Abstract
Objectives
To evaluate the value of MRI in differentiating benign (b-MCN) and malignant (m-MCN) MCN. European guidelines suggest that certain mucinous cystic neoplasms (MCN) of the pancreas can be conservatively managed.
Methods
A retrospective single-center study of consecutive patients with resected MCN. MRIs were independently reviewed by two readers blinded to the pathological results. The authors compared b-MCN (i.e., mucinous-cystadenoma comprising high-grade dysplasia (HGD)) and m-MCN (i.e., cystadenocarcinoma).
Results
Sixty-three patients (62 women [98%]) with 63 MCN (6 m-MCN, 2 HGD) were included. m-MCN tumors had a tendency to be larger than b-MCN (median 86 [25–103] vs. 45 [17–130] mm, p = .055). The combination of signal heterogeneity on T2-weighted imaging, wall thickness ≥ 5 mm, the presence of mural nodules ≥ 9 mm, and enhancing septa had an area under the ROC curve of 0.97 (95% CI 0.91–1.00) for the diagnosis of m-MCN. A total of 24 (37%), 20 (32%), 10 (16%), 5 (8%), and 4 (6%) out of 63 MCNs showed 0, 1, 2, 3, and 4 of these features, respectively. The corresponding rate of m-MCN was 0%, 0%, 10%, 20%, and 100%, respectively, with a good-to-excellent inter-reader agreement. Patterns with a high NPV for m-MCN included an absence of enhancing septa or walls (NPV 97% and 100%, respectively), wall thickness < 3 mm (NPV 100%), and no mural nodules (NPV 100%).
Conclusions
A combination of 4 imaging features suggests malignant MCN on MRI. On the other hand, visualization of a thin non-enhancing wall with no mural nodules suggests benign MCN.
Key Points
• A heterogenous signal on T2-weighted MRI, a ≥ 5-mm-thick wall, mural nodules ≥ 9 mm, and/or enhancing septa suggest malignant MCNs.
• A thin non-enhancing wall with no mural nodules suggests benign MCNs.
• MRI should be performed in the pre-therapeutic evaluation of MCN to help determine the therapeutic strategy in these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mucinous cystic neoplasms (MCNs) of the pancreas are a group of cystic lesions that mainly occur in women. Like many other epithelial tumors, they follow the dysplasia-carcinoma sequence. Reported rates of high-grade dysplasia and malignant transformation into carcinoma ranged between 0 and 34% [1,2,3].
The prognosis of patients is highly dependent on the severity of epithelial lesions. While patients with dysplasia, including high-grade-dysplasia, have a favorable long-term prognosis without recurrence or related death, those with invasive m-MCN have a much poorer prognosis with a median survival of 22.9 months [4].
Besides its diagnostic value, imaging plays an important role in helping to determine the treatment strategy. Indeed, when the features of local invasion are identified, an endoscopic ultrasound is usually performed to confirm invasiveness and to biopsy the suspected solid malignant component [5,6,7,8]. In confirmed cases of invasive carcinoma, neoadjuvant chemotherapy is the recommended treatment [9] and curative-intent radical resection is recommended [10, 11]. In other cases, imaging can help the surgeon decide on the type of surgery needed. Since the recurrence rate of MCNs with no suspected local invasion is low, especially in small lesions, parenchyma-sparing resection tends to be the preferred approach (such as distal pancreatectomy without splenectomy, middle pancreatectomy, or enucleation) to reduce the risk of post-operative endocrine and/or exocrine pancreatic insufficiency and to preserve the spleen [9]. This approach is usually a preventive option for benign MCN, whatever the grade of dysplasia [9,10,11,11].
Recent European guidelines suggest that certain MCNs of the pancreas can be conservatively managed by surveillance if they are < 40 mm, with no risk features such as suspicious mural nodules or symptoms, or associated with other risk factors such as age, comorbidities, and increased surgical risk. Patient preference may also be taken into account [9].
Results of imaging are encouraging to distinguish patients who require radical resection from those who do not [9,10,11,12,12]. Tumor size and the presence of mural nodules have been reported to be the most predictive features of malignant transformation [13]. In a systematic review including studies published between 1970 and 2015, a 0.03% rate of malignant transformation was reported in MCNs < 4 cm with no mural nodules on preoperative imaging [3]. Many other features, such as the number of locules, septal or wall thickness, and locules of various intensities on T1-weighted imaging (T1-WI), have also been reported [7,8,9,10,11,12,13,13].
The aim of the present study was to reassess the value of MRI in differentiating benign from malignant mucinous cystic neoplasms of the pancreas in a large series of resected tumors.
Materials and methods
This single-center retrospective study was approved by our Institutional Review Board, and the requirement for informed consent was waived. (Institutional Review Board - IRB 00006477- of HUPNVS, Paris 7 University, AP-HP).
Patient population
We extracted all consecutive patients who underwent pancreatic resection for a MCN in our institution between November 2009 and January 2020, from a pathological database. Five of the 121 identified consecutive patients with no ovarian stroma on the pathology specimen were excluded, to exclude mucinous non-neoplastic cysts of the pancreas, which is a different entity from MCN [15]. We then excluded 53 patients without preoperative MRI or in whom it was performed more than one year before the surgery. Finally, 63 patients were included. Figure 1 shows the flow chart of the study.
Image analysis
All MRI examinations were performed on a 1.5-T (from November 2009 to November 2012) or a 3-T (since November 2012) clinical MRI scanner (Intera®, Ingenia®; Philips Healthcare). Table 1 describes the MRI acquisition protocol. MRI examinations were independently reviewed on a picture archive and communication system (PACS) workstation (Directview®, v11.3, Carestream Health Inc.) by two abdominal radiologists (J.G. and M.P.V. with 8 and 25 years of experience in MRI of the pancreas). Readers were aware that patients had MCNs but were blinded to the results of the pathological examinations. Readings were performed using a standardized data collection form. Maximum tumor diameter was assessed either on axial or coronal plane images in millimeters.
Readers were asked to report the following items for each tumor (Figs. 2, 3, 4, and 5):
-
Location: “head, body, or tail” according to conventional anatomical landmarks; “posterior” or “anterior” part of the parenchyma (yes/no); “exophytic aspect” (part of the lesion contours arising from the outer surface of the pancreas with no surrounding pancreatic parenchyma ≥ 1/3 of the circumference (yes/no); close to the left adrenal gland (yes/no). (Fig. 2)
-
Outer wall: wall thickness (mm); thick wall (i.e., > 3 mm); enhancement (yes/no); peripheral hypointense rim on diffusion-weighted imaging (DWI) (yes/no). When a preoperative CT was available, the presence of wall calcifications was recorded.
-
Shape and contours: oval or round (yes/no); locules (absent if the cyst had completely regular outer contour); unilocular (< 2 inner cysts) or multilocular (≥ 2 cysts).
-
Signal intensities: Signal intensity on T1- and T2-WI images considered to be hypointense, isointense, or hyperintense compared to the adjacent pancreas; homogeneity (yes/no, all inner components could be responsible for heterogeneity, including nodules, septa, fluid level); presence of inner cysts with various signal intensities on T1-WI (yes/no); inner fluid-containing cysts with a signal drop on high b-value DWI (yes/no) with a corresponding apparent diffusion coefficient (ADC) value.
-
Inner septa: presence (yes/no); thick septa (i.e., > 2 mm) (yes/no); enhancement (yes/no); indentation of the external wall facing septa (yes/no).
-
Mural nodules: presence (yes/no); if present, number and size (in mm, in case of multiple nodules, we selected the size of the largest; enhancement (yes/no); signal intensity on DWI separating low, intermediate, and high signal intensity; the ADC value of the mural nodule was not reported due to their small size.
-
Central scar (yes/no) or microcysts defined as multiple cysts < 2 cm separated by thin fibrous septa [16] (yes/no).
-
Adjacent pancreas: enlargement of the upstream main pancreatic duct (MPD) if > 3 mm (yes/no), hypointense signals of the upstream pancreas parenchyma on precontrast T1-WI images (yes/no).
Benign mucinous cystic neoplasm (b-MCN) of the pancreas in a 49-year-old woman. Contrast-enhanced portal-phase axial T1-weighted MRI shows a 27-mm exophytic (i.e., not surrounded with pancreas parenchyma at its posterior part) posterior cyst, located in the body of the pancreas in front of the left adrenal gland (arrow). The lesion has a thin wall (i.e., < 3 mm), with no enhancement
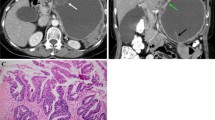
Pancreatic malignant mucinous cystic neoplasm (m-MCN) in a 59-year-old woman. a Axial T2-weighted image shows a large heterogeneous cyst, with septa perpendicular to the wall (arrow heads in b), and with a thick wall (arrows in b). On portal-phase contrast-enhanced axial T1-weighted MRI (c), the mural nodules (arrow in c) and the septa (black arrow in d) show contrast enhancement. The wall is thickened up to 9 mm. The patient underwent neoadjuvant chemotherapy based on a MRI-based suspicion of m-MCN confirmed by the results of an endoscopic ultrasound–guided puncture. The lesion was resected and was classified as pT2N0R0. Pathological analysis revealed many large papillae and numerous malignant portions, the largest measuring 5 cm, with focal invasion of the wall, but without invasion of the parenchyma of the pancreas
Pancreatic malignant mucinous cystic neoplasm (m-MCN) in a 60-year-old woman. a Unenhanced axial T1-weighted MRI shows a 90-mm hypointense posterior lesion, located in the body-tail of the pancreas, next to the left adrenal gland, containing an ill-defined nodule (arrow). b On a portal-phase contrast-enhanced axial T1-weighted image, the mural nodule shows enhancement (arrow). c On diffusion-weighted imaging (b 800 s/mm2), the mural nodule appears hyperintense (arrow) and with a low apparent diffusion coefficient (ADC) (arrow in d). A second nodule is depicted (arrowhead). The lesion was resected and was classified as pT1N0R0. Pathological analysis showed three portions with large papilla (2.5, 2, and 1.5 cm) containing four malignant mural nodules (ranging from 1 to 5 mm), with focal invasion of the wall but without invasion of the parenchyma of the pancreas and without peri-pancreatic invasion
In case of disagreement between the two observers, a consensus was reached, and these results were used for further statistical analysis.
Pathological analysis
Pathological analyses were performed by expert pathologists in the field of pancreatic tumors. The size of all lesions was recorded, and fresh and surgical specimens were carefully examined to identify areas with papillae, nodules (or solid masses in larger nodules), or wall thickening. After formalin fixation, all suspicious areas were sampled together with a thorough sampling of less suspicious areas before paraffin embedding. The 2019 World Health Organization (WHO) histological criteria were used to make a diagnosis of mucinous cystic neoplasms (namely the presence of a mucinous epithelial lining of the cyst underlined by an ovarian-type stroma, positive for anti-progesterone immunohistochemistry) [1]. Dysplasias were also graded according to WHO 2019 guidelines. Tumors were separated into two groups: benign lesions (b-MCN) including mild-, moderate-, or high-grade dysplasia but with no traces of malignant tissue, and malignant lesions corresponding to invasive m-MCN [1, 2].
Statistical analysis
Categorical variables are presented as numbers and percentages, and continuous variables as means and standard deviations (SD) or medians and ranges, as appropriate. Comparisons were performed with the chi-2 or Fisher exact tests for categorical variables, and with Mann-Whitney U test for continuous variables. Positive predictive values (PPV) and negative predictive values (NPV) of imaging features for the differentiation of b-MCN and m-MCN are provided. Inter-reader agreement was assessed by the Cohen’s kappa statistics. All tests were two-sided and p < 0.05 was considered to be significant. Analyses were performed using SPSS software (v23.0; SPSS Inc.).
Results
Patients and tumors
Sixty-three patients were included with 62 women (98%), median age 49 (24–85). Median delay between surgery and MRI was 4 months (0–12).
The median size of the lesions was 47 mm (17–130 mm). Sixty MCN were located in the body/tail (60/63, 95%). Fifty-six were posterior in the parenchyma (56/63, 89%), 57 were exophytic (57/63, 91%), and 50 were located close to the left adrenal gland (50/63, 79%).
Comparison between benign and malignant tumors
A total of 55/63 (87%) tumors displayed low-grade dysplasia, two (3%) displayed HGD, and six (10%) were associated with m-MCN. Tables 2 and 3 describe the imaging features of MCNs and the comparison between b-MCN and m-MCN.
Patients and tumor characteristics
Patients with m-MCN were significantly older than those with b-MCN (median 63 [52–85] vs. 48 [24–76] years old, p = .006). There was a trend towards larger tumors in m-MCN compared to b-MCN (median 86 [25–103] vs. 45 [17–130] mm, p = .055). Tumors > 80 mm were more frequently m-MCN (5/6, 83%) (p = 0.01). Other clinical characteristics were not significantly different between benign and malignant tumors.
Imaging features
Inter-reader agreement for imaging reading was found to be good to excellent for most features except for ADC values in cysts, which were poor (Table 3). Results of the most experienced readers are provided here. Details of both readings are provided in Table 3.
Most lesions were hyperintense on T2-WI (62/63, 98%) and hypointense on T1-WI (55/63, 87%). There were no central scars or microcysts. Inner cysts with various signal intensities on T1-WI (p = .032) were found more frequently in the six m-MCN, as well as enhancing septa (p = .026), wall ≥ 5 mm thick (p < .001), and mural nodules (p < .001). When present, mural nodules more frequently enhanced (p = < .001), with a hypersignal on DWI (p = .018) in m-MCN. Nodules ≥ 9 mm (including solid masses) were never observed in b-MCN, but were identified in all malignant tumors (p = .011) (Figs. 3 and 4). Other features including tumor shape, location, and lobulations were not different between groups.
Diagnostic value of MRI features
Features favoring malignancy
We selected the four features that were significantly more frequent in m-MCN for both readers (multivariate analysis was performed to identify features because of the limited number of malignant cases) and were not collinear: signal heterogeneity on T2-WI, wall thickness ≥ 5 mm, mural nodules ≥ 9 mm, and enhancing septa. The “presence of mural nodules” and “enhancing nodules” were not selected because they were collinear with “nodules ≥ 9 mm.”
A total of 24 (37%), 20 (32%), 10 (16%), 5 (8%), and 4 (6%) of the 63 tumors showed 0, 1, 2, 3, and 4 of the selected features, respectively. The corresponding rate of m-MCN was 0%, 0%, 10%, 20%, and 100%, respectively. The association of the four criteria of interest had an area under the ROC curve of 0.969 (95% CI 0.917–1.0) for the diagnosis of m-MCN. Addition of size > 4 cm did not improve the diagnostic performance. Details on the two patients with high-grade dysplasia are provided as Supplemental material.
Features favoring benignity
The absence of several features was shown to have > 90% NPV for the diagnosis of m-MCN (Table 4), namely the absence of low-intensity signals for cyst fluid on high b DWI (NPV 91%), the absence of enhancing septa (NPV 97%) or of an enhancing wall (NPV 100%), wall thickness < 3 mm (NPV 100%), the absence of mural nodules (NPV 100%), hypointense signal of mural nodules on high b-value DWI (NPV 100%), no upstream MPD enlargement (NPV 92%), and no wall calcifications on CT (NPV 95%) (Fig. 2).
Discussion
The goal of this retrospective study was to reassess the value of MRI in differentiating benign from malignant mucinous cystic neoplasms of the pancreas. Our results suggest that malignancy should be suspected in MCN with heterogenous signals on T2-WI, wall thickness ≥ 5 mm, mural nodules ≥ 9 mm, and enhancing septa. On the other hand, visualization of thin non-enhancing walls with no mural nodules suggests benign lesions.
As in previous studies, our results confirm that MCNs present with a fairly typical pattern: most were round or focally lobulated cystic lesions found in women, located in the body/tail of the pancreas, and with septa [2, 3, 9, 17–20]. It is important to note that we also report additional and as yet rarely described findings, in particular a posterior location close to the left adrenal gland, and an exophytic appearance that can mimic an extra-pancreatic origin. Since we did not include patients with other pancreatic cystic lesions, we could not assess the diagnostic value of these features for the diagnosis of MCNs. Nevertheless, it is consistent with the hypothesis of a dysembryogenic origin for these cysts suggested by Zamboni et al (ectopic primary yolk cells) with the pancreatic and ovarian ridges that are strictly adjacent during early embryogenesis [2, 20].
We showed that several features were more frequent in m-MCNs. Since the malignant transformation occurs on the epithelium lining the cysts, some of these features are likely to represent the local proliferation and the abnormal angiogenesis observed during the dysplasia-carcinoma transition (e.g., thickness of tumor walls, nodules). The presence of nodules and solid masses in malignant MCNs has been previously reported. In a clinico-pathological study describing 163 resected MCNs, and 19/163 (12%) invasive carcinomas, nodules were reported in 16/19 invasive carcinomas [9]. In a cohort of 52 patients with MCNs, Procacci et al reported the presence of nodules on CT in 5/16 (31%) malignant MCNs (HGD/carcinoma in situ) and 4/36 (11%) benign MCNs [21]. Mural nodules were found in all tumors on CT in a study of 60 MCNs by Le Baleur et al that pooled patients with HDG (n = 7/60, 12%) and those with invasive carcinoma (n = 3/60, 5%). The median nodule size was 19 mm (range 4–65). The presence of nodules had a sensitivity of 91% and a specificity of 98% for malignancy [12]. Assessing the presence of nodules on MRI has previously been described in a study by Di Paola et al, which also pooled HGD MCNs with invasive carcinoma and found 20/22 malignant lesions and 0/43 benign MCNs [14].
We chose to pool low-grade and HDG MCNs because of the good prognosis of these lesions. However, it can be argued that surveillance could be sufficient in low-grade dysplasia, while preventive resection should be performed in HDG. The two patients with HGD MCN were very different. We retrospectively applied our imaging criteria to one of these patients who was well classified for the need for resection based on the extent of HGD. On the other hand, resection of the other HGD lesion could be considered preventive because of the very small size of dysplasia. The latter case shows one weakness of radiopathological correlations, in which very small and flat hot spots of high-grade dysplasia may be sufficient to classify the entire lesion, while they are too small to be detectable with cross-sectional imaging.
Evidence suggests that a size of > 4 cm is associated with a higher risk of malignancy [3]. Our results do not confirm this because nearly 60% of b-MCN were > 4 cm in our cohort. Garces-Descovich et al found that a threshold of 8.5 cm was highly sensitive for malignancy [22]. However, in our cohort, 50% of m-MCN were < 8.5 cm and one was 2.5 cm. Interestingly, in this case, the only suspicious feature was a 9-mm nodule which only contained a small malignant area of < 3 mm on pathology. Although we retrospectively added the 4-cm threshold to the four imaging criteria we identified, this did not improve the results.
A hyperintense signal of the cyst on T1-WI, as well as a multilocular pattern, have also been described as being associated with malignancy [15, 22]. We did not confirm these findings, as hyperintense T1-WI images were found in both malignant (1/6, 17%) and benign (4/57, 7%) lesions (p = 0.617), and a multilocular pattern was present in 5/6 (83%) malignant lesions and in 40/57 (70%) benign lesions (p = 0.664). It should be noted that 22/26 lesions presenting with more than 3 locules were benign (Fig. 5). We did not find any significant difference according to the presence of calcifications in the wall and/or septa as reported by Proccaci et al. These authors reported their presence in seven (44%) malignant MCNs (HGD/carcinoma in situ) and three (8%) benign MCNs (p = 0.006) [21]. In our study, a lack of wall calcifications suggested a benign lesion.
Postlewait et al suggested that male gender was associated with malignancy [23]. In their study, up to 11% of patients were men with 29% presenting with a malignant lesion [23]. This proportion of men was surprisingly high compared to the large systematic review by Nilsson et al [3] or to the 2018 study by Keane et al which included 4.3% men, with one third having invasive cancer [24]. In our cohort, only one man who did not present with m-MCN was included. Finally, the patients in our series with m-MCN were older, like in previous studies [10,11,12,13,14,15,16,17,18,19,20,21,22,22].
The rate of m-MCN in our series was low, close to 10%, and only two more patients had HGD (3%). This is consistent with published studies. A large US series of 349 resected MCNs reported a 12.6% rate of invasive carcinoma and 2.3% of HGD [23]. Di Paola et al reported a higher rate of malignancies (24%), but the authors chose to include patients with liver metastases whom we excluded from our surgical study population [14]. We chose to separate patients with MCNs and HGD from those with invasive carcinoma, because the latter have been shown to have a poor prognosis, similar to that in patients with ductal carcinoma [4], while patients with MCN and HGD have a similar prognosis to those with a lower grade of MCN [13]. Nevertheless, preventive resection should be performed in these high-grade lesions and they should not simply be observed, especially when our combined malignant criteria are present.
Importantly, we report that visualization of a thin, non-enhancing wall with no mural nodules is a pattern that strongly suggests benign tumors. This is important because it can be used as criteria for the surgeon to perform parenchyma-sparing resection [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,25]. As previously mentioned, European guidelines suggest that some MCNs of the pancreas (i.e., < 4 cm without nodule) could be conservatively managed by surveillance. Those two additional features (thin non-enhancing wall) may be added to the two suggested by the guidelines. Overall, our study confirms both the diagnostic and prognostic value of MRI in the evaluation MCNs, as well as its role in excluding the presence of an invasive component.
Our study has certain limitations. First, it is a retrospective and single-center study. However, the patients were well documented and the study population was fairly large. Second, two different MRI machines, a 1.5-T and a 3.0-T MRI scanner have been used. The imaging protocols remained largely unchanged, as we only adjusted parameters to the field strength, and the manufacturer was the same for both machines. Moreover, the vast majority of imaging features we analyzed were qualitative and categorical and not quantitative. Third, this is a surgical series. Thus, non-resected lesions were not included. This may have introduced biases, and have favored certain MRI features in the analysis. Moreover, DWI was not available in all patients. Although we identified several valuable DWI features associated with MCN malignancy, they should be interpreted with caution. Finally, we did not compare MCNs to other round, unicystic lesions such as pseudocysts or macrocystic serous cystadenomas that may be difficult to differentiate from MCNs as this was not the aim of our study.
In conclusion, MCNs appear as round or oval cystic lesions of the left pancreas, which are typically posterior, exophytic, and located next to the left adrenal. On MRI, signal heterogeneity on T2-WI, wall thickness ≥ 5 mm, mural nodules (especially when ≥ 9 mm or enhancing), and enhancing septa were independently associated with malignancy. On the other hand, visualization of a thin, non-enhancing wall with no mural nodules suggests benign lesions.
Abbreviations
- b-MCN:
-
Benign mucinous cystic neoplasm
- HGD:
-
High-grade dysplasia
- MCN:
-
Mucinous cystic neoplasms
- m-MCN:
-
Malignant mucinous cystic neoplasm
References
Nagtegaal ID, Odze RD, Klimstra D et al (2020) The 2019 WHO classification of tumours of the digestive system. Histopathology 76:182–188
Zamboni G, Scarpa A, Bogina G et al (1999) Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 23:410–422
Nilsson LN, Keane MG, Shamali A et al (2016) Nature and management of pancreatic mucinous cystic neoplasm (MCN): a systematic review of the literature. Pancreatology 16:1028–1036
Hui L, Rashid A, Foo WC et al (2018) Significance of T1a and T1b carcinoma arising in mucinous cystic neoplasm of pancreas. Am J Surg Pathol 42:578
O'Toole D, Palazzo L, Hammel P et al (2004) Macrocystic pancreatic cystadenoma: the role of EUS and cyst fluid analysis in distinguishing mucinous and serous lesions. Gastrointest Endosc 59:823–829
Cizginer S, Turner B, Bilge AR, Karaca C, Pitman MB, Brugge WR (2011) Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas 40:1024–1028
Zhong N, Zhang L, Takahashi N et al (2012) Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin Gastroenterol Hepatol 10:192–198. e192
Genevay M, Mino-Kenudson M, Yaeger K et al (2011) Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann Surg 254
The European Study Group on Cystic Tumours of the Pancreas (2018) European evidence-based guidelines on pancreatic cystic neoplasms. Gut 67:789–804
Park JW, Jang J-Y, Kang MJ, Kwon W, Chang YR, Kim S-W (2014) Mucinous cystic neoplasm of the pancreas: is surgical resection recommended for all surgically fit patients? Pancreatology 14:131–136
Crippa S, Salvia R, Warshaw AL et al (2008) Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg 247:571
Yamao K, Yanagisawa A, Takahashi K et al (2011) Clinicopathological features and prognosis of mucinous cystic neoplasm with ovarian-type stroma: a multi-institutional study of the Japan Pancreas Society. Pancreas 40:67–71
Kosmahl M, Egawa N, Schröder S, Carneiro F, Lüttges J, Klöppel G (2002) Mucinous nonneoplastic cyst of the pancreas: a novel nonneoplastic cystic change? Mod Pathol 15:154–158
Jais B, Rebours V, Malleo G et al (2016) Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 65(2):305–312
Le Baleur Y, Couvelard A, Vullierme MP et al (2011) Mucinous cystic neoplasms of the pancreas: definition of preoperative imaging criteria for high-risk lesions. Pancreatology 11:495–499
Di Paola V, Manfredi R, Mehrabi S et al (2016) Pancreatic mucinous cystoadenomas and cystoadenocarcinomas: differential diagnosis by means of MRI. Br J Radiol 89:20150536
Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P (2008) Pancreatic insufficiency after different resections for benign tumours. Br J Surg 95:85–91
Regi P, Salvia R, Cena C, Girelli R, Frigerio I, Bassi C (2013) Cystic “feminine” pancreatic neoplasms in men. Do any clinical alterations correlate with these uncommon entities? Int J Surg 11:157–160
Procacci C, Carbognin G, Accordini S et al (2001) CT features of malignant mucinous cystic tumors of the pancreas. Eur Radiol 11:1626–1630
Garces-Descovich A, Beker K, Castillo-Angeles M et al (2018) Mucinous cystic neoplasms of the pancreas: high-resolution cross-sectional imaging features with clinico-pathologic correlation. Abdom Radiol (NY) 43:1413–1422
Postlewait LM, Ethun CG, McInnis MR et al (2017) Association of preoperative risk factors with malignancy in pancreatic mucinous cystic neoplasms: a multicenter study. JAMA Surg 152:19–25
Keane MG, Shamali A, Nilsson LN et al (2018) Risk of malignancy in resected pancreatic mucinous cystic neoplasms. Br J Surg 105:439–446
Goh BK, Tan Y-M, Chung Y-FA et al (2006) A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg 30:2236–2245
Manfredi R, Ventriglia A, Mantovani W et al (2015) Mucinous cystic neoplasms and serous cystadenomas arising in the body-tail of the pancreas: MR imaging characterization. Eur Radiol 25:940–949
Mamone G, Barresi L, Tropea A, Di Piazza A, Miraglia R (2020) MRI of mucinous pancreatic cystic lesions: a new updated morphological approach for the differential diagnosis. Updates Surg 72:617–637
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Marie-Pierre Vullierme, MD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Dr. Maxime Ronot and Dr. Jules Gregory kindly provided statistical advice for this manuscript. Both authors have significant statistical expertise. No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because the study was retrospective upon preoperative MRI.
Ethical approval
Institutional Review Board approval was obtained, Board -IRB 00006477- of HUPNVS, Paris 7 University, AP-HP.
Methodology
• retrospective
• case-control study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Vullierme, MP., Gregory, J., Rebours, V. et al. MRI is useful to suggest and exclude malignancy in mucinous cystic neoplasms of the pancreas. Eur Radiol 32, 1297–1307 (2022). https://doi.org/10.1007/s00330-021-08091-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08091-6