Abstract
The use of adipose tissue transfer in plastic and reconstructive surgery is not new and has been studied extensively. Due to different results with regard to adipose cell damage and the level of survival of the transferred tissue in clinical practice, the authors aimed to investigate the effects of centrifugation on fat aspirates to optimize the centrifugal force for fat transplantation and to obtain an increased number of intact adipose progenitor cells. The following different centrifugation forces were evaluated in vitro in terms of fat decantation: 3,000 rpm (1,500×g), 1,300 rpm (250×g), and 500 rpm (50×g). Moreover, the density level, morphology of fat cells, cell viability, and progenitor cell number also were evaluated. Centrifugation leads to a good fat tissue density, with a significant number of progenitor cells, and efficiently removes the liquid portion. High centrifugal forces (at 3,000 rpm) caused significant damage to fat cells with low cell viability, whereas very low centrifugal forces (at 500 rpm) showed little effect on adipose tissue density, resembling fat decantation. Fat aspirates, withdrawn from 30 healthy donors in vivo, were centrifuged at different rotations per minute (rpm), as follows. For the 10 patients in group A, Coleman’s technique was used with a centrifugation of the aspirated fat at 3,000 rpm (1,500×g) for 3 min. For the 10 patients in group B, the authors’ technique was used, with centrifugation of the aspirated fat at 1,300 rpm (250×g) for 5 min. For the 10 patients in group C, simple decantation of fat was used. In conclusion, a centrifugal force of 1,300 rpm resulted in better density of adipose tissue, with good cell viability and increased ability to preserve a significant number of progenitor cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The idea of using fat as a filling material is not recent. Neuber [1] was the first to use autologous fat grafts in 1893 to treat facial defects. Lexer [2] examined fat used in plastic surgery to increase the malar regions and to fill wrinkles and pleating. In 1911, Bruning [3] was the first to inject autologous fat into the subcutaneous tissue to increase the soft tissue. Later, in the 1950s, the first articles describing the behavior of autologous adipose tissue were published [4, 5]. In those studies, adipose tissue was infiltrated for the correction of the body profile, proving that the survival rate for grafts of autologous fat was higher than 50%.
An autologous fat tissue transplant is a very common material for increasing soft tissue in reconstructive and cosmetic surgery [6]. Illouz [7, 8] and Fournier [9] developed a different approach for fat transfer, which previously was collected with a needle or cannula (syringe harvesting).
The international literature is rich in articles regarding the transfer of autologous fat using a variety of existing techniques [10, 11]. Some articles highlight graft survival rates ranging from 0% to 80% [12]. The main disadvantage of these methods is the high rate for absorption of injected fat, which reaches 70% of its entire volume [13].
The methods for fat collection and injection can greatly influence the longevity of the correction. In this regard, the survival of fat cells has been analyzed only clinically, with observation for the vascularity of the donor and receiving site, the pressure used to suction the fat, the diameter of the cannulas, the possible overcorrection of the receiving site, and the depth and technique of positioning [14]. Finally, Coleman [15] introduced an innovative technique with injection of adipocytes purified by centrifugation.
The first problem observed by Coleman was a decrease in the number of fat cells damaged by the aspiration procedure. The second aspect was the requirement to infiltrate cells in direct contact with well-vascularized tissues. In fact, this new technique of fat infiltration was performed using numerous small channels [16]. Centrifugation is an important process for isolating vital fat cells [17–19] and separating them from substances that can degrade the adipocytes such as blood cells, lipids, proteases, and lipases [20]. This study therefore aimed to test the effectiveness of alternative methods by assessing the survival of adipocytes after centrifugation through clinical and in vitro studies in an attempt to optimize the parameters for centrifugation of autologous fat for transplantation.
Materials and Methods
In Vitro Experiments
Adipose Tissue Extraction and Digestion
Adipose tissue was obtained by liposuction in the Plastic and Reconstructive Surgery Clinic at the Second University of Naples. The liposuction aspirate was placed in NaCl 0.9%, washed twice in phosphate-buffered saline (PBS: NaCl 137 mmol/l, KCl 2.7 mmol/l, Na2HPO4 10 mmol/l, KH2PO4 1.8 mmol/l), and centrifuged at 500 rpm (50×g) for 10 min, at 1,300 rpm (250×g) for 5 min, and at 3,000 rpm (1500×g) for 3 min. A part of the liposuction aspirate also was decanted.
After centrifugation and decantation, specimens were divided into three portions, namely, oil (top), adipose (middle) and fluid (bottom). Only the adipose portion was placed in a digestion solution consisting of collagenase type 1 (3 mg/ml) and dispase (4 mg/ml) supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and clarythromicin (500 μg/ml) in PBS at 37°C agitated for 60 min. This digestion solution was filtered through 70-μm filters (Becton & Dickinson, Sunnyvale, CA, USA).
Cell Culture
After filtration and washing, the pellet was resuspended in erythrocyte lysis buffer consisting of NH4Cl 155 mmol/l, KHCO3 10 mmol/l, and ethylenediaminetetraacetic acid (EDTA) 0.1 mmol/l, pH 7.3, for 10 min at room temperature. The cell suspension was centrifuged at 1,300 rpm for 5 min, and the pellet was resuspended in 5 ml of Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS). Flasks were incubated at 37°C with 5% carbon dioxide (CO2), and the medium was changed twice a week. Experiments were performed in triplicates.
Flow Cytometry
The cells were detached using a solution of trypsin-EDTA (EDTA 200 mg/l, trypsin 500mg/l; Cambrex, Verviers, Belgium). At least 500,000 cells were incubated with primary antibody for 30 min at 4°C, washed twice in PBS, and incubated with a secondary antibody. Samples were analyzed at days 0 (day of withdrawal), 15, and 30. The antibodies used in this study were anti-CD117 (or c-kit) phycoerythrin (PE) (Miltenyi-Biotech, Calderara di Reno, Bologna, Italy), anti-CD34 fluorescein isothiocyanate (FITC) and PE (Miltenyi-Biotech), anti-CD90 FITC (Chemicon, Prodotti Gianni, Milan, Italy), anti-CD105 FITC (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-CD29 Cy (Miltenyi-Biotech), anti-CD31 FITC (Miltenyi-Biotech), anti-CD133 PE (Miltenyi-Biotech), anti-hVEGF (Santa Cruz Biotechnology), anti-VEGFR-2 (Santa Cruz Biotechnology), anti-CD54 PE (Miltenyi-Biotech), anti-CD44 FITC (Miltenyi-Biotech), anti-CD45 Cy and PE (Becton & Dickinson, Franklin Lakes, NJ, USA), and anti-CD14 PE (Miltenyi-Biotech). A FACS Vantage (Becton & Dickinson) was used.
Adipogenic Differentiation
The cells were induced in the following adipogenic medium for 2 to 3 weeks: DMEM supplemented with 10% FBS plus dexamethasone 1 μmol/l (Sigma, Milan, Italy), human recombinant insulin 10 μmol/l (Sigma), indomethacin 200 μmol/l (Fluka, Milan, Italy), and 3-isobutyl-1-methyl-xantine (IBMX) 0.5 mmol/l (Sigma). Cells cultured in basal medium (see earlier description) were used as control subjects.
Angiogenic Differentiation Using Methylcellulose
To analyze in vitro capillary-like morphology, 2 × 105 to 5 × 105 cells/ml were plated in 24-well plates in a semisolid growth medium that consisted of 0.9% methylcellulose in DMEM, 30% FBS, 1% bovine serum albumin (BSA), mercaptoethanol 10−4 mol/l, and L-glutamine 2 mmol/l. In parallel experiments, cultures were stimulated in addition with vascular endothelial growth factor (VEGF, 50 ng/ml). All cultures were performed in triplicate, incubated at 37°C under 5% CO2, and left for 7 days to develop a capillary-like morphology.
Apoptosis Assay
Apoptosis analyses were performed with an Annexin V-FITC Apoptosis Detection Kit I (BD Pharmigen, Buccinasco, Milan, Italy) according to the manufacturer’s protocol.
Histologic Staining, Immunofluorescence, and Immunohistochemistry
Liposuction aspirates were fixed in formalin or stored at −80°C. The specimens fixed in formalin then were dehydrated in alcohol, clarified in xylene, and embedded in paraffin. Sections (5 μm thick) were stained with hematoxylin-eosin and Mallory’s trichrome, then observed using light microscopy.
Cells in the P6 multiwell were washed in PBS and fixed with 4% paraformaldehyde (PFA) for 30 min at 4°C, then washed three times in PBS for 10 min and incubated in PBS/5%FBS for 60 min at 4°C. After a double washing in PBS for 10 min at room temperature, the cells were incubated overnight at 4°C with monoclonal antihuman antibodies (diluted 1:100 in PBS). The wells were washed in PBS three times for 10 min at room temperature and incubated for 90 min at 4°C with the secondary FITC- or PE-conjugated antibody (diluted 1:200 in PBS × 1) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Moreover, the cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen; San Giuliano Milanese, Milan, Italy) diluted 1:10000 (5 μg/ml) in PBS for 7 min at room temperature. Cells incubated for 90 min at 4°C only with conjugated secondary antibodies were used as negative control cells. The cells then were observed under a fluorescence microscope (Nikon Instruments Italia, Calenzano, Firenze, Italy). Primary antibodies included anti-VEGF (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Immunohistochemical analyses were performed with a DAKO CYTOMATION kit (En Vision + System-HRP-AEC; Dako Italia, Milan, Italy) according to the manufacturer’s protocol. The antibodies used were anti-adiponectin (AbCam, Cambridge, UK).
In Vivo Experiments
Patients
From June 2007 to January 2009, 30 patients were divided into three different groups:
-
Group A: For 10 patients, a routine Coleman’s technique was used with centrifugation of the aspirated fat at 3,000 rpm (1,500×g) for 3 min.
-
Group B: For 10 patients, our technique was used, with centrifugation of the aspirated fat at 1,300 rpm (250×g) for 5 min.
-
Group C: For 10 patients, the traditional technique was used with simple decantation of fat.
The following data were collected: date of surgery, amount of aspirated fat, amount of produced filler, time of follow-up evaluation, and possible complications. Patients were examined by two different surgeons not involved in the operation. All the patients were informed of the surgery treatment and the time of follow-up assessment, after which they signed the informed consent.
The follow-up period was 12 months, and the method of postoperative evaluation was standardized. Clinical results were documented by pre- and postoperative digital photos. Positioning, focal distance, and camera adjustments were standardized. Results were evaluated at the end of the follow-up period by the operating surgeons, by the patients themselves, and by an independent medical observer. Patient evaluations were obtained by a questionnaire completed at the end of the follow-up period. Postoperative satisfaction was rated on a scale of 0 to 10.
Surgical Technique
The usual preoperative instructions for liposuction were given to the patients, including the use of antibiotics, to avoid the use of aspirin and derivatives at least 1 week before the surgery. The donor sites were chosen based on the facility of access and could include the medial portions of the knee, the trochanteric region, the root of the thigh, and the region below the gluteal sulcus.
By means of 3-mm incisions, the collection site was infiltrated by NaCl 0.9% cooled to 4°C, and the fat was removed through a 20-ml syringe connected by a Luer-lock attached to a cannula for infiltration using a 3-mm exit hole. The cannula was pushed through the site of access, creating a small negative pressure inside the syringe. The mechanical dissection of the adipose tissue associated with the cannula’s movement was used to fill the syringe placed under negative pressure with the aspirated fat. Subsequently, the syringe was closed with a plug after the cannula was removed, and the syringe was either left on the table for decantation or placed inside the centrifuge.
Both centrifugation (3,000 rpm for 3 min and 1,300 rpm for 5 min) and decantation separated the various components of the aspirated fat into three levels. The top level was the least dense and consisted mainly of oil. The central part was composed primarily of adipose tissue. The lower level was constituted of blood, water, and any other watery element. After the layer of oil was decanted, the plug was removed from the syringe to release it from the bottom layer. At this point, the purified fat was transferred into syringes equipped with attached Luer-locks of 2 ml with cannulas adequate for infiltration ready to be injected into the involved points.
Results
In Vitro Experiments
The aspirate fat was treated with three different centrifugal forces at different times: 500 rpm for 10 min, 1,300 rpm for 5 min, and 3,000 rpm for 3 min. In vitro studies were conducted to evaluate the compactness, volume, number of stem isolated cells, and cell survival. After these results had been obtained, we evaluated the different techniques of centrifugation in vivo compared with others described in the literature [19, 21, 22].
The aspirated fat with various forces of centrifugation was subjected to a careful histologic analysis including the decanted samples of aspirated fat (Fig. 1a–d). Histologic analysis showed that the nucleus center of the aspirated fat maintained approximately 70% of the vital adipocytes (intact cells) in each sample of fat subjected to various forces of centrifugation, whereas the cells at the periphery showed slight damage to the cell wall in samples centrifuged at 500 rpm for 10 min (15%). The cell wall damage increased when the centrifugal force was 1,300 rpm for 5 min (30%), and when 3,000 rpm was used for 3 min (55%), a high peripheral destruction of aspirated fat was found. Regarding fat decantation, the results showed minimal damage to the cell walls similar to that of 500-rpm experiments.
a Adipose tissue by liposuction using the decantation technique. Adipocytes are intact at this level. Hematoxylin-eosin staining was used (original magnification, ×100). b Adipose tissue by liposuction centrifuged at 500 rpm for 10 min, stained with Mallory’s trichrome. Adipocytes are partially intact but lose their density (original magnification, ×100). c Adipose tissue by liposuction centrifuged at 1,300 rpm for 5 min, stained with Mallory’s trichrome. Adipocytes are partially intact and compacted (original magnification, ×100). d Adipose tissue by liposuction centrifuged at 3,000 rpm for 3 min, stained with Mallory’s trichrome. Adipocytes are completely destroyed (original magnification, ×100)
The propidium iodide (PI) and V-FITC annexin method was used to evaluate the apoptosis. Actually, annexin binds to residues of phosphatidylserine when it is exposed on the outer leaflet of the plasma membrane, and the PI binds to DNA only when the cell is dead. This method allows live cells to be distinguished from those in apoptosis and those with necrosis. The lower left quadrant of the cytogram shows the percentage of viable cells, which exclude PI, and they are negative for the bond with annexin V-FITC (AX FITC-/PI-). The lower right quadrant represents the cells in early apoptosis, positive for annexin V-FITC and negative for PI (AX FITC+/PI−). The upper left quadrant shows necrotic cells, positive only for PI and negative for annexin (AX FITC−/PI+). The cells that coexpress both annexin and PI are the cells in late apoptosis.
We used this method to evaluate the degree of cell membrane destruction. Hence, we could evaluate the effects of different centrifugation forces on cellular integrity. For the samples centrifuged at 500 rpm for 10 min and at 1,300 rpm for 5 min, the results were similar. In fact, the viable cells were about 72% of the total cell population tested for centrifugation at both 500 and 1,300 rpm. The cells in total apoptosis, including early and late apoptosis, were 17% of the total cells in both cases, whereas the cells in necrosis were 9% of the total cells in both cases. In the samples treated with a centrifugal force of 3,000 rpm for 3 min, total apoptosis increased to 28%. The cells in necrosis were 4% of the total cells, whereas the viable cells were 68% of the total cells. Any apoptosis was detectable for fat decantation (Fig. 2a–d).
The propidium iodide (PI) and V-FITC annexin method used to evaluate apoptosis. The control is the decantation method (a). In the samples centrifuged at 500 rpm for 10 min (b) and at 1,300 rpm for 5 min (c), the results are similar. In fact, the viable cells are about 72% of the total cell population tested for centrifugation at both 500 and 1,300 rpm. The cells in total apoptosis, including early and late apoptosis, are 17% of the total cells in both cases, whereas the cells in necrosis are 9% of the total cells in both cases. In the samples treated at the centrifugal force of 3,000 rpm for 3 min (d), total apoptosis increases to 28%, and the cells in necrosis are 4% of the total cells, whereas the viable cells are 68% of the total cells
The phenotype of the cells in the samples centrifuged at 1,300 rpm for 5 min was studied by examining specific antigens for mesenchymal stem cells. In particular, we focused our attention on a population of stem/progenitor cells expressing the following markers: c-kit, CD34, CD90, CD105, CD29, and CD45. The flow cytometric analysis detected the presence of a cell population that we had identified previously [23]: cells positive for CD29 (β1-integrin), CD34, and CD90 (Thy-1) eCD105 (Fig. 3a, b), markers of the mesenchymal stemness. The cells in culture, sorted for CD34+/CD90+ (45%), were able to differentiate into endothelial cells, as demonstrated by expression of markers such as CD44+/CD54+ (50%) and VEGF+ (15%), and into adipocytes under appropriate stimuli, expressing markers such as adiponectin and PPARγ (Fig. 4a–d). This shows that centrifugation at 1,300 rpm for 5 min preserves not only the cell integrity but also the characteristics of stemness and differentiation with respect to centrifugation at 3,000 rpm for 3 min.
a Adipose stem cells (ASCs) in adipogenic medium exhibit an adipocyte morphology (original magnification, ×100). b CD34+/CD90+ cells in adipogenic medium show positivity for adiponectin by immunohistochemistry (original magnification, ×100). c Differentiated endothelial cells that after day 7 in methylcellulose formed an extensive intercellular tube network (original magnification, ×400). d Vascular endothelial growth factor (VEGF) (original magnification, ×100)
In Vivo Experiments
For the study, 30 patients ranging in age from 30 to 50 years were recruited. They were free of systemic pathology, and all were experiencing posttraumatic lipoatrophy, postinfiltration lipoatrophy of cortisones, or postsuppuration after intramuscular injection. The site of the defect, selected to standardize the experiment, was the gluteal region.
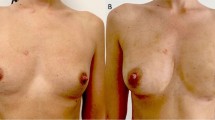
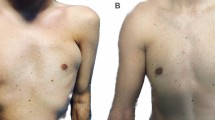
On the basis of results obtained in vitro, we decided to test in vivo only two centrifugation forces (3,000 and 1,300 rpm) and fat decantation. The patients were divided into three groups including a control group. All the patients underwent surgery in the Plastic and Reconstructive Surgery Clinic at the Second University of Naples. Aspirate fat (50 ml) was injected into each patient (the defect was filled with minimal tension without producing overcorrection). The injection of the aspirated fat was achieved in multiple directions to increase the surface contact between the implanted cells and the receiving tissue to ensure the survival of the transplanted fat cells. They tolerated the postoperative period fairly well. During the first 48 h in some cases, slight swelling of the tissues (edema) was observed, but it was reabsorbed in approximately 5 days. After the edema’s reabsorption and possible ecchymosis, the result began to appear within 2 to 3 weeks afterward. The final result, however, was visible within 3 to 6 months after the implantation (Fig. 5).
In group A (3,000 rpm for 3 min), all the patients, after a follow-up period of 12 months, showed almost 50% infiltration resorption of a purified fat. Eight group B patients (1,300 rpm for 5 min) showed no evidence of resorption. In the two remaining group B patients, minimal absorption (<20%) was observed. All the patients were satisfied with the aesthetic results. In addition, all the patients had the advantage of a significantly improved skin surrounding the site of the defect. All the patients belonging to group C (fat decantation) showed consistent fat absorption with an unsatisfying aesthetic result.
The mean postoperative follow-up period was 12 months. The satisfaction degree was scored by a questionnaire submitted to the patient, surgeon, and external observer. The general satisfaction score of the patients, surgeons, and external observer was 8.3.
Discussion
Various techniques of lipoinjection to correct dermal and subcutaneous defects have been developed [22, 24, 25]. The main indication for lipostructure is face-lift and profile correction involving cheekbones, lips, furrows genieni-nose, chin, jaw, or localized atrophies. Because the volume of the lost soft tissue often is due to aging of skin and subcutaneous tissues, with a loose consistency, lipostructure in these cases may represent an ideal solution [26]. In addition, the techniques of fat grafting often are used for rejuvenation of hands [18] and correction of localized tissue atrophy; fat atrophy that appears in HIV-positive patients [27]; congenital diseases such as Romberg and Poland syndromes [28]; congenital diseases of the chest such as the pectus excavatum [29], ectropion [30], soft tissue defects after loss of substance [31]; and paralysis of the vocal cords [32].
However, many variables affect the final result such as the caliber of cannula, possible use of epinephrine and lidocaine [33], and the time and force of centrifugation [19]. The main drawback is the necessity to overcorrect or to repeat the operation due to reabsorption of the injected material [34]. Regardless the technique, the injected fat encounters problems as well, mainly consisting of collection methods. In fact, adipocytes can lead to a physical trauma capable of inducing a wide range of effects culminating in cellular apoptosis. Remaining adipocytes, especially the central components, lead to ischemia due to lack of adequate vascularization [35].
Another problem that limits the use and the success of fat graft is the lack of adequate donor-site vascularization because many of the transplanted cells are mature adipocytes that do not have the capacity to replicate, and their survival is linked to blood supply [36]. Therefore, our study aimed to improve the current technique of adipocyte isolation by centrifugation of the collected fat with liposuction. Based on the results, the centrifugation at 1,300 rpm for 5 min has the advantage of a minimal overcorrection compared with current techniques in which corrections reaching 50% usually are done, with poor results after 2 years of follow-up evaluation.
Our patients displayed minimal resorption of the injected tissue (only in two cases that we considered) compared with control groups. This is due to lower cellular stress, as evidenced by the method of the annexin V-FITC and PI commonly used to evaluate apoptosis. Indeed, annexin binds to phosphatidylserine’s residues only when it is exposed on the outer leaflet of the plasma membrane, whereas PI binds to DNA only when the cell is dead. Indeed, this method allows optimal differentiation of living cells from those in apoptosis and those in necrosis. Excluding the apoptotic cells that automatically trigger a process of programmed death, depending on the percentage of annexin bind to phosphatidylserine and of PI bind to DNA, we evaluated the effects of different centrifugations on cellular integrity and found that with centrifugation at 1,300 rpm for 5 min, we obtained a lower number of damaged cells with respect to Colemann’s technique. This, although the numbers of damaged cells were greater than those obtained using a 500-rpm centrifugation force for 10 min, although in the latter case, the fat density was decreased.
Moreover, we tested stemness characteristics and differentiation capability of cells after centrifugation at both 1,300 rpm for 5 min and 3,000 rpm for 3 min. Furthermore, we demonstrated that the centrifugation at 1,300 rpm for 5 min preserves both the phenotype of stem cells and their ability to differentiate into endothelial cells and adipocytes compared with Colemann’s technique.
At the 12-month follow-up evaluation, the patients treated by centrifugation at 1,300 rpm for 5 min showed good aesthetic results with minimal absorption in two cases. Fat graft resorption has been attributed to traumatic handling of the graft during the harvest and injection stages. The needle may produce adipocyte damage, resulting in decreased cell viability. The use of a 3-mm-diameter blunt cannula prevented traumatization of harvested fat tissue.
The most important uncertainties with the use of aspirated fat graft injections concern the quantity of the graft and the required degree of overcorrection. Quantitative evidence of clinical fat survivability of volume restoration does not exist. Overcorrection is a vague concept, and there is no agreement on the amount or even the need of it. We did not perform overcorrection in this study. Patients rated the aesthetic result of their treatment as acceptable, and many patients were satisfied with the aesthetic outcome. Similarly, the cosmetic outcomes were significantly enhanced after our procedure. This stems primarily from the resolution of superficial irregularities, reduction of fibrosis-related deformities, and scar improvement.
Some uncertainties are the appropriate fat graft injection level and whether to perform the injection in a multilaminar manner or not. In the literature, several injection techniques are described. We suggest that the ideal plane is that of the subcutaneous tissue, which has a rich blood supply. This increases the chances of adipocyte survival and integration with the microenvironment. In this study, the microwheal technique was used, and injections were performed in different planes for an increased chance of adipocyte survival.
We also evaluated the problems of blood supply to already differentiated transplanted cells. It is possible to overcome this problem by culturing cells [23, 37] and transferring to the patient not only differentiated cells. We previously highlighted that within the stromal vascular fraction of adipose tissue it is possible to isolate a large number of stem cells identified to be CD34+/CD90+ [38]. It is possible to expand these cells on biocompatible scaffolds, and this is the future therapy.
References
Neuber GA (1893) Fettransplantation. Verh Dtsch Ges Chir 22:66
Lexer E (1910) Freire fettgewebstranplantation. Dtsch Med Wochenschr 36:46
Bruning P. Cited by Broeckaert TJ, Steinhaus J (1914) Contribution e l’etude des greffes adipueses. Bull Acad Roy Med Belgique 28:440
Peer LA (1950) Loss of weight and volume in human fat grafts. Plast Reconstr Surg 5:217
Peer LA (1956) The neglected free fat graft. Plast Reconstr Surg 18:233
Gurney CE (1937) Studies on the fate of free transplants of fat. Proc Staff Meet Mayo Clin 12:317
Illouz YG (1986) The fat cell graft: a new technique to fill depressions. Plast Reconstr Surg 78:122
Illouz YG (1988) Present results of fat injection. Aesthetic Plast Surg 12:175–181
Fournier PF (1985) Microlipoextration et microlipoinjection. Rev Chir Esthet Lang Franc 10:36–40
Pinski KS, Roenigk HH Jr (1992) Autologous fat transplantation. Long-term follow-up. J Dermatol Surg Oncol 18:179–184
Matsudo PK, Toledo LS (1988) Experience of injected fat grafting. Aesthetic Plast Surg 12:35–38
Elenbogen R (2000) Fat transfer: current use in practice. Clin Plast Surg 27:545–556
Horl HW, Feller AM, Biemer E (1991) Technique for liposuction fat reimplantation and long-term volume evaluation by magnetic resonance imaging. Ann Plast Surg 26:248–258
Sadick NS, Hudgins LC (2001) Fatty acid analysis of transplanted adipose tissue. Arch Dermatol 137:723–727
Coleman SR (1995) Long-term survival of fat transplants: controlled demonstrations. Aesthetic Plast Surg 19:421–425
Coleman SR (1997) Facial recontouring with lipostructure. Clin Plast Surg 24:347
Boschert MT, Beckert BW, Puckett CL, Concannon MJ (2002) Analysis of lipocyte viability after liposuction. Plast Reconstr Surg 109:761
Butterwick KJ (2002) Lipoaugmentation for aging hands: a comparison of the longevity and aesthetic results of centrifuged versus noncentrifuged fat. Dermatol Surg 28:987–991
Kurita M, Matsumoto D, Shigeura T, Sato K, Gonda K, Harii K, Yoshimura K (2008) Influences of centrifugation on cells and tissues in liposuction aspirates: optimized centrifugation for lipotransfer and cell isolation. Plast Reconstr Surg 121:1033–1041
Shiffman MA (2000) Effect of various methods of fat harvesting and reinjection. Am J Cosmet Surg 17:91
Shiffman MA, Mirrafati S (2001) Fat transfer techniques: the effect of harvest and transfer methods on adipocyte viability and review of the literature. Dermatol Surg 27:819–826
Coleman SR (2006) Structural fat grafting: more than a permanent filler. Plast Reconstr Surg 118:108S
D’Andrea F, De Francesco F, Ferraro G, Desiderio V, Tirino V, Papaccio G (2008) Large-scale production of human adipose tissue from stem cells: a new tool for regenerative medicine and tissue banking. Tissue Eng Part C Methods 14:233–242
Chajchir A, Benzaquen I (1989) Fat-grafting injection for soft tissue augmentation. Plast Reconstr Surg 84:921–935
Niechajev I, Sevcuk O (1994) Long-term results of fat transplantation: clinical and histologic studies. Plast Reconstr Surg 94:496–506
Glasgold M, Lam SM, Glasgold R (2007) Autologous fat grafting for cosmetic enhancement of the perioral region. Facial Plast Surg Clin North Am 15:461–470
Nelson L, Stewart KJ (2008) Experience in the treatment of HIV-associated lipodystrophy. J Plast Reconstr Aesthet Surg 61:366–371
Pinsolle V, Chichery A, Grolleau JL, Chavoin JP (2008) Autologous fat injection in Poland’s syndrome. J Plast Reconstr Aesthet Surg 61:784–791
Pereira LH, Sterodimas A (2008) Free fat transplantation for the aesthetic correction of mild pectus excavatum. Aesthetic Plast Surg 32:393–396
Caviggioli F, Klinger F, Villani F, Fossati C, Vinci V, Klinger M (2008) Correction of cicatricial ectropion by autologous fat graft. Aesthetic Plast Surg 32:555–557
Klinger M, Marazzi M, Vigo D, Torre M (2008) Fat injection for cases of severe burn outcomes: a new perspective of scar remodeling and reduction. Aesthetic Plast Surg 32(3):465–469
de Souza Kruschewsky L, de Mello-Filho FV, Saggioro F, Serafini LN, Rosen CA (2007) Histologic study of an autologous fat graft in the larynx of dogs with unilateral vocal fold paralysis. Laryngoscope 117:2045–2049
Shoshani O, Berger J, Fodor L, Ramon Y, Shupak A, Kehat I, Gilhar A, Ullmann Y (2005) The effect of lidocaine and adrenaline on the viability of injected adipose tissue—an experimental study in nude mice. J Drugs Dermatol 4:311–316
Ullmann Y, Hyams M, Ramon Y, Beach D, Peled IJ, Lindenbaum ES (1998) Enhancing the survival of aspirated human fat injected into nude mice. Plast Reconstr Surg 101:1940–1944
Sommer B, Dsttler G (2000) Current concepts of fat graft survival: histology of aspirated adipose tissue and review of the literature. Dermatol Surg 26:1159–1166
Sajjadian A, Tandav Magge K (2007) Treating facial soft tissue deficiency: fat grafting and adipose-derived stem cell tissue engineering. Aesthetic Surg J 27:100–104
Huss FR, Kratz G (2002) Adipose tissue processed for lipoinjection shows increased cellular survival in vitro when tissue engineering principles are applied. Scand J Plast Reconstr Surg Hand Surg 36:166–171
De Francesco F, Tirino V, Desiderio V, Ferraro G, D’Andrea F, Giuliano M, Libondi G, Pirozzi G, De Rosa A, Papaccio G (2009) Human CD34+/CD90+ ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS ONE 4:e6537
Acknowledgments
The authors thank Gianpaolo Papaccio, Department of Experimental Medicine, Section of Histology and Embryology, Second University of Naples, for his suggestions, criticisms, and laboratory technical support. The authors also thank Giuseppe Pirozzi, Department of Experimental Oncology, Section of Cellular Biology and Biotherapy, INT “Pascale,” for flow cytometry and apoptosis assays.
Conflict of interest
All authors declare that they have no actual or potential conflict of interest including any financial, personal or other with other people or organizations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferraro, G.A., De Francesco, F., Tirino, V. et al. Effects of a New Centrifugation Method on Adipose Cell Viability for Autologous Fat Grafting. Aesth Plast Surg 35, 341–348 (2011). https://doi.org/10.1007/s00266-010-9613-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-010-9613-8