21–33), p = 0.03, Fig. 2].
In 40% of patients, thrombocyte count was detected as greater than 300.000/mm3 and in 13% of patients, >400.000/mm3. Three-years OS rate was 64% and the median survival time was 41 months (95% CI; 34–48 months) in patients whose platelets counts were less than or equal to 300.000/mm3. On the other hand, for patients whose platelets counts were greater than 300.000/mm3, three-years OS rate and the median survival interval were 40% and 30 months (95% CI; 23–37), respectively. These differences were not statistically significant (p = 0.24, Fig. 3). The association of hematological parameters with the median survival time is listed in Table 2.
One hundred and seven patients (63.6%) were detected whose NLR was less than 2.56, while there were 61 patients (36.4%) whose NLR was greater than or equal to 2.56. The median OS duration was significantly higher in patients with NLR < 2.56 than those with NLR ≥ 2.56 (42 months (95% CI; 36–49) vs. 27 months (95% CI; 21–34), p = 0.001). The relationship between NLR, PLR and lymph node involvement or tumor grade was not found significant (p > 0.05).
Discussion
Gastric cancer is often diagnosed at an advanced staged, because screening is not performed routinely in most of the world, except for Japan [15, 16]. Infections, autoimmune diseases, benign and malign tumors are caused chronic inflammations [8]. Inflammatory cells can suppress or stimulate tumor growth. Moreover, inflammation plays a major role in the development and progress of various organ cancers [17–19]. Therefore, hematological markers including neutrophils, lymphocytes, NLR or PLR are recommended as diagnostic and prognostic factors in various types of cancers in recently published studies [1–5].
Firstly, Levin and Conley have been reported thrombocytosis in patients with gastric cancer, but the prognostic significance of thrombocytosis has not been investigated [20]. However, its’ prognostic importance have been observed frequently in patients with gynecological cancers, renal cell carcinoma, and lung cancer [21–23]. The prevalence of thrombocytosis in patients with gynecological cancer was 9.5–38%, 13–60% with primary lung cancer, 56.8% with renal cell carcinoma, and 33% with colon cancer [4, 14, 24, 25]. We found that thrombocyte count was greater than 400.000/mm3 in 13% of patients. Our results were similar to the study of Ikeda et al. [14].
Shimada et al. found that thrombocytosis was present in only 5.1% of patients with esophageal cancer. Furthermore, they showed that the patients with high platelet counts had significantly worse survival than the low platelet group [4]. Similarly, Ikeda et al., indicated that the patients without thrombocytosis had significantly better survival than those with thrombocytosis in gastric cancer patients [14]. In our study, we suggested that the patients whose platelet counts were less than or equal to 300.000/mm3, had better the median survival time than those with platelet counts >300.000/mm3. Although this difference was not statistically significant, it may be noteworthy for clinical practice.
Preoperative PLR has been defined as an independent significant prognostic marker by Smith et al. in resected pancreatic ductal adenocarcinoma [26]. In the same study, the median overall survival in patients with a PLR of 150 or less was 19.7 months, 13.7 months in those with a PLR of 151–300, and 5.8 months in patients with a value of greater than 300. Smith et al. showed elevated preoperative CA 19-9 levels and PLR were associated with poorer survival in patients underwent resection for ampullary adenocarcinoma [3]. In our study, for cases where PLR was less than or equal to 160 (n = 54), the median survival time was 45 months. Nonetheless, the median survival interval was 27 months in patients whose PLR was higher than 160. This difference was significant (p = 0.006).
Some studies indicated that a decreased lymphocyte count in the peripheral blood is a predictor of a poor prognosis in cancer patients [27–29]. Bruckner et al. suggested that a pretreatment absolute neutrophil count <6,000/mm3 and lymphocyte count >1,500/mm3 were independent prognostic indicators of a good prognosis for patients with metastatic gastric cancer [30]. Moreover, Elias et al. investigated mononuclear cell percentages in patients with head and neck cancer, and they showed that high percentages of lymphocytes (≥30%) and low monocyte percentages (<10%) related to a better prognosis [31]. The relation between low lymphocyte counts and poor prognosis has been reported in patients with various tumor types, including ovarian, pancreatic, renal and breast tumors [27, 28, 32]. In our study, we found that the median survival interval was significantly higher in patients with lymphocyte counts which were greater than or equal to 1,500/mm3 (n = 118) than those with <1,500/mm3 (n = 50) (41 months vs. 27 months, p = 0.03).
NLR is considered as a simple indicator of the systemic inflammatory response in critically ill patients [6]. Elevated NLR at the time of diagnosis accompanies low survival rates in ovarian cancer [8]. In addition, an elevated NLR is also a potentially poor prognostic predictor after curative resection for hepatocellular carcinoma [33]. Walsh et al. demonstrated that for patients with colorectal cancers, preoperative NLR value greater than 5 associated with a poor survival rates [34]. Yamanaka et al. found that in patients with NLR value less than 2.5, the median survival time was significantly higher than those with NLR of >2.5 in advanced gastric cancer (363 days vs. 239 days.) [2]. Similarly, in the present study, the patients with an NLR value lower than 2.56 had significantly higher median duration of survival than those with an NLR value of 2.56 or above (42 vs. 27 months, p = 0.001). The reports associated with the effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer were shown in Table 3.
In conclusion, we found that pretreatment routine hematological parameters including lymphocyte, and NLR and PLR were correlated with prognosis in patients with gastric cancer who had undergone curative gastrectomy. Our finding may be useful for evaluating the accuracy of risk stratification and lead to more appropriate clinical management of patients with radically resected gastric cancer, but, our results need to be confirmed by study including larger sample size in future.
References
Teramukai S, Kitano T, Kishida Y, Kawahara M, Kubota K, Komuta K, et al. Pretreatment neutrophil count as an independent prognostic factor in advanced non small cell lung cancer: an analysis of Japan multinational trial organisation LC00–03. Eur J Cancer. 2009;45:1950–8.
Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215–20.
Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, Neoptolemos JP. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19–9 levels and platelet-lymphocyte ratio. J Gastrointest Surg. 2008;12:1422–8.
Shimada H, Oohira G, Okazumi S, Matsubara H, Nabeya Y, Hayashi H, et al. Thrombocytosis associated with poor prognosis in patients with esophageal carcinoma. J Am Coll Surg. 2004;198:737–41.
Rodriguez GC, Clarke-Pearson DL, Soper JT, Berchuck A, Synan I, Dodge RK. The negative prognostic implications of thrombocytosis in women with stage IB cervical cancer. Obstet Gynecl. 1994;83:445–8.
Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14.
Hirashima M, Higuchi S, Sakamoto K, Nishiyama T, Okada H. The ratio of neutrophils to lymphocytes and the phenotypes of neutrophils in patients with early gastric cancer. J Cancer Res Clin Oncol. 1998;124:329–34.
Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23.
Gwak MS, Choi SJ, Kim JA, Ko JS, Kim TH, Lee SM, et al. Effects of gender on white blood cell populations and neutrophil–lymphocyte ratio following gastrectomy in patients with stomach cancer. J Korean Med Sci. 2007;22(Suppl):S104–8.
Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–72.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Hong WS, Hong SI, Kim CM, Kang YK, Song JK, Lee MS, et al. Differential depression of lymphocyte subsets according to stage in stomach cancer. Jpn J Clin Oncol. 1991;21:87–93.
Hong WS, Min YI, Son YS, Hong SI. Peripheral blood lymphocyte subsets in patients with stomach cancer. J Korean Med Sci. 1995;10:164–8.
Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, et al. Satomi T Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9:287–91.
Lawrence W, Menck HR, Steele GD, Winchester DP. The national cancer data base report on gastric cancer. Cancer. 1995;75:1734–44.
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50.
Hussein MR, Ahmed RA. Analysis of the mononuclear inflammatory cell infiltrates in the non-tumorigenic, pre-tumorigenic and tumorigenic keratinocytic hyperproliferative lesions of the skin. Cancer Biol Ther. 2005;4:819–21.
Altinoz MA, Korkmaz R. NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer. Neoplasma. 2004;51:239–47.
Jaiswal M, LaRusso NF, Gores GJ. Nitric oxide in gastrointestinal epithelial cell carcinogenesis: linking inflammation to oncogenesis. Am J Physiol Gastrointest Liver Physiol. 2001;281:626–34.
Levin J, Conley CL. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964;114:497–500.
Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996;9:1826–30.
Monreal M, Fernandez-Llamazares J, Piñol M, Julian JF, Broggi M, Escola D, et al. Platelet count and survival in patients with colorectal cancer-a preliminary study. Thromb Haemost. 1998;79:916–8.
Lopes A, Daras V, Cross PA, Robertson G, Beynon G, Monaghan JM. Thrombocytosis as a prognostic factor in women with cervical canceri. Cancer. 1994;74:90–2.
Symbas NP, Townsend MF, El-Galley R, Keane TE, Graham SD, Petros JA. Poor prognosis associated with thrombocytosis in patients with renal cell carcinoma. BJU Int. 2001;87:715–6.
Costantini V, Zacharski LR, Moritz TE, Edwards RL. The platelet count in carcinoma of the lung and colon. Thromb Haemost. 1990;64:501–5.
Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–72.
Blake-Mortimer JS, Sephton SE, Carlson RW, Stites D, Spiegel D. Cytotoxic T lymphocyte count and survival time in women with metastatic breast cancer. Breast J. 2004;10:195–9.
Fumagalli LA, Vinke J, Hoff W, Ypma E, Brivio F, Nespoli A. Lymphocyte counts independently predict overall survival in advanced cancer patients: a biomarker for IL-2 immunotherapy. J Immunother. 2003;26:394–402.
Fogar P, Sperti C, Basso D, Sanzari MC, Greco E, Davoli C, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32:22–8.
Bruckner HW, Lavin PT, Plaxe SC, Storch JA, Livstone EM. Absolute granulocyte, lymphocyte, and moncyte counts. Useful determinants of prognosis for patients with metastatic cancer of the stomach. JAMA. 1982;247:1004–6.
Elias EG, Leuchten JM, Buda BS, Brown SD. Prognostic value of initial mononucleated cell percentages in patients with epidermoid carcinoma of the head and neck. Am J Surg. 1986;152:487–90.
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13.
Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757–62.
Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ, et al. Neutrophil–lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–4.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aliustaoglu, M., Bilici, A., Ustaalioglu, B.B.O. et al. The effect of peripheral blood values on prognosis of patients with locally advanced gastric cancer before treatment. Med Oncol 27, 1060–1065 (2010). https://doi.org/10.1007/s12032-009-9335-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12032-009-9335-4