Abstract
Purpose
To determine if gadolinium is necessary for the diagnosis of a pancreatic cystic lesion (PCL) as benign or malignant by assessing inter- and intra-observer agreement and diagnostic accuracy for the presence of worrisome features/high-risk stigmata on non-contrast MRI compared to MRI with and without contrast, with cytopathology as a reference standard.
Methods
The institutional database was searched to identify consecutive patients that underwent EUS/FNA or surgical resection of an asymptomatic PCL performed from 01/01/2015 to 01/01/2019. Two abdominal radiologists independently evaluated PCLs on MRI with all sequences except for contrast-enhanced sequences followed by a second reading with data from the entire MRI including pre- and post-contrast sequences. Cyst size, growth, and the presence of worrisome features/high-risk stigmata were assessed for each cyst on both datasets.
Results
There were 87 patients with 87 pancreatic cysts; 76(87.4%) were benign and 11 (12.7%) were malignant. The presence of any worrisome features/high-risk stigmata for reader 1 was concordant on both MRIs in 95.4% (83/87; k = 0.874) of cases and for reader 2 was concordant in 96.6% (84/87; k = 0.920) of cases. The diagnostic accuracy of the two datasets when the presence of any worrisome feature/high-risk stigmata was predictive of malignancy was identical for reader 1 (AUC = 0.622 for both; p = 1.0) and similar for reader 2 (AUC 0.569 and 0.589; p = 0.08) for both MRI datasets.
Conclusion
The addition of gadolinium had no significant impact in the diagnosis of a benign versus malignant PCL, with similar intra-observer agreement and diagnostic accuracy for both readers when using contrast-enhanced and unenhanced MRI datasets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic cystic lesions (PCL) are commonly encountered at cross-sectional imaging [1]. These are detected more frequently on MRI than CT, likely due to the increased soft tissue contrast of MRI [2], with an incidental PCL prevalence as high as 41.6% in some abdomen MRI series [3]. Most PCLs are mucin-containing lesions, with the majority being intraductal papillary mucinous neoplasms (IPMNs) [4]. The frequency of malignant transformation from PCL to adenocarcinoma is low [5, 6], allowing for conservative management algorithms. Various clinical societies, including the American College of Radiology (ACR) and International Consensus Fukuoka Guidelines have issued management guidelines for IPMNs [7,8,9]. Both guidelines consider cyst size, cyst growth, and additional imaging features such as the presence of an enhancing mural nodule, thickened cyst wall, or pancreatic duct dilatation in the diagnosis of cyst malignancy and overall management decision; either EUS/FNA, resection, or continued surveillance, with the length and frequency of surveillance varying between guidelines [7,8,9].
Although the ACR recommends either contrast-enhanced MRI or CT for PCL surveillance, MRI is often preferred due to the lack of ionizing radiation and superior performance for characterizing cystic lesions [10, 11]. Given the frequency and potential duration of surveillance, patients with PCLs that undergo MRI surveillance may be subject to multiple repeated administrations of gadolinium-based contrast agents. This is a point of concern for some patients and referring clinicians given reports of gadolinium deposition in various body tissues and the yet unknown clinical effects [12,13,14]. Two prior studies have assessed the need for intravenous contrast in the surveillance of PCLs by assessing the interobserver and intra-observer agreement for final management decision or cyst size/presence of mural nodule using MRI without contrast as well as MRI with and without contrast [10, 15]. However, there is a sparsity of studies evaluating whether contrast is needed in the diagnosis of a benign or malignant PCL by assessing individual worrisome features/high-risk stigmata against a cytopathology reference standard.
Therefore, the aim of our study is to determine if gadolinium is necessary for the diagnosis of a PCL as benign or malignant by assessing interobserver and intra-observer agreement and diagnostic accuracy for the presence of worrisome features/high-risk stigmata on non-contrast MRI compared to MRI with and without contrast, with cytopathology as a reference standard.
Material and methods
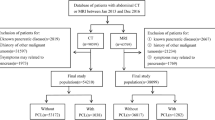
This Health Insurance Portability and Accountability Act–compliant study was approved by the institutional review board with a waiver of informed consent. In this single institutional retrospective study, the institutional database was searched to identify consecutive patients > 18-years-old that underwent EUS/FNA or surgical resection of an asymptomatic PCL performed from 01/01/2015 to 01/01/2019 (Fig. 1). Exclusion criteria were as follows: no dynamic contrast-enhanced abdomen MRI prior to EUS/FNA or surgery (n = 81); interval between MRI and either EUS/FNA or surgical resection > 90 days (n = 15); cysts without any cyst contents on EUS/FNA and therefore were considered non-diagnostic (n = 12); patients with chronic pancreatitis, epigastric abdominal pain, jaundice, or unexplained weight loss (n = 5), as the management recommendation in these cases are based on clinical criteria and not imaging criteria; and suboptimal image quality of MRI due to severe motion artifact (n = 1). In patients with more than one pancreatic cyst that underwent EUS/FNA or resection, the largest lesion was chosen for inclusion in our study cohort.
Imaging technique
MR examinations were performed by using either 1.5-T or 3-T MR imagers: Magnetom Skyra, Trio, or Aera (Siemens Healthcare, Erlangen, Germany) or LX Signa Excite 2, (GE Healthcare, Milwaukee, Wis; HD, GE Healthcare). The MRI with and without contrast included the following sequences: axial and coronal breath-hold single-shot fast spin-echo T2-weighted sequence (repetition time ms/echo time ms, TR/TE: 1100-1100/80-85, matrix 320 × 256, section thickness 5 mm, interslice gap 1 mm, acceleration factor of 1.5-2x), axial breath-hold three-dimensional dual-echo sequence (TR/TE 150/2.3 and 4.6 (1.5 T), 2.3 and 5.8 (3 T), matrix 288 × 230, section thickness 3 mm, acceleration factor of 2x), axial diffusion-weighted images (b values of 0, 50, 800, TR/TR: 5900/52, matrix 134 × 134, section thickness 5 mm, acceleration factor of 2x). 3D fat-suppressed T1-weighted sequence (TR/TE: 2.68/1.1, matrix 288 × 170, section thickness 3 mm, acceleration factor 2x) before and after the administration of gadobenate dimeglumine (Multihance, Bracco Diagnostics, Princeton NJ; 0.1 mmol of gadolinium per kilogram of body weight) or gadobutrol (Gadavist, Bayer Pharma; 0.05 mmol of gadolinium per kilogram of body weight) in the following phases: axial arterial phase (either 2 or 3 separate consecutive acquisitions obtained within a single breath-hold or single arterial phase); axial portal venous phase; coronal delayed phase, and axial equilibrium phase. Contrast was injected at 1 ml/s (gadobutrol) or 2 ml/s (gadobenate dimeglumine) followed by a 20 ml saline flush at 2 ml/s. MR cholangiopancreatography, when available, was performed with coronal respiratory-triggered three-dimensional fast spin-echo heavily T2-weighted sequence (4000/614; flip angle, 90°; section thickness, 1.8 mm) and coronal breath-hold thick-slab single-shot fast spin-echo T2-weighted sequence (6000/800; flip angle, 90°; section thickness, 30 mm).
Image analysis
All observations were reviewed by the study coordinator who was not involved in study readings (an abdominal imaging radiologist with 6 post-fellowship years of experience) and the series and image number of each PCL on T2-weighted imaging was recorded. Two fellowship trained abdominal radiologists (R1, R2) with 2 and 7 years of post-fellowship experience independently reviewed every pancreatic cyst on MRI. The readers were aware that patients had a pancreatic cyst, but were blinded to all clinical, pathologic data, and follow-up imaging. First, the two reviewers independently analyzed data from the MRI examination utilizing all sequences except for contrast-enhanced sequences [unenhanced T1-, T2-weighted, and DWI]. Following at least a 4-week interval to decrease recall bias [16], a second session was performed where the readers independently analyzed data from the entire MRI including pre- and post-contrast sequences.
Each reader evaluated the following features based on the International Consensus Fukuoka and the ACR Guidelines for the Management of IPMNs [8, 9]: cyst size in longest dimension, cyst growth, communication of cyst with the main pancreatic duct (MPD) and the following worrisome features/high-risk stigmata: the presence of non-enhancing mural nodule, enhancing mural nodule, thickened cyst wall, and pancreatic duct dilatation. A thickened cyst wall was defined as an enhancing or non-enhancing wall measuring > 2 mm. A non-enhancing or enhancing mural nodule was defined as any non-enhancing or enhancing solid protuberance into the cyst, respectively. The main pancreatic duct was measured as the maximal duct diameter anywhere in the gland and was considered dilated when ≥ 7 mm. Cyst growth was defined as: for cysts < 0.5 cm, a 100% increase in long-axis diameter; for cysts ≥ 0.5 cm and < 1.5 cm, a 50% increase in long-axis diameter; and for cysts ≥ 1.5 cm, a 20% increase in long-axis diameter as per the ACR guidelines on the management of pancreatic cysts [8]. All measurements were performed on axial or coronal images.
Reference standard
Fine needle aspiration with fluid analysis (n = 64) or surgical resection (n = 23) were used as the reference standard. For pancreatic cysts that underwent resection, the pathologic grades of IPMN were classified as low-grade dysplasia, intermediate-grade dysplasia, high-grade dysplasia, or invasive carcinoma associated with IPMN as per the World Health Organization guidelines [17]. Those with low- and intermediate-grade dysplasia were considered as benign and those with high-grade dysplasia and associated invasive carcinoma were malignant. For cysts that underwent EUS/FNA, cyst contents demonstrating high-grade dysplasia were considered malignant and cyst contents without evidence of high-grade dysplasia were considered benign. Those without evidence of cyst contents on cytology (and no histology available) were considered non-diagnostic (n = 12) and were excluded as described above. Twenty-seven cysts that underwent EUS-FNA also underwent biopsy with results that were concordant with cytology.
Statistics
To evaluate the strength of interobserver and intra-observer agreement, weighted k value and intraclass correlation coefficient values were calculated for all features and interpreted as follows: 0.00–0.20, poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and 0.81–1.00, excellent agreement. Receiver operating characteristic analyses were performed using the presence of any worrisome feature/high-risk stigmata as predictive of malignancy and the presence of cyst growth as predictive of malignancy. We compared the area under the receiver operating characteristic curve values of contrast-enhanced and non-contrast MRI datasets for both readers. A two-tailed p < 0.05 was considered to indicate statistical significance. All statistical analysis was performed by using SPSS (version 25; IBM, Armonk, NY) and R (version 3.5.2; R Foundation for Statistical Computing, Vienna, Austria) software.
Results
Patients
The study cohort consisted of 87 patients with 87 pancreatic cysts (mean age 65.5 years+—13.2; 59 women, 28 men). Seventy-six cysts (87.4%) were benign, defined as low or moderate grade dysplasia. Eleven cysts (12.7%) were malignant and included 6 with high-grade dysplasia, 4 with invasive carcinoma, and 1 pancreatic neuroendocrine tumor. The mean interval between CT and MRI examinations and surgery was 1.7 months (range, 2 days to 3 months). Sixty-six of the 87 patients (75.9%) had a prior contrast-enhanced CT or MRI at least 6 months prior to the baseline MRI to assess for cyst growth.
Interobserver agreement
Kappa coefficients to assess interobserver agreement for pancreatic cyst features is outlined in Table 1. For MRI without gadolinium, reader 1 and reader 2 agreed on the presence of any worrisome features/high-risk stigmata in 77/87 (88.5%; k = 0.722) of cases and for MRI with and without gadolinium in 76/87 (87.4%; k = 0.739) of cases. Reader 1 and 2 agreed on the presence of cyst growth in 61/66 (92.4%; k = 0.800) of cases on MRI without gadolinium and in 61/66 (92.4%; k = 0.800) of cases on MRI with and without gadolinium. Thickened cyst wall demonstrated the lowest interobserver agreement for both MRIs (k = 0.282 and 0.274 respectively) and pancreatic cyst size demonstrated the highest interobserver agreement for both MRIs (ICC = 0.953 and 0.937 respectively).
Intra-observer agreement
Kappa coefficients to assess intra-observer agreement for pancreatic cyst features is outlined in Table 2. The presence of any worrisome features/high-risk stigmata for reader 1 was concordant on both MRIs in 95.4% (83/87; k = 0.874) of cases and for reader 2 was concordant in 96.6% (84/87; k = 0.920) of cases. In 2 cases, reader 1 recorded the presence of a worrisome feature on MRI without contrast that was not recorded on MRI with and without contrast (both nodule without enhancement), both cysts were benign on EUS/FNA. In two cases, reader 1 recorded the presence of a worrisome feature on MRI with and without contrast that was not recorded on MRI without contrast (1 with thickened cyst wall and one with a nodule without enhancement), both cysts were benign on EUS/FNA. In 3 cases, reader 2 recorded the presence of a non-enhancing nodule on MRI without contrast that was not recorded to be present on MRI with and without contrast (Fig. 2), all three cysts were benign on EUS. The presence of cyst growth showed the greatest intra-observer agreement and was concordant in 100% (66/66; k = 1.0) of cases for both readers. Communication with the duct demonstrated the lowest intra-observer agreement when using both datasets for reader 1 (k = 0.494) and non-enhancing mural nodule demonstrated the lowest intra-observer agreement for reader 2 (k = 0.772). Reader 1 recorded the presence of an enhancing mural nodule in 3 cases (2 of which were malignant on cytopathology), and reader 2 recorded the presence of an enhancing mural nodule in 5 cases (2 of which were malignant on cytopathology). In all cases where an enhancing nodule was recorded, the presence of a non-enhancing nodule on the same case on MRI without contrast was recorded for both readers.
66-year-old female with an incidentally detected pancreatic cyst in the pancreatic body. Axial and coronal single-shot fast spin-echo (SSFSE) T2-weighted image (a, c) and axial and coronal 3D gradient-recalled echo T1-weighted image (b, d) obtained during the portal venous phase shows a 16 mm cyst (arrows) in the pancreatic body. In this case, reader 2 recorded the presence of a non-enhancing mural nodule on MRI without contrast that was recorded as having no worrisome features/high-risk stigmata on MRI with and without contrast. Reader 1 did not record the presence of any worrisome features/high-risk stigmata for either MRI dataset. This patient underwent EUS/FNA demonstrating no evidence of high-grade dysplasia
Diagnostic accuracy
The diagnostic accuracy of MRI without and MRI with and without gadolinium for the diagnosis of a benign or malignant PCL, when the absence of any worrisome feature/high-risk stigmata is considered a true negative and the presence of any of these features is considered a true positive is outlined in Table 3. The diagnostic performance of the two MRIs when using the above criteria, for reader 1 was identical (area under the receiver operating characteristic curve (AUC), 0.622 [95% CI 0.44, 0.74] and 0.622 [95% CI 0.44, 0.74], respectively; P = 1.0) (Fig. 3). The diagnostic performance of MRI without and MRI with and without, when using the above criteria, for reader 2 was similar (AUC, 0.569 [95% CI 0.39, 0.71] and 0.589 [95% CI 0.40, 0.72], respectively; P = 0.08) (Fig. 4). There were no false negative cases using MRI without contrast that were not noted on concurrent MRI with and without contrast for either reader. In three cases, reader 2 recorded the presence of a non-enhancing nodule on MRI without contrast that was not recorded for MRI with and without contrast, with all three pancreatic cysts being benign on EUS/FNA, leading to the decreased specificity for reader 2 between the two datasets (68.4% vs. 72.4%).
Graph shows comparison of area under the receiver operating characteristic curve (AUC) between MRI without gadolinium and MRI with and without gadolinium for the diagnosis of benign or malignant pancreatic cystic lesion for reader 1 and reader 2. wo gad = MRI without gadolinium. w/wo gad = MRI with and without gadolinium
79-year-old male with pancreatic cystic lesion within the tail incidentally detected on MRI. Axial and coronal single-shot fast spin-echo (SSFSE) T2-weighted image (a, c) and axial and coronal 3D gradient-recalled echo T1-weighted image (b, d) obtained during the portal venous phase show a 26-mm cyst (white arrows) in the tail of the pancreas. This cyst was not present on contrast-enhanced CT performed 14 months prior (not shown). In this case, both readers recorded the presence of an enhancing and non-enhancing mural nodule (red arrows), thickened cyst wall, and cyst growth on the MRI without as well as MRI with and without contrast. This patient underwent EUS/FNA and biopsy, both demonstrating pancreatic adenocarcinoma
The diagnostic accuracy of MRI without and MRI with and without gadolinium for the diagnosis of a benign or malignant pancreatic cyst, when the absence of cyst growth is considered a true negative and the presence of cyst growth is considered a true positive is outlined in Table 4. The diagnostic performance of the two MRIs when using the above criteria, was identical for reader 1 (AUC for both MRIs, 0.692 [95% CI 0.59, 0.80]) and identical for reader 2 (AUC for both MRIs, 0.735 [95% CI 0.56, 0.84]) (p = 1.0).
The diagnostic accuracy of each additional individual worrisome feature/high-risk stigmata is present in Tables E1–E5 in Supplemental Material.
Discussion
We demonstrated excellent intra-observer agreement for the detection of worrisome features/high-risk stigmata, cyst size, and cyst growth for both readers using both MRI without and MRI with and without intravenous contrast (k = 0.851–1.00). The diagnostic accuracy of the two datasets when the presence of any worrisome feature/high-risk stigmata was predictive of malignancy was identical for reader one (AUC = 0.622 for both) and similar for reader 2 (AUC 0.569 and 0.589) for both MRI datasets.
Asymptomatic pancreatic cysts are a common clinical problem and are being detected with increasing frequency given the growing use of MRI in clinical practice [8, 18]. MRI offers several advantages for the detection and characterization of pancreatic cysts over CT, with the main benefit being in the superior contrast resolution [11, 19]. T2-weighted imaging allows assessment of cyst morphology, internal contents (including nodularity), presence of pancreatic ductal communication, and presence of ductal dilatation. Given these advantages, MRI is currently the preferred modality for surveillance of pancreatic cysts, and patients with pancreatic cysts often undergo annual MRI follow-up for several years, usually with intravenous contrast as recommended by the America College of Radiology Guidelines [8]. However, given the recent findings that gadolinium deposition in the brain is cumulative over a patient’s lifetime [12], the necessity of repeat doses of GBCAs is being questioned [13, 14].
In our study, all high-risk stigmata and worrisome features demonstrated fair to excellent inter-reader agreement (k = 0.274–0.953), for each dataset (MRI without contrast and MRI with and without contrast). Our results are in line with a prior study that also demonstrated good to moderate interobserver variability for these features (kappa range 0.48–0.74) on MRI with and without intravenous contrast [20]. Like this prior investigation, our results also demonstrated that a thickened cyst wall had the lowest inter-reader agreement for both datasets (k = 0.282 and 0.274 respectively) and cyst size demonstrated the highest agreement (ICC = 0.953 and 0.937 respectively). The presence of an enhancing mural nodule demonstrated moderate inter-reader agreement (k = 0.739) in our study, which is also in line with this prior investigation (k = 0.66) [20].
There was excellent intra-observer agreement when assessing both datasets for the evaluation of any worrisome features/high-risk stigmata for both readers (R1 k range = 0.851–1.00, R2 k range = 0.920–1.00). Interestingly, most of the discordance for both readers occurred as a worrisome feature/high-risk stigma that was noted on MRI without contrast that was not noted for MRI with and without contrast. This suggests that this discordance may be due to inherent intra-observer variability and not because contrast-enhanced sequences added any new information. Similarly, the intra-observer agreement for cyst size (R1 ICC = 0.941; R2 ICC = 1.0) and the presence of cyst growth (R1 k = 1.0; R2 k = 1.0) between the two datasets was excellent for both readers. A prior study by Nougeret et al. similarly evaluated the intra-observer agreement of MRI without and MRI with and without contrast for final pancreatic cyst classification as either benign, indeterminant, or malignant, also demonstrating high concordance between the two datasets of 94.6–96.4% [15]. Similarly, a study by Macari et al. evaluated the intra-observer agreement in overall management decision for pancreatic cysts on MRI without and with/without contrast, demonstrating similar high concordance of 95.5% (k = 0.67) between the two datasets [10]. However, in both studies, individual cyst features were not assessed and diagnostic accuracy with a cytopathology reference standard was not evaluated.
The presence of a non-enhancing mural nodule is considered a worrisome feature and an enhancing mural nodule is considered a high-risk stigmata as per the American College of Radiology Guidelines on the management of IPMN [8]. However, as per the revised International Consensus Fukuoka Guidelines, the presence of a non-enhancing nodule is not considered a worrisome feature or high-risk stigmata [9]. In our study, we demonstrated relatively high specificity of non-enhancing mural nodule for predicting cyst malignancy for both readers (80.3–86.8%). However, the majority of the intra-observer variability between the two datasets was when a non-enhancing mural nodule was recorded for MRI without contrast that was not recorded for MRI with and without contrast. It is possible this was due to greater reader confidence in classifying a PCL as having no worrisome features/high-risk stigmata when contrast-enhanced sequences were available, although this was an uncommon occurrence in our cohort. In rare cases of uncertainty on MRI without contrast, we propose that it is reasonable for a patient to be recommended to return for MRI with contrast prior to rendering a final management recommendation.
While few prior studies have evaluated intra-observer agreement [10, 21], there is a sparsity of investigations assessing diagnostic accuracy of the two imaging techniques for cyst malignancy against a cytopathology reference standard. The diagnostic accuracy of the two datasets when the presence of any worrisome feature/high-risk stigmata was considered predictive of malignancy was identical for reader one (AUC = 0.622 for both) and similar for reader 2 (AUC 0.569 and 0.589) for both datasets. Similarly, the diagnostic accuracy when using cyst growth as predictive of malignancy was identical for both readers for both datasets (R1: 0.692 for both datasets and R2: 0.735 for both datasets). The presence of worrisome features/high-risk stigmata (which is inclusive of cyst size ≥ 3 cm) and cyst growth are the primary features that dictate management recommendations for both the American College of Radiology and Internal Consensus Fukuoka Guidelines for the management of IPMNs [8, 15]. Therefore, the similar diagnostic accuracy of these features between the two datasets is reassuring.
There were no cases of malignancy in our cohort that were separate from the PCL. However, a widespread neoplastic field defect is believed to underlie the development of IPMNs [9, 22, 23] and therefore, the entire pancreatic parenchyma is at increased risk for synchronous or metachronous adenocarcinoma in these patients. This complicates radiologic screening in patients with pancreatic cysts, however, additional features on non-contrast MRI (not evaluated in this study) may alert the radiologist to the presence of a separate pancreatic malignancy, including pancreatic ductal dilatation with duct cut-off, a hypointense lesion on non-contract T1-weighted sequences, and the presence of focal diffusion or T2-weighted signal abnormality. In fact, a prior study demonstrated no significant difference in diagnostic accuracy between the two MRI examinations (without contrast and with/without contrast) for the detection of pancreatic ductal adenocarcinoma [24]. However, further studies are needed to evaluate diagnostic accuracy between the two datasets for the detection of synchronous or metachronous pancreatic malignancy in patients with IPMNs.
Our study has several limitations. We aimed to simulate normal clinical practice, in which the majority of PCL s do not undergo surgical resection, using a combination of EUS/FNA and pancreatic resection as our reference standard. The utilization of an exclusively surgical cohort would skew the results to predominantly suspicious appearing lesions. Second, given the inherent infrequency of malignant PCL s in clinical practice, we had a relatively small number of malignant cysts, and therefore our study may have been under-powered to detect a difference in diagnostic accuracy between the two MRI imaging studies. As EUS/FNA was used as the reference standard in most cases, we did not know the histology of most of the cystic lesions and some had microcystic morphology (suggesting serous cystadenoma), however, our aim was not to evaluate lesion characterization but to compare the diagnostic accuracy of MRI without and MRI with and without contrast for detecting malignant PCL s. Imaging prior to the baseline MRI to assess for cyst growth was only present for 76% of the patient cohort. We chose to include consecutive cases of PCLs on MRI, as inclusion of a cohort of only patients with follow-up imaging would possibly skew our population toward lesions that likely underwent cyst growth, prompting EUS or surgery, or lesions that had features on baseline imaging that warranted follow-up, as opposed to all-comer PCLs mimicking what is encountered on MRI in clinical practice.
In conclusion, our study showed that the addition of gadolinium had no significant impact in the diagnosis of a benign versus malignant PCL with similar intra-observer agreement and diagnostic accuracy for both readers when using contrast-enhanced and unenhanced MRI datasets.
References
de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol 2010;8(9):806-811. https://doi.org/10.1016/j.cgh.2010.05.017
Zhu S, Wang WT, Shang XS, Ni T, Wu WC, Lou WH, Zeng MS, Rao SX. Difference analysis in prevalence of incidental pancreatic cystic lesions between computed tomography and magnetic resonance imaging. BMC Med Imaging 2019;19(1):43. https://doi.org/10.1186/s12880-019-0341-5
Moris M, Bridges MD, Pooley RA, Raimondo M, Woodward TA, Stauffer JA, Asbun HJ, Wallace MB. Association Between Advances in High-Resolution Cross-Section Imaging Technologies and Increase in Prevalence of Pancreatic Cysts From 2005 to 2014. Clin Gastroenterol Hepatol 2016;14(4):585-593.e583. https://doi.org/10.1016/j.cgh.2015.08.038
Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B, Klöppel G. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch 2004;445(2):168-178. https://doi.org/10.1007/s00428-004-1043-z
Gardner TB, Glass LM, Smith KD, Ripple GH, Barth RJ, Klibansky DA, Colacchio TA, Tsapakos MJ, Suriawinata AA, Tsongalis GJ, Pipas JM, Gordon SR. Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in US adults. Am J Gastroenterol 2013;108(10):1546-1550. https://doi.org/10.1038/ajg.2013.103
Chernyak V, Flusberg M, Haramati LB, Rozenblit AM, Bellin E. Incidental pancreatic cystic lesions: is there a relationship with the development of pancreatic adenocarcinoma and all-cause mortality? Radiology 2015;274(1):161-169. https://doi.org/10.1148/radiol.14140796
Hasan A, Visrodia K, Farrell JJ, Gonda TA. Overview and comparison of guidelines for management of pancreatic cystic neoplasms. World J Gastroenterol 2019;25(31):4405-4413. https://doi.org/10.3748/wjg.v25.i31.4405
Megibow AJ, Baker ME, Morgan DE, Kamel IR, Sahani DV, Newman E, Brugge WR, Berland LL, Pandharipande PV. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2017;14(7):911-923. https://doi.org/10.1016/j.jacr.2017.03.010
Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, Salvia R, Shimizu Y, Tada M, Wolfgang CL. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017;17(5):738-753. https://doi.org/10.1016/j.pan.2017.07.007
Macari M, Lee T, Kim S, Jacobs S, Megibow AJ, Hajdu C, Babb J. Is gadolinium necessary for MRI follow-up evaluation of cystic lesions in the pancreas? Preliminary results. AJR American journal of roentgenology 2009;192(1):159-164. https://doi.org/10.2214/ajr.08.1068
Sainani NI, Saokar A, Deshpande V, Fernández-del Castillo C, Hahn P, Sahani DV. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR American journal of roentgenology 2009;193(3):722-731. https://doi.org/10.2214/ajr.08.1253
Zivadinov R, Bergsland N, Hagemeier J, Ramasamy DP, Dwyer MG, Schweser F, Kolb C, Weinstock-Guttman B, Hojnacki D. Cumulative gadodiamide administration leads to brain gadolinium deposition in early MS. Neurology 2019;93(6):e611-e623. https://doi.org/10.1212/wnl.0000000000007892
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 2014;270(3):834-841. https://doi.org/10.1148/radiol.13131669
McDonald RJ, Levine D, Weinreb J, Kanal E, Davenport MS, Ellis JH, Jacobs PM, Lenkinski RE, Maravilla KR, Prince MR, Rowley HA, Tweedle MF, Kressel HY. Gadolinium Retention: A Research Roadmap from the 2018 NIH/ACR/RSNA Workshop on Gadolinium Chelates. Radiology 2018;289(2):517-534. https://doi.org/10.1148/radiol.2018181151
Nougaret S, Reinhold C, Chong J, Escal L, Mercier G, Fabre JM, Guiu B, Molinari N. Incidental pancreatic cysts: natural history and diagnostic accuracy of a limited serial pancreatic cyst MRI protocol. European radiology 2014;24(5):1020-1029. https://doi.org/10.1007/s00330-014-3112-2
Boone D, Halligan S, Mallett S, Taylor SA, Altman DG. Systematic review: bias in imaging studies - the effect of manipulating clinical context, recall bias and reporting intensity. European radiology 2012;22(3):495-505. https://doi.org/10.1007/s00330-011-2294-0
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76(2):182-188. https://doi.org/10.1111/his.13975
Pergolini I, Sahora K, Ferrone CR, Morales-Oyarvide V, Wolpin BM, Mucci LA, Brugge WR, Mino-Kenudson M, Patino M, Sahani DV, Warshaw AL, Lillemoe KD, Fernández-Del Castillo C. Long-term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology 2017;153(5):1284-1294.e1281. https://doi.org/10.1053/j.gastro.2017.07.019
Visser BC, Yeh BM, Qayyum A, Way LW, McCulloch CE, Coakley FV. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR American journal of roentgenology 2007;189(3):648-656. https://doi.org/10.2214/ajr.07.2365
Lee JE, Choi SY, Min JH, Yi BH, Lee MH, Kim SS, Hwang JA, Kim JH. Determining Malignant Potential of Intraductal Papillary Mucinous Neoplasm of the Pancreas: CT versus MRI by Using Revised 2017 International Consensus Guidelines. Radiology 2019;293(1):134-143. https://doi.org/10.1148/radiol.2019190144
Pozzi-Mucelli RM, Rinta-Kiikka I, Wünsche K, Laukkarinen J, Labori KJ, Ånonsen K, Verbeke C, Del Chiaro M, Kartalis N. Pancreatic MRI for the surveillance of cystic neoplasms: comparison of a short with a comprehensive imaging protocol. European radiology 2017;27(1):41-50. https://doi.org/10.1007/s00330-016-4377-4
Griffin JF, Poruk KE, Wolfgang CL. Is It Time to Expand the Role of Total Pancreatectomy for IPMN? Dig Surg 2016;33(4):335-342. https://doi.org/10.1159/000445019
Remotti HE, Winner M, Saif MW. Intraductal papillary mucinous neoplasms of the pancreas: clinical surveillance and malignant progression, multifocality and implications of a field-defect. Jop 2012;13(2):135-138.
Jang KM, Kim SH, Kim YK, Song KD, Lee SJ, Choi D. Missed pancreatic ductal adenocarcinoma: Assessment of early imaging findings on prediagnostic magnetic resonance imaging. Eur J Radiol 2015;84(8):1473-1479. https://doi.org/10.1016/j.ejrad.2015.05.012
Funding
The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kierans, A.S., Gavlin, A., Wehrli, N. et al. Utility of gadolinium for identifying the malignant potential of pancreatic cystic lesions. Abdom Radiol 47, 1351–1359 (2022). https://doi.org/10.1007/s00261-022-03446-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03446-z