Abstract
Background
We investigated the effect of a combination of pregabalin and dexamethasone, when used as part of a multimodal analgesic regimen, on pain control after rhinoplasty operations.
Methods
Sixty patients were enrolled in this study. They were randomly assigned into three groups: Group C (placebo + placebo), Group P (pregabalin + placebo), and Group PD (pregabalin + dexamethasone). Patients received either pregabalin 300 mg orally 1 h before surgery, dexamethasone 8 mg intravenously during induction, or placebo according to their allocation. Postoperative pain was treated with intravenous patient-controlled analgesia (tramadol, 20-mg bolus dose, 45-min lockout time). The numeric rating scale (NRS), side effects, and consumption of tramadol, pethidine, and ondansetron were assessed.
Results
The median NRS scores at 0, 1, and 6 h after surgery were significantly higher in Group C than in Group PD (p < 0.001 for all). The 24-h consumption of tramadol and pethidine was significantly reduced in Groups P and PD compared to Group C (p < 0.01 and p < 0.01). The total tramadol consumption was decreased by 54.5 % in Group P and 81.9 % in Group PD compared to Group C (p < 0.001 for both). The incidence of nausea was higher in Group C than in Groups P and PD between the postoperative 0–2 and 0–24-h periods (p < 0.05 for both). The frequency of blurred vision was significantly higher in Groups P and PD than in Group C within the 0–24-h period (p < 0.05 for both).
Conclusion
We found that the addition of a single dose of pregabalin and dexamethasone to multimodal analgesia in rhinoplasty surgeries provided efficient analgesia and thus decreased opioid consumption.
Level of Evidence I
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhinoplasty surgeries are more traumatic and lead to more painful surgical procedures in the postoperative period than septoplasty surgeries [32, 33, 38]. Pain is more intense during the first 3 days (especially in the first day) after the procedure [33]. The control of this pain will decrease the hospitalization time of the patient and prevent complications due to pain, thus enhancing the comfort of the patient [30, 35]. Multimodal analgesia is frequently used to combat acute pain [20, 36, 39]. The combined utilization of opioid and nonopioid analgesics increases the analgesic effect and decreases the drug dose and the side effects [20].
Pregabalin is a synthetic analog of γ-aminobutyric acid that exerts analgesic, antiepileptic, and anxiolytic properties [9]. Its antihyperalgesic efficiency in a human pain model has been demonstrated [4]. Its oral bioavailability is approximately 90 %. Following intake, it is absorbed by the gastrointestinal system and reaches a maximum plasma concentration within 1 h [1, 9, 18]. Clinical studies have demonstrated the efficiency of this drug in controlling postoperative pain and in decreasing analgesic consumption [17, 21, 22].
The strong anti-inflammatory effect and the antiemetic and analgesic efficiency of glucocorticoids have been demonstrated [19, 28]. These effects have been shown for dexamethasone and methylprednisolone in many clinical studies [2, 13, 14, 26, 27, 34]. Dexamethasone has been used alone and in combination with other analgesics to increase their effects [3, 15]. However, there is no study showing the effect of pregabalin either alone or in combination with dexamethasone used after rhinoplasty operations. The aim of this study was to determine the contribution of pregabalin and the addition of dexamethasone to pregabalin to the efficiency of the multimodal analgesic system before rhinoplasty surgeries.
Methods
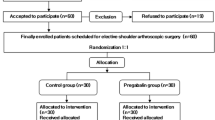
Sixty patients (ASA I-II, age = 18–75 years) who were waiting for elective rhinoplasty were included in this prospective, randomized, double-blind study. Exclusion criteria were insufficient cooperation (e.g., psychiatric disorder, dementia), regular use of drugs (benzodiazepines, corticosteroids, tricyclic antidepressants, NSAIDs, or other analgesic drugs), preoperative current use of pregabalin or gabapentin, a history of allergy to any of the study medications, pregnancy, obesity (body mass index >35 kg/m2), or significant cardiac, pulmonary, hepatic, or renal disease.
A numeric rating scale (NRS) was used to quantify pain (0 mm = no pain, 10 mm = worst pain imaginable). The patients were informed about the NRS and the use of patient-controlled analgesia (PCA).
The patients were randomly placed into one of three study groups in a 1:1 ratio by means of a computer-generated list of random numbers: Group P (pregabalin group), Group PD (pregabalin + dexamethasone group), and Group C (control group). Pregabalin (Lyrica, Pfizer) in a 300-mg capsule was administered orally with 10 ml of water 1 h before surgery to the patients in the study groups (Groups P and PD), and a similar-looking placebo capsule was given to the controls (Group C) by the same researcher who was not involved in the intraoperative or postoperative treatment and data collection. The exact same packaging was used for the placebo capsules as the active capsules.
In the operating room, intravenous (i.v.) access was established with a 20-G i.v. cannula, and blood pressure, peripheric oxygen saturation (SpO2), and ECG were monitored. The induction of anesthesia was achieved with remifentanil (Ultiva Flk., GlaxoSmithKline) 1 mcg kg−1 min−1, propofol (Propofol Amp. Fresenius Kabi) 2 mg kg−1, and rocuronium (Esmeron Flk., Organon) 0.6 mg kg−1. After 1 min, the infusion dose of remifentanil was reduced to 0.5 mcg kg−1 min−1. The patients in Group PD were administered dexamethasone (Deksamet 8 mg, Osel) intravenously after endotracheal intubation and stabilization. Group P and Group C received 2 ml i.v. saline solution as a placebo. During the first 10 min, the anesthesia was maintained with 2 % sevoflurane, which was inspired at a fresh gas flow rate of 3 l min−1, in combination with 65 % nitrous oxide. Subsequently, sevoflurane was reduced to 1 % by keeping a fixed concentration of nitrous oxide. To maintain the patients’ end-tidal CO2 values between 34 and 38 mmHg, they were mechanically ventilated. The infusion of remifentanil was adjusted to maintain a mean arterial pressure (MAP) of 65–100 mmHg. The fluid infusion was increased when the MAP was below 60 mmHg. In the event of hypotension, the infusion of remifentanil was ceased, and ephedrine HCl i.v. 10 mg (ephedrine 0.05 mg ml−1, Osel) was given. Intravenous atropine sulfate (atropine sulfate 1 mg ml−1, Galen) 0.5 mg was administered if bradycardia occurred.
The surgeons injected a mixture of lidocaine HCl (20 mg ml−1) and epinephrine (0.0125 mg ml−1) locally to the septal tissue before surgery. Thirty minutes before the end of surgery, 50 mg tramadol HCl was administered intravenously, and 75 mg diclofenac sodium (Diclomec, 75 mg ml−1, Abdi Ibrahim) was injected intramuscularly for postoperative pain. Remifentanil infusion and sevoflurane inhalation were stopped, and the patient was ventilated with 100 % O2 when the operation was completed. A combination of atropine sulfate (0.15 mg kg−1) and neostigmine (40 mcg kg−1) was used to reverse the residual neuromuscular block. The patients were extubated in the operating room when adequate spontaneous ventilation was established. Intraoperative data (total remifentanil dose, duration of surgery, and hemodynamic parameters) were recorded.
The patients were admitted to the post anesthesia care unit (PACU) for at least 1 h until complete recovery. They were connected to a PCA device (Abbot Pain Management Provider, Abbot Laboratories, North Chicago, IL, USA) and given tramadol HCl (20-mg bolus dose, 45-min lockout time). The NRS values were recorded at 30 min and 1 h in the PACU and at 2, 4, 8, 12, and 24 h in the inpatient unit by the same researcher who was totally unaware of the patient groups. Side effects were also evaluated by the same researcher. Pethidine HCl (Aldolan 100 mg 2 ml−1, Gerot) was administered (50 mg i.v.) as a rescue analgesia if the NRS value was >4. Nausea and vomiting were treated with 4 mg ondansetron HCl i.v. (Zofran 4 mg 2 ml−1, GlaxoSmithKline).
The amount of pethidine used, the number of patients who received pethidine, the time of the first administration of pethidine, side effects (nausea, vomiting, dizziness, blurred vision, headache, loss of concentration, itching, and other), tramadol consumption between the 0–1, 0–12, and 12–24 h, and total tramadol consumption were recorded. PCA ended on the second day, and diclofenac was started, given orally twice a day for analgesia.
This study was conducted in accordance with the ethical principles described in the most recent version of the Declaration of Helsinki. Ethical approval was provided by the Clinical Ethical Committee of Abant Izzet Baysal University, Bolu, Turkey. Written informed consent was obtained from all the participants.
Statistical Methods and Sample Size
Data analysis was performed using SPSS statistical software ver. 11.5 for Windows (SPSS, Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used to determine whether the distributions were significantly different. The data are given as mean ± SD for the continuous variables, median (minimum–maximum) for the ordinal variables, and frequency with percent for the categorical variables. One-way analysis of variance (ANOVA) was used to assess the significance of differences between the groups where appropriate. The Kruskal–Wallis test was used to test for significant differences between median values. When the p values from the ANOVA and the Kruskal–Wallis tests were statistically significant, Tukey’s HSD post hoc test or Conover’s nonparametric multiple comparison test was used to determine which measurements differed from the others. Categorical comparisons were made using the χ 2 test. A p value less than 0.05 was considered significant. The Bonferroni adjustment was used for all multiple comparisons to control type I errors.
Results
Sixty patients were enrolled in the study. The patients were randomly assigned to three groups. No statistically significant differences were found between the groups in terms of their age, sex, demographics, and clinical characteristics (p > 0.05 for all) (Table 1). The groups did not differ with respect to mean heart rate and mean arterial blood pressure at all time points (p > 0.00625 for all). The changes in percent of mean heart rate and mean arterial blood pressure values were not statistically different in the intraoperative period compared to the preoperative baselines (p > 0.002 for both).
Group P and Group PD exhibited statistically significant lower tramadol consumption compared to Group C within the 0–24-h postoperative period (p < 0.01 for all). There was a statistically significant difference between Group P and Group PD with respect to tramadol consumption (p = 0.016) only in the first 1-h period. Table 2 and Fig. 1 give the mean tramadol consumption (mg) of the groups within the first 24-h period.
There was no statistically significant difference between the groups according to the median NRS scores, except at the 0, 1, and 6 h (p > 0.00625). The median NRS scores at the 0, 1, and 6 h were significantly higher in Group C than in Group PD (p < 0.001 for all). Table 3 and Fig. 2 give the mean NRS scores within the first postoperative 24 h.
The frequency of pethidine use in Group P and Group PD was significantly lower than in Group C (p < 0.01 for both). The administration time of pethidine was not different among the groups. The frequency of ondansetron use was significantly lower for patients in Group P than for those in Group C (p < 0.05). The administration time of pethidine in patients who were given ondansetron was not significantly different among the groups (p = 0.068). Table 4 gives the frequency of postoperative rescue analgesia and ondansetron use of the groups.
The incidence of nausea was higher in Group C than in Group P and Group PD between the postoperative 0–2 and 0–24-h periods (p < 0.05 for both). The frequency of blurred vision was significantly higher in Group P and Group PD than in Group C within the 0–24-h period (p < 0.05 for both). The groups did not differ with respect to other complications (p > 0.05 for all). Table 5 gives the incidence of observed adverse effects in the groups.
Discussion
This prospective, randomized, double-blinded study showed that a preoperative pregabalin and PD combination decreased NRS values and tramadol and rescue analgesic needs after rhinoplasty surgeries with osteotomy. Rhinoplasty surgeries are more painful than septoplasty surgeries. Osteotomy also leads to more acute pain in the postoperative period [10, 31–33]. In addition to this painful surgery, the nasal packing increases the pain level [29].
Tramadol is associated with fewer side effects than other opioids, thus increasing its use after surgeries with moderate to severe pain. In studies of tramadol and diclofenac, combined use has been shown to be more efficient than individual use [25, 37]. In our study, the control group needed more rescue analgesic in the early postoperative period. The higher nausea rate of the control group was attributed to the greater need for pethidine because the nausea was observed mostly after patients used pethidine. Tramadol also presents an antinociceptive effect in common with pregabalin and dexamethasone, but it has a greater analgesic effect [11, 12, 24]. In third-molar surgery, when tramadol was used in combination with dexamethasone, the drug was more efficient, especially in the first 12 h during which the rescue analgesic needs are lower [7].
Pregabalin has also been used in pain control in many studies, and its efficiency in decreasing pain has been demonstrated [17, 21, 22]. The most commonly encountered side effects of pregabalin are dizziness, dry mouth, somnolence, peripheral edema, and blurred vision [9]. Pregabalin should be tapered gradually to minimize the potential of increased seizure frequency in patients who have seizures. Known hypersensitivity to pregabalin is a contraindication for its use. The current study revealed a decrease in the NRS scores of the group that received pregabalin in the postoperative period. Moreover, pregabalin contributed to a decrease in daily tramadol consumption (54.5 %) and rescue analgesic needs (60 %), thus contributing significantly to the analgesia. In the study groups, the low opioid consumption decreased the incidence of nausea and led to lower ondansetron administration. In the control group, the low NRS scores at the 1–24 h-time point were attributed to the administration of pethidine to 50 % of the patients and to the high tramadol consumption.
The most frequently observed side effect in the study groups was blurred vision (30 % of Group P and 25 % of Group PD). Other studies determined that the incidence of pregabalin-associated side effects increases proportionally to the dose [9, 16, 17]. Jokela et al. [8] reported 20 and 27 % of blurred vision in study groups administered 75 and 150 mg pregabalin, respectively, in laparoscopic gynecological surgery. In another study [17], 150 and 300 mg of pregabalin was administered before surgery and 12 h after surgery, respectively. The authors observed that side effects increased proportionally with the dose. The incidence of blurred vision in 150- and 300-mg pregabalin groups on the first postoperative day in that study was 50 and 63 %, respectively [14]. In the current study, the lower incidence of side effects associated with the 300-mg dose of pregabalin compared to that reported by Jokela et al. [17] is associated with the single-dose administration. In most of the patients in the present study, blurred vision disappeared within 1 h following the operation.
The analgesic efficiency of dexamethasone has been shown in many studies [3, 14, 26]. Some studies have demonstrated that treatment with pregabalin combined with dexamethasone increases the analgesic efficiency [5] but that the addition of dexamethasone does not have any additional effect [21–23]. The current study showed that the addition of dexamethasone to pregabalin leads to a greater decrease in the NRS and in opioid consumption compared to pregabalin alone. These findings can be explained by the analgesic efficiency of dexamethasone which has been reported in the literature [3, 14, 26]. Although the antiemetic efficiency of dexamethasone has been cited in the literature [6], the rate of nausea and vomiting in the present study in the dexamethasone group was similar to that of the pregabalin group. The other side effects were similar to those of the pregabalin group.
One of the main limitations of our study is its small sample size. We also did not evaluate the NRS scores on the second and third postoperative days because all the patients were discharged one day after surgery.
We conclude that the addition of single-dose pregabalin and dexamethasone to multimodal analgesia in rhinoplasty surgeries provides efficient analgesia and thus decreases opioid consumption. We think that this combination will provide efficient and reliable analgesia in rhinoplasty surgeries.
References
Ben-Menachem E (2004) Pregabalin pharmacology and its relevance to clinical practice. Epilepsia 45(suppl 6):13–18. doi:10.1111/j.0013-9580.2004.455003.x
Bigat Z, Boztug N, Hadimioglu N, Cete N, Coskunfirat N, Ertok E (2006) Does dexamethasone improve the quality of intravenous regional anesthesia and analgesia? A randomized, controlled clinical study. Anesth Analg 102:605–609. doi:10.1213/01.ane.0000194944.54073.dd
Bisgaard T, Klarskov B, Kehlet H, Rosenberg J (2003) Preoperative dexamethasone improves surgical outcome after laparoscopic cholecystectomy: a randomized double-blind placebo-controlled trial. Ann Surg 238:651–660. doi:10.1097/01.sla.0000094390.82352.cb
Chizh BA, Gohring M, Troster A, Quartey GK, Schmelz M, Koppert W (2007) Effects of oral pregabalin and aprepitant on pain and central sensitization in the electrical hyperalgesia model in human volunteers. Br J Anaesth 98:246–254. doi:10.1093/bja/ael344
Choi YS, Shim JK, Song JW, Kim JC, Yoo YC, Kwak YL (2013) Combination of pregabalin and dexamethasone for postoperative pain and functional outcome in patients undergoing lumbar spinal surgery: a randomized placebo-controlled trial. Clin J Pain 29:9–14. doi:10.1097/AJP.0b013e318246d1a9
De Oliveira GS, Castro-Alves LJ Jr, Ahmad S, Kendall MC, McCarthy RJ (2013) Dexamethasone to prevent postoperative nausea and vomiting: an updated meta-analysis of randomized controlled trials. Anesth Analg 116:58–74. doi:10.1213/ANE.0b013e31826f0a0a
de Sousa SJA, da Silva LC, de Santana ST, Junior LRM, de Assuncao OAC, Brandao JR (2012) Comparative study of tramadol combined with dexamethasone and diclofenac sodium in third-molar surgery. J Craniomaxillofac Surg 40:694–700. doi:10.1016/j.jcms.2012.01.001
Dursteler C, Miranda HF, Poveda R, Mases A, Planas E, Puig MM (2007) Synergistic interaction between dexamethasone and tramadol in a murine model of acute visceral pain. Fundam Clin Pharmacol 21:515–520. doi:10.1111/j.1472-8206.2007.00511.x
Gajraj NM (2007) Pregabalin: its pharmacology and use in pain management. Anesth Analg 105:1805–1815. doi:10.1213/01.ane.0000287643.13410.5e
Granier M, Dadure C, Bringuier S, Bonnet-Boyer MC, Ryckwaert Y, Loriaux E, Capdevila X (2009) Intranasal lidocaine plus naphazoline nitrate improves surgical conditions and perioperative analgesia in septorhinoplasty surgery. Can J Anaesth 56:102–108. doi:10.1007/s12630-008-9020-7
Grond S, Sablotzki A (2004) Clinical pharmacology of tramadol. Clin Pharmacokinet 43:879–923
Guneli E, Yavasoglu NUK, Apaydin S, Uyar M (2007) Analysis of the antinociceptive effect of systemic administration of tramadol and dexmedetomidine combination on rat models of acute and neuropathic pain. Pharmacol Biochem Behav 88:9–17. doi:10.1016/j.pbb.2007.06.006
Holte K, Kehlet H (2002) Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg 195:694–712
Hong JY, Han SW, Kim WO, Kim EJ, Kil HK (2010) Effect of dexamethasone in combination with caudal analgesia on postoperative pain control in day-case paediatric orchiopexy. Br J Anaesth 105:506–510. doi:10.1093/bja/aeq187
Hval K, Thagaard KS, Schlichting E, Raeder J (2007) The prolonged postoperative analgesic effect when dexamethasone is added to a nonsteroidal antiinflammatory drug (rofecoxib) before breast surgery. Anesth Analg 105:481–486. doi:10.1213/01.ane.0000267261.61444.69
Jokela R, Ahonen J, Tallgren M, Haanpaa M, Korttila K (2008) Premedication with pregabalin 75 or 150 mg with ibuprofen to control pain after day-case gynaecological laparoscopic surgery. Br J Anaesth 100:834–840. doi:10.1093/bja/aen098
Jokela R, Ahonen J, Tallgren M, Haanpaa M, Korttila K (2008) A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain 134:106–112. doi:10.1016/j.pain.2007.04.002
Kavoussi R (2006) Pregabalin: from molecule to medicine. Eur Neuropsychopharmacol 16(suppl 2):S128–S133. doi:10.1016/j.euroneuro.2006.04.005
Kehlet H (2007) Glucocorticoids for peri-operative analgesia: how far are we from general recommendations? Acta Anaesthesiol Scand 51:1133–1135. doi:10.1111/j.1399-6576.2007.01459.x
Kehlet H, Dahl JB (1993) The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg 77:1048–1056
Mathiesen O, Jacobsen LS, Holm HE, Randall S, Adamiec-Malmstroem L, Graungaard BK, Holst PE, Hilsted KL, Dahl JB (2008) Pregabalin and dexamethasone for postoperative pain control: a randomized controlled study in hip arthroplasty. Br J Anaesth 101:535–541. doi:10.1093/bja/aen215
Mathiesen O, Jorgensen DG, Hilsted KL, Trolle W, Stjernholm P, Christiansen H, Hjortso NC, Dahl JB (2011) Pregabalin and dexamethasone improve post-operative pain treatment after tonsillectomy. Acta Anaesthesiol Scand 55:297–305. doi:10.1111/j.1399-6576.2010.02389.x
Mathiesen O, Rasmussen ML, Dierking G, Lech K, Hilsted KL, Fomsgaard JS, Lose G, Dahl JB (2009) Pregabalin and dexamethasone in combination with paracetamol for postoperative pain control after abdominal hysterectomy. A randomized clinical trial. Acta Anaesthesiol Scand 53:227–235. doi:10.1111/j.1399-6576.2008.01821.x
Meymandi MS, Keyhanfar F (2012) Pregabalin antinociception and its interaction with tramadol in acute model of pain. Pharmacol Rep 64:576–585
Mitra S, Khandelwal P, Sehgal A (2012) Diclofenac-tramadol vs. diclofenac–acetaminophen combinations for pain relief after caesarean section. Acta Anaesthesiol Scand 56:706–711. doi:10.1111/j.1399-6576.2012.02663.x
Parrington SJ, O’Donnell D, Chan VW, Brown-Shreves D, Subramanyam R, Qu M, Brull R (2010) Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. Reg Anesth Pain Med 35:422–426. doi:10.1097/AAP.0b013e3181e85eb9
Romundstad L, Breivik H, Roald H, Skolleborg K, Haugen T, Narum J, Stubhaug A (2006) Methylprednisolone reduces pain, emesis, and fatigue after breast augmentation surgery: a single-dose, randomized, parallel-group study with methylprednisolone 125 mg, parecoxib 40 mg, and placebo. Anesth Analg 102:418–425. doi:10.1213/01.ane.0000194358.46119.e1
Romundstad L, Stubhaug A (2007) Glucocorticoids for acute and persistent postoperative neuropathic pain: what is the evidence? Anesthesiology 107:371–373. doi:10.1097/01.anes.0000279487.27940.5c
Samad I, Stevens HE, Maloney A (1992) The efficacy of nasal septal surgery. J Otolaryngol 21:88–91
Savoia G, Alampi D, Amantea B, Ambrosio F, Arcioni R, Berti M, Bettelli G, Bertini L, Bosco M, Casati A, Castelletti I, Carassiti M, Coluzzi F, Costantini A, Danelli G, Evangelista M, Finco G, Gatti A, Gravino E, Launo C, Loreto M, Mediati R, Mokini Z, Mondello E, Palermo S, Paoletti F, Paolicchi A, Petrini F, Piacevoli Q, Rizza A, Sabato AF, Santangelo E, Troglio E, Mattia C (2010) Postoperative pain treatment SIAARTI recommendations 2010. Short version. Minerva Anestesiol 76:657–667
Sener M, Yilmazer C, Yilmaz I, Caliskan E, Donmez A, Arslan G (2008) Patient-controlled analgesia with lornoxicam vs. dipyrone for acute postoperative pain relief after septorhinoplasty: a prospective, randomized, double-blind, placebo-controlled study. Eur J Anaesthesiol 25:177–182. doi:10.1017/S0265021507002827
Sommer M, Geurts JW, Stessel B, Kessels AG, Peters ML, Patijn J, van Kleef M, Kremer B, Marcus MA (2009) Prevalence and predictors of postoperative pain after ear, nose, and throat surgery. Arch Otolaryngol Head Neck Surg 135:124–130. doi:10.1001/archoto.2009.3
Szychta P, Antoszewski B (2010) Assessment of early post-operative pain following septorhinoplasty. J Laryngol Otol 124:1194–1199. doi:10.1017/S0022215110001519
Wang JJ, Ho ST, Lee SC, Liu YC, Liu YH, Liao YC (1999) The prophylactic effect of dexamethasone on postoperative nausea and vomiting in women undergoing thyroidectomy: a comparison of droperidol with saline. Anesth Analg 89:200–203
White PF (2005) The changing role of non-opioid analgesic techniques in the management of postoperative pain. Anesth Analg 101:S5–S22
White PF (2008) Multimodal analgesia: its role in preventing postoperative pain. Curr Opin Investig Drugs 9:76–82
Wilder-Smith CH, Hill L, Dyer RA, Torr G, Coetzee E (2003) Postoperative sensitization and pain after cesarean delivery and the effects of single im doses of tramadol and diclofenac alone and in combination. Anesth Analg 97:526–533 (table of contents)
Wittekindt D, Wittekindt C, Schneider G, Meissner W, Guntinas-Lichius O (2012) Postoperative pain assessment after septorhinoplasty. Eur Arch Otorhinolaryngol 269:1613–1621. doi:10.1007/s00405-011-1854-x
Yilmaz YF, Ozlugedik S, Titiz A, Tuncay A, Ozcan M, Unal A (2008) Comparison of levo-bupivacaine and lidocaine for postoperative analgesia following septoplasty. Rhinology 46:289–291
Conflict of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Demirhan, A., Tekelioglu, U.Y., Akkaya, A. et al. Effect of Pregabalin and Dexamethasone Addition to Multimodal Analgesia on Postoperative Analgesia Following Rhinoplasty Surgery. Aesth Plast Surg 37, 1100–1106 (2013). https://doi.org/10.1007/s00266-013-0207-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-013-0207-0