Abstract
Background
Septorhinoplasty is a traumatic procedure that is associated with epistaxis and postoperative pain. The primary objective of this randomized double-blind controlled trial was to determine whether intranasal 5% lidocaine plus naphazoline decreases postoperative pain and lessens the use of rescue analgesics.
Methods
After induction of general anesthesia and laryngeal topical anesthesia with 5% lidocaine, 28 adult patients, scheduled to undergo septorhinoplasty, were randomly assigned to one of two groups, either topical intranasal saline 20 ml (control group) or intranasal 5% lidocaine plus naphazoline solution 0.2 mg ml−1 (lidocaine group). The perioperative dose of sufentanil, the mean end-tidal concentration of isoflurane, and surgeon satisfaction with the operative field were recorded. In the lidocaine group, plasma lidocaine concentrations were sampled 15, 20, 25, 35, 45, and 55 min after induction of anesthesia. Visual analogue scale pain scores were recorded 30, 60, 90, and 120 min after the patients arrived in the postanesthesia care unit and 24 h after surgery. Consumption of morphine rescue analgesia and the occurrence of any side effects were recorded at the end of the 24-h study period.
Results
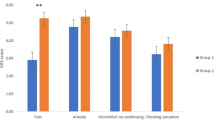
The intranasal lidocaine-naphazoline application decreased isoflurane requirements [median values: 0.8% (0.7–1.5) vs. 1.2% (0.9–1.8), respectively; P = 0.04] and enhanced surgical conditions. Patients in the lidocaine group experienced less postoperative pain than the control group [1 h after surgery: median values of visual analogue scale: 0 (0–20) vs. 50 (30–80), respectively; P = 0.001], and they required fewer doses of subcutaneous morphine. Total plasma concentrations of lidocaine remained below 4 μg ml−1 throughout the study period.
Conclusions
Intranasal lidocaine plus naphazoline is a simple and efficient technique for decreasing intra- and postoperative pain and for lessening rescue analgesic requirements in the postoperative period after septorhinoplasty. Toxic plasma concentrations of lidocaine were not reached.
Résumé
Contexte
La rhinoseptoplastie est une procédure traumatisante associée à une épistaxis et des douleurs postopératoires. L’objectif primaire de cette étude randomisée contrôlée à double insu était de déterminer si une solution intranasale de lidocaïne 5 % plus naphazoline réduisait la douleur postopératoire et diminuait l’utilisation d’analgésiques de sauvetage.
Méthode
Aprèsl’induction de l’anesthésie générale et une anesthésie laryngée topique avec de la lidocaïne 5 %, 28 patients adultes subissant une rhinoseptoplastie ont été randomisés en deux groupes: 20 mL de solution saline intranasale topique (groupe témoin) ou solution de 5% lidocaïne intranasale plus naphazoline 0,2 mg·mL−1 (groupe lidocaïne). La dose périopératoire de sufentanil, la concentration d’isoflurane moyenne télo-expiratoire et la satisfaction du chirurgien quant au champ opératoire ont été enregistrés. Dans le groupe lidocaïne, les concentrations plasmatiques de lidocaïne ont été échantillonnées à 15, 20, 25, 35, 45 et 55 min aprèsl’induction de l’anesthésie. Les scores de douleur sur une échelle visuelle analogique ont été enregistrés 30, 60, 90 et 120 minutes aprèsl’arrivée des patients dans l’unité de soins postanesthésiques et 24 h après la chirurgie. La consommation de morphine en analgésie de sauvetage et la survenue d’effets secondaires ont été enregistrées à la fin de la période d’étude de 24 heures.
Résultats
L’application de lidocaïne-naphazoline intranasale a diminué les besoins en isoflurane [valeurs médianes : 0,8% (0,7–1,5) vs 1,2% (0,9–1,8), respectivement; P = 0,04] et amélioré les conditions chirurgicales. Les patients du groupe lidocaïne ont souffert de moins de douleurs postopératoires que ceux du groupe témoin [une heure après la chirurgie: valeurs médianes sur l’échelle visuelle analogique : 0 (0–20) vs 50 (30–80), respectivement; P = 0,001], et ont nécessité moins de doses de morphine sous-cutanée. Les concentrations plasmatiques totales de lidocaïne sont demeurées au dessous de 4 μg·mL−1 tout au long de la période d’étude.
Conclusion
La lidocaïne intranasale plus naphazoline est une technique simple et efficace pour diminuer la douleur per- et postopératoire et pour réduire les besoins en analgésiques de sauvetage durant la période postopératoire suivant une rhinoseptoplastie. Un niveau toxique de concentrations plasmatiques de lidocaïne n’a pas été atteint.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Septorhinoplasty is generally considered to be a traumatic procedure associated with epistaxis, periorbital hematoma, and postoperative pain.1 The leading causes of hospital readmission and/or overstay after day-care septorhinoplasty surgery are epistaxis and a high level of postoperative pain.2 A multimodal analgesia regimen can be used, but many patients suffer from adverse events after opioid administration.3,4 Also, the use of high doses of nonsteroidal anti-inflammatory drugs is associated with patient readmission after septorhinoplasty surgery.5 Facial nerve blocks and/or anesthesia with intranasal topical cocaine have been proposed in association with general anesthesia. However, these techniques are associated with frequent block failure and high plasma concentrations of cocaine.6 A 5% solution of lidocaine plus naphazoline 0.2 mg ml−1 is often used for topical anesthesia during dental surgery, intranasal surgery, and bronchoscopy. However, information is unavailable about the use of this compound for intra- and postoperative analgesia in septorhinoplasty surgery. We hypothesized that an intranasal topical application of 5% lidocaine plus naphazoline could reduce pain and enhance intraoperative surgical conditions during and after septorhinoplasty surgery. The primary objective of this randomized double-blind controlled trial was to determine whether intranasal 5% lidocaine plus naphazoline reduces postoperative pain and lessens consumption of rescue analgesics. We also investigated intraoperative surgical conditions, patient and surgeon satisfaction, and plasma lidocaine concentrations as secondary outcomes.
Methods
After obtaining approval from the Institutional Review Board of Lapeyronie University Hospital Center (Montpellier, France) and written informed consent from the patients, we prospectively enrolled 28 adults who were scheduled to undergo septorhinoplasty surgery. The inclusion criteria were patients who were 18 years or older with ASA physical status I and an understanding of the possible complications related to local anesthetics and the study protocol. Patients who failed to cooperate and those who had psychological disorders or linguistic difficulties that might interfere with pain assessments were excluded. The medical exclusion criteria included blood clotting impairment, hepatic or renal insufficiency, a history of recent local or systemic infection, known allergy to the trial drugs, and cardiac conduction problems (second or third degree atrioventricular block). Patients with pre-existing facial pain and/or chronic pain medication were not included. In addition, patient refusal, patients who had participated in a therapeutic trial within the previous month, and those who were already participating in another study were not included.
Patients were premedicated with oral midazolam 0.15 mg kg−1 1 h before surgery. A peripheral intravenous catheter was inserted, and the patients were placed in the supine position. Patients were monitored according to the standard guidelines published by the French Society of Anesthesiology and Critical Care Medicine (Art D 712-43 and 44 of the “Code de la Santé Publique”). General anesthesia was induced with propofol 3–5 mg kg−1 iv and sufentanil 0.5 μg kg−1 iv. No muscle relaxant was administered. After laryngoscopy, an 8 mm diameter tracheal tube was inserted. The patients’ lungs were mechanically ventilated with a 1:1 mixture of nitrous oxide and oxygen. Patients received isoflurane and sufentanil (0.5 μg kg−1 h−1), intraoperatively, for maintenance of anesthesia. The initial isoflurane target end-tidal concentration was set at 0.7%. Additional sufentanil (0.1 μg kg−1 iv) and increases in the end-tidal concentration of isoflurane (titrated in 0.2% increments) were administered when there were signs of inadequate anesthesia, as determined by a 20% increase in systolic blood pressure and/or heart rate persisting above respective baseline values for >60 s. In order to allow for isoflurane and sufentanil adaptations, the values of end-tidal concentrations of isoflurane, as well as arterial pressure and heart rate values, were noted 15 min after surgical incision.
A randomization list with a computer-generated table was prepared before the study began, and a physician, who was not involved in the study, enrolled patients and assigned treatments sequentially, i.e., saline or 5% lidocaine plus naphazoline 0.2 mg ml−1 solution. An envelope containing the group assignment was prepared, sealed, and sequentially numbered for each patient. The anesthesiologists performing the study were also blinded to the use of lidocaine-naphazoline vs. saline. According to the protocol, the same physician (as mentioned above) applied pharyngeal and laryngeal topical anesthesia using 5% lidocaine spray (1 spray/10 kg), then inserted either cotton-tipped sticks soaked with 6 ml of 5% lidocaine plus naphazoline (lidocaine group) or 20 ml saline (control group) into each nasal turbinate for 10 min bilaterally. The same surgeon performed a septorhinoplasty using a standardized technique. Afterwards, the surgeon graded his satisfaction with the technique (very satisfied, mildly satisfied, or not satisfied) based on surgical conditions and bleeding during surgery. The surgeon did not apply additional infiltrative mucosal anesthesia. The duration of the surgery was recorded. In addition, venous blood samples were withdrawn at intervals 15, 20, 25, 35, 45, and 55 min after laryngeal topical anesthesia to determine total plasma lidocaine concentrations in the patients receiving intranasal anesthesia. The clinical research assistant immediately brought each sample to the laboratory. The total concentration of lidocaine in plasma was determined by gas chromatography with a nitrogen-sensitive detector. The limit of detection was 5 ng ml−1 and the limit of quantification was 10 ng ml−1. The interassay coefficient of variation for lidocaine was 8%.
After surgery, the patients were admitted to the postanesthesia care unit (PACU) where they all received ondansetron 4 mg iv as standard antiemetic prophylaxis. Visual analogue scale (VAS) pain scores (ranging from 0 mm, for no pain, to 100 mm, for the worst imaginable pain) were recorded 30, 60, 90, and 120 min after arrival in the PACU, 6 and 12 h after surgery by the nursing staff, and 24 h after surgery by the physician. Throughout the 24-h study period, patients were directed to take 1 g of paracetamol orally four times a day and 100 mg of ketoprofen twice daily. If the VAS score remained more than 30 mm, patients received a subcutaneous injection of morphine 0.1 mg kg−1, and a new VAS value was recorded 1 h later. Consumption of morphine rescue analgesia was noted at the end of the 24-h study period, and follow-up evaluations were performed by an anesthesiologist to determine the cumulative subcutaneous morphine requirement and the occurrence of any side effects (e.g., epistaxis, nausea and vomiting, pruritus, urinary retention, ileus, sleep disturbance, sedation, or fever). Each patient’s primary physician formally assessed the side effects at 30, 60, 90, and 120 min after arrival in the PACU and 24 h after surgery. The patients graded their satisfaction regarding analgesia (very satisfied, mildly satisfied, or not satisfied) at the end of the 24-h period.
Statistical analysis
Statistical analysis was performed using SAS software version 8.2 (SAS Institute, Cary, NC, USA) in the Medical Computer Programming Department of the University Hospital of Montpellier, France. A preliminary descriptive study demonstrated that the mean pain value for patients who did not benefit from the application of lidocaine-naphazoline (control group) was 40/100 (SD 15) at 60 min. Sample size calculations were centred around our primary hypothesis that intranasal 0.5% lidocaine plus naphazoline decreases postoperative pain compared with intranasal saline. A 50% reduction in pain scores was considered clinically relevant; at 60 min, the mean pain score decreased from 40 to 20 mm on the scale of 0–100. Based on SD 15 for the pain score values, and assuming a two-sided type 1 error of 0.05 and a power (1 – β) of 80%, 12 patients in each group were required to attain a clinically significant difference. The normality of distribution was determined using the Shapiro–Wilk test. Summary data are reported using the median (range) or are graphically reported as the median with 25–75th percentiles and extremes. For normally distributed data, multiple comparisons were made using a one-way analysis of variance. The non-parametric Kruskal–Wallis test was used for continuous data. Categorical data were analyzed using the Chi-squared test. A P value < 0.05 was considered significant.
Results
The study was conducted from September 1st, 2004 to March 31st 2005. Of the 33 patients screened, five were excluded: three due to use of chronic pain medication, one due to a recent local infection, and one for refusing to participate. Twenty-eight patients, 14 in each group, were enrolled in the study. There were no patient drop outs, and the data from all 28 subjects are reported. The patient characteristics of the two groups were similar (Table 1). Intraoperative details regarding anesthesia and the duration and conditions of surgery are presented in Table 2. Compared to the control group, topical intranasal lidocaine plus naphazolin application decreased intraoperative isoflurane and sufentanil requirements (P = 0.04 and P = 0.02, respectively) (Table 2). In the lidocaine plus naphazolin group, 6/14 of the patients required supplemental sufentanil, compared to 14/14 of the patients in the saline group. There were significant differences between groups regarding surgeon and patient satisfaction (Table 3; P < 0.05). The use of topical intranasal lidocaine-naphazoline was associated with decreased perioperative bleeding and enhanced surgical conditions.
Postoperatively, patients in the lidocaine group experienced significantly less postoperative pain compared with the control group (Table 4). The total amount of rescue analgesia with subcutaneous morphine was significantly increased in the control group [14 (10–16) mg vs. 0 mg in the lidocaine group; P = 0.01]. Total plasma lidocaine concentrations increased slightly to 4 μg ml−1 during the study period, but remained below toxic levels (Fig. 1). The highest individual plasma concentration of total lidocaine, 3.9 μg ml−1, was observed at 20 min after drug administration, and the highest median plasma concentration of total lidocaine was established at the end of the study period. Two patients experienced nausea-vomiting episodes; one patient had urinary retention and one experienced postoperative epistaxis. All complications were observed in patients in the control group. No systemic complications were observed.
Discussion
This double-blind comparative trial demonstrates that, compared with intranasal saline, a topical intranasal application of 5% lidocaine plus naphazoline 0.2 mg ml−1 improves postoperative analgesia in the 24 h after septorhinoplasty surgery. Also, lidocaine-naphazoline application decreases the requirements for intraoperative anesthetic drugs and rescue analgesics and enhances surgical conditions. Total plasma concentrations of lidocaine remained below 4 μg ml−1 throughout the study period.
The intranasal topical application of 5% lidocaine-naphazoline 0.2 mg ml−1 for septorhinoplasty surgery is a simple and effective procedure for decreasing pain and rescue analgesics in the postoperative period. Surgeons and anesthesiologists in France frequently use naphazoline nitrate to decrease intraoperative bleeding (naphazoline) while allowing optimal postoperative analgesia (lidocaine). The pharmacological profile of naphazoline nitrate closely resembles that of oxymetazoline chlorydrate, which is regarded as the vasoconstrictor, with or without anesthetic. The two compounds have the same risk/benefit ratio.7,8 The use of local anesthetics and vasoconstrictors in rhinoseptoplasty surgery is well known. Hogg et al.1 reported the following treatment: First the nose should be prepared with xylometazoline 0.1% spray. Next, the septum should be infiltrated before surgery with 4 ml of 0.2% lidocaine with 1:80,000 epinephrine. Finally, the operative area should be infiltrated with 10 ml of 0.5% bupivacaine. Unfortunately, despite this technique, the authors reported a 22% incidence of patient dissatisfaction and/or unexpected readmission. The leading causes for these events included postoperative pain and bleeding. Similarly, Singh et al.2 reported that 24% of patients had unacceptable levels of postoperative pain, delaying their hospital discharge. In our study, the VAS pain scores in the lidocaine group were very low throughout the 24-h study period, and no patient required rescue analgesia. This prolonged duration of analgesia was not expected. For septoplasty surgery, Molliex et al.9 used nasociliary and infraorbital nerve blocks with 0.25% bupivacaine and 1% lidocaine plus epinephrine 1:400,000, as well as intranasal 5% lidocaine and 0.25% phenylephrine. The nerve blocks facilitated good surgical conditions, mainly because the combination of a local anesthetic and a vasoconstrictor drug reduced bleeding during surgery. However, the mean duration of sensory blockade was only 91 min, and the authors did not investigate postoperative pain relief. Schonemann et al.10 studied the onset and duration of analgesia after a spray application of lidocaine on the oral mucosa and reported that maximal analgesia was attained within five minutes of administration. The hypoalgesic effect lasted for only 14 min. Repeated applications were found to be without any additional hypoalgesic effect.
Two mechanisms might account for the prolonged sensory block observed in trial. The combination of intranasal lidocaine and napahazoline as a vasoconstrictor leads to a prolonged infiltrative sensory block of the nasal mucosa and a progressive transmucosal spread of local anesthetic to the nasociliary and infraorbital nerve branches. Buchanan et al.11 reported less pain in patients whose nostrils contained local anesthetic-soaked packs than in patients with saline-soaked packs at 2, 4, and 6 h after surgery. Recent studies evaluating the use of trigeminal nerve blocks reported prolonged postoperative analgesia in endoscopic endonasal maxillary surgery, as well as dramatic decreases in isoflurane requirements.12–14 These results are comparable with those of our study, and we assume that the progressive transmucosal spread of lidocaine with naphazoline could explain the “trigeminal nerve branches”-like block. On the other hand, as recently demonstrated in other surgical procedures, the plasma lidocaine concentrations may have been sufficient to produce sufficient analgesia as an independent effect at any time point during surgery and in the early postoperative period.15,16
In our study, the highest recorded plasma lidocaine concentration was 3.9 μg ml−1, but in all other patients the peak concentration was <3 μg ml−1; toxic total plasma concentrations of lidocaine (between 4 and 8 μg ml−1) were never attained. We note that the blood sampling period did not extend beyond 1 h after lidocaine-naphazoline administration; however, lidocaine absorption is rapid.17–20 Absorption of lidocaine varies according to both the site, and the mode of delivery and fluctuates with the use of vasoconstrictors or cholinergic drugs.17,18 Williams et al.17 observed peak plasma lidocaine concentrations between 20 and 60 min after a 9 mg kg−1 maximal dose of intranasal and nebulized lidocaine via a fibreoptic endoscope. The highest individual plasma lidocaine concentration they observed was 4.5 mg l−1 at 60 min. Watanabe et al.18 reported that peak plasma lidocaine concentrations were attained between 17 and 29 min after an oral mucosal application of 100 mg of lidocaine. Many factors, such as the site of topical application, the delivery method, and even the position of the patient, may influence the speed of absorption of lidocaine. Some cardiovascular risks have been reported with naphazoline applications. They are mainly due to the use of massive doses of naphazoline in children or adults and/or because of intravenous rapid absorption during submucosal intranasal injections.21–24 Each method must be assessed with regard to potential local anesthetic toxicity, particularly when high doses are administered.
In conclusion, we report that the topical intranasal application of 5% lidocaine-naphazoline 0.2 mg ml−1 is a simple and effective technique to decrease intraoperative anesthetic requirements and to provide sustained postoperative analgesia after septorhinoplasty surgery.
References
Hogg RP, Prior MJ, Johnson AP. Admission rates, early readmission rates and patient acceptability of 142 cases of day case septoplasty. Clin Otolaryngol Allied Sci 1999; 24: 213–5.
Singh G, McCormack D, Roberts DR. Readmission and overstay after day case nasal surgery. BMC Ear Nose Throat Disord 2004; 4: 2.
Chung F, Mezei G. Factors contributing to a prolonged stay after ambulatory surgery. Anesth Analg 1999; 89: 1352–9.
Marshall SI, Chung F. Discharge criteria and complications after ambulatory surgery. Anesth Analg 1999; 88: 508–17.
Georgalas C, Obholzer R, Martinez-Devesa P, Sandhu G. Day-case septoplasty and unexpected re-admissions at a dedicated day-case unit: a 4-year audit. Ann R Coll Surg Engl 2006; 88: 202–6.
Liao BS, Hilsinger RL Jr, Rasgon BM, Matsuoka K, Adour KK. A preliminary study of cocaine absorption from the nasal mucosa. Laryngoscope 1999; 109: 98–102.
Meyer DR. Comparison of oxymetazoline and lidocaine versus cocaine for outpatient dacryocystorhinostomy. Ophtal Plast Reconstr Surg 2000; 16: 201–5.
Riegle EV, Gunter JB, Lusk RP, Muntz HR, Weiss KL. Comparison of vasoconstrictors for functional endoscopic sinus surgery in children. Laryngoscope 1992; 102: 820–3.
Molliex S, Navez M, Baylot D, Prades JM, Elkhoury Z, Auboyer C. Regional anaesthesia for outpatient nasal surgery. Br J Anaesth 1996; 76: 151–3.
Schonemann NK, van der Burght M, Arendt-Nielsen L, Bjerring P. Onset and duration of lidocaine spray applied to oral mucosa—a dose response study. Acta Anaesthesiol Scand 1992; 36: 733–5.
Buchanan MA, Dunn GR, Macdougall GM. A prospective double-blind randomized controlled trial of the effect of topical bupivacaine on post-operative pain in bilateral nasal surgery with bilateral nasal packs inserted. J Laryngol Otol 2005; 119: 284–8.
Higashizawa T, Koga Y. Effect of infraorbital nerve block under general anesthesia on consumption of isoflurane and postoperative pain in endoscopic endonasal maxillary sinus surgery. J Anesth 2001; 15: 136–8.
Nicodemus HF, Ferre MJ, Cristobal VC, de Castro L. Bilateral infraorbital block with 0.5% bupivacaine as post-operative analgesia following cheiloplasty in children. Scand J Plast Reconstr Surg Hand Surg 1991; 25: 253–7.
Rajamani A, Kamat V, Rajavel VP, Murthy J, Hussain SA. A comparison of bilateral infraorbital nerve block with intravenous fentanyl for analgesia following cleft lift repair in children. Paediatr Anaesth 2007; 17: 133–9.
Groudine SB, Fisher HA, Kaufman RP Jr, et al. Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg 1998; 86: 235–9.
Kaba A, Laurent SR, Detroz BJ, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology 2007; 106: 11–8.
Williams KA, Barker GL, Harwood RJ, Woodall NM. Combined nebulization and spray-as-you-go topical local anaesthesia of the airway. Br J Anaesth 2005; 95: 549–53.
Watanabe H, Lindgren L, Rosenberg P, Randell T. Glycopyrronium prolongs topical anaesthesia of the oral mucosa and enhances absorption of lignocaine. Br J Anaesth 1993; 70: 94–5.
British Thoracic Society Bronchoscopy Guidelines Committee, a Subcommittee of Standards of Care Committee of the British Thoracic Society. British Thoracic Society on diagnostic flexible bronchoscopy. Thorax 2001; 56: i1–21.
Mostafa SM, Murthy BV, Hodgson CA, Beese E. Nebulized 10% lignocaine for awake fibreoptic intubation. Anaesth Intensive Care 1998; 26: 222–3.
Fukushima H, Norimoto K, Seki T, et al. Acute pulmonary edema associated with naphazoline ingestion. Clinical Toxicol (Phila) 2008; 46: 254–6.
Musshoff F, Gerschlauer A, Madea B. Naphazoline intoxication in a child—a clinical and forensic toxicological case. Forensic Sci Int 2003; 134: 234–7.
Stamer UM, Buderus S, Wetegrove S, Lentze MJ, Stuber F. Prolonged awakening and pulmonary edema after general anesthesia and naphazoline application in an infant. Anesth Analg 2001; 93: 1162–4.
Villeret I, Tellier AC, Erhmann S, Rea D, Delalande JP. Cardiac arrest and secondary pulmonary oedema following accidental intramucosal injection of naphazoline in an adult (French). Ann Fr Anesth Réanim 2003; 22: 477–80.
Acknowledgment
Support was provided solely from institutional and/or departmental sources.
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Granier, M., Dadure, C., Bringuier, S. et al. Intranasal lidocaine plus naphazoline nitrate improves surgical conditions and perioperative analgesia in septorhinoplasty surgery. Can J Anesth/J Can Anesth 56, 102–108 (2009). https://doi.org/10.1007/s12630-008-9020-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-008-9020-7