Abstract
Purpose
Meningiomas have an excellent survival prognosis, and radiotherapy (RT) is a central component of interdisciplinary treatment. During treatment planning, the definition of the target volume remains challenging using MR and CT imaging alone. This is the first study to analyze the impact of additional PET-imaging on local control (LC) and overall survival (OS) after high-precision RT.
Methods
We analyzed 339 meningiomas treated between 2000 and 2018. For analyses, we divided the patients in low-grade (n = 276) and high-grade (n = 63) cases. We performed RT in an adjuvant setting due to subtotal resection or later due to recurrent tumor growth. The target volumes were delineated based on diagnostic CT and MRI and, if available, additional PET-imaging (low-grade: n = 164, 59.4%; high-grade: n = 39, 61.9%) with either 68Ga-Dotanoc/Dotatoc, 18F-fluoroethyltyrosine or 11C-methionine tracer. Patients were treated with fractionated stereotactic RT with a median total dose and dose per fraction of 54 Gy and 1.8 Gy, respectively.
Results
Median follow-up was 5.6 years. For low-grade meningiomas, mean OS was 15.6 years and mean LC was 16.9 years; for high-grade cases mean OS was 11.6 years, and mean LC was 11.1 years. In univariate analyses, PET-imaging had a significant impact on OS (p = 0.035) and LC (p = 0.041) for low-grade meningiomas and remained significant (p = 0.015) for LC in the multivariate analysis. For high-grade cases, PET did not influence both OS and LC. Further prognostic factors could be identified.
Conclusions
For low-grade meningiomas, we showed that the addition of PET-imaging for target volume definition led to a significantly enhanced LC. Thus, PET improves the detection of tumor cells and helps distinguish between healthy tissue and meningioma tissue, especially during the treatment planning process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Radiotherapy (RT) is an alternative treatment to surgery for low-grade meningiomas, and it is often applied in an adjuvant setting after incomplete resection or for local tumor progression after surgery alone [1, 2]. In high-grade meningiomas, RT after resection should be recommended especially in WHO Grade III histology [3,4,5].
Modern RT techniques such as intensity modulated radiotherapy (IMRT) combined with image-guided radiotherapy (IGRT) increased safety and precision over the years. To date, meningiomas have an excellent survival prognosis of 85% and 62% at 10 years and 64% and 50% at 15 years for low-grade and high-grade cases, respectively [6].
It has been shown that using positron-emission tomography (PET) can be helpful in diagnostics of meningiomas. Studies by Afshar et al. [7] and Rachinger et al. [8] showed that the detection rate increases using PET-imaging. Several PET-tracers are available, including 18F-Fluoroethyltyrosine (18F-FET), 11C-Methionine (11C-MET), and 68Ga-Dotanoc/Dotatoc (68Ga-DOTA). However, most studies have focused on 68Ga-DOTA-imaging which seems to correlate more precisely with actual meningioma extension, especially when compared with 18F-FET-imaging [9]. For methionine PET, a similar sensitivity and specificity can be assumed as with 68Ga-DOTA-PET.
During radiation treatment planning, the definition of the planning target volume (PTV) remains challenging, and differentiation between healthy tissue, i.e., meninges, post-operative changes, and residual tumor, can be difficult using magnetic resonance imaging (MRI) and computed tomography (CT) alone. PET imaging has been used for meningioma treatment planning for more than 10 years. It helps to identify active tumor cells after subtotal resection for adjuvant RT and to precise tumor extent, e.g., for infiltration in bony structures, orbita, and cavernous sinus. Thus, for treatment planning, the addition of PET-imaging improves target volume definition [10,11,12,13].
To date, no randomized trials exist investigating the long-term impact of PET-imaging on outcomes. Hence, in this study, we compared our patients documented prospectively in our database to analyze the impact of additional PET-imaging on local control (LC) and overall survival (OS) after high-precision RT.
Methods
Patients
We analyzed 332 patients with 339 cranial meningiomas consecutively treated between 2000 and 2018 at the department of radiation oncology at the Klinikum rechts der Isar, Munich. Of all 332 patients, seven had meningiomas at a secondary location and two treatments. Cases with spinal meningiomas, early termination of treatment, or re-irradiation due to local recurrence were not included in these analyses. Patient data were collected prospectively and documented in the institutional database. For analyses, we divided the patients in low-grade (benign cases, n = 276) and high-grade (atypical/anaplastic cases, n = 63) meningiomas. Table 1 shows the patient characteristic. Histology grading was done by pathology or in cases with no resection based on imaging.
Treatment planning
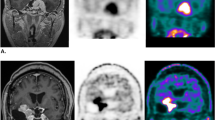
Treatment planning was performed using a stereotactic treatment setup with a thermoplastic mask system (Brainlab, Germany) and daily image-guidance (IGRT) by robotic ExacTrac positioning (Brainlab, Germany). An advanced radiation oncologist delineated gross tumor volume (GTV) on contrast-enhanced computed tomography (CT) and T1-weighted magnetic resonance images (MRI) images and, if available, additional PET-imaging (low-grade: n = 164, 59.4%; high-grade: n = 39, 61.9%) with either 68Ga-DOTA, 18F-FET or 11C-MET tracer was used for target definition (Fig. 1). The GTV was defined manually slice by slice by experienced radiation oncologist taking into account PET-tracer uptake in correlation with CT and MRI findings as described previously [9, 14, 15]; to date, no semiautomatic delineation tool is available since no standardized and widely transferable SUV has been published in those cases. In all patients, all three imaging modalities available were integrated into target volume delineation and observed in parallel in the treatment planning system. Previously, we had shown that the integration of PET-imaging improves target volume delineation and leads to substantial modification of target volumes [10, 14].
MRI and PET scans of two patients. GTVMRI is displayed in green, GTVPET is displayed in pink. a, b shows patient 1: GTVMRI = 24.9 ml, GTVPET = 33.94 ml, GTVintersection = 22.41 ml, GTV proportion due to PET 11.53 ml; c, d shows patient 2: GTVMRI = 5.86 ml, GTVPET = 16.33 ml, GTVintersection = 1.73 ml, GTV proportion due to PET 14.6 ml
For low-grade meningiomas, the clinical target volume (CTV) resulted from the GTV with an additional margin of 1–2 mm and 5–7 mm along the meninges. This was expanded further for 1–2 mm, resulting in the planning target volume (PTV). For high-grade meningiomas, the CTV resulted from GTV with an additional margin of 1–2 cm for WHO II and 2–3 cm for WHO III, and the PTV as CTV + 1–2 mm.
We used only PET-images acquired within a maximum of 50 days before RT. In our clinic, PET-planned meningioma treatment started in 2001 with 11C-MET until 2010, between 2004 and 2011 18F-FET tracer was used, and since 2011 only 68Ga-DOTA PETs are acquired.
Treatment and follow-up
RT was performed in an adjuvant setting due to subtotal resection or later due to recurrent tumor growth. The resection status was determined according to Simpson classification; in older and external cases the status was determined by postoperative MRI.
We treated all patients with high-precision fractionated stereotactic radiotherapy (FSRT) The median total dose and dose per fraction were 54 Gy (range 45–66 Gy) and 1.8 Gy (range 1.8–2 Gy), respectively.
Clinical neurological assessment, including contrast-enhanced MR imaging, was scheduled initially 4–6 weeks after treatment. Patients were followed up every 3 months in the first year and then every 6–12 months in the years after that or earlier with signs of tumor recurrence. As meningiomas tend to get lost to follow-up, we established a PRO assessment (patient-reported outcome) via mail and web portal to gain also long-term information [6]. A questionnaire was sent to all living patients within Germany. It contained questions about the last clinic visit, imaging, and its findings, as well as current symptoms.
Local control (LC) was defined as tumor growth of the treated lesion determined by the radiologist according to RECIST criteria. The response was then discussed and assessed in an interdisciplinary board. In most cases, follow-up treatment, e.g., resection, RT, confirmed the diagnosis.
Statistics
Statistical calculations were performed using SPSS Statistics v25 (IBM, USA). For patients treated with multiple courses, we used the first treatment date for overall survival (OS). LC was determined per meningioma treated. We calculated OS from the end of treatment until death or last follow-up; LC from the end of treatment until the date of local progression or until death or last follow-up. Outcome analyses were based on the Kaplan-Meier and Cox regression methods. The latter was also used for univariate (UVA) and multivariate analyses (MVA).
The following prognostic factors were analyzed: gender, age, Karnofsky Performance Score (KPS) before RT, target volume (PTV), previous resection and resection status, PET-imaging for treatment planning, PET-tracer, as well as the time between resection and RT. A p value < 0.05 was considered to indicate statistical significance.
Results
Outcome
Median follow-up was 5.6 years (range 0–18.4 years). For low-grade meningiomas, the mean OS was 15.6 years (95% confidence interval (CI), 14.9–16.4) and the mean LC was 16.9 years (95% CI 16.2–17.5); for high-grade cases mean OS was 11.6 years (95% CI 9.1–14.2), and mean LC was 11.1 years (95% CI 8.4–13.7) (median was not reached) (see also Table 2).
Prognostic factors
In univariate analyses (UVA, Table 3), PET-imaging had a significant impact on OS (p = 0.034) and LC (p = 0.042) for low-grade meningiomas (Fig. 2); for high-grade cases, PET had no influence.
UVA further identified a high KPS, small PTV, short time between resection and RT, young age, and female gender as prognostic factors for OS of low-grade meningiomas. The three factors KPS, PTV, and age were also significant in high-grade meningiomas. UVA was also calculated grouped by PET imaging for low-grade cases only.
Significant for LC of low-grade cases was also female gender and small PTV. For high-grade cases, small PTV and high KPS had a significant impact on LC in the UVA.
At the time of the analyses, the number of patients dead or with local recurrence was 49 and 20 patients (18%, 7.2%) for low-grade, and 15 and 18 patients (2.5%, 2.9%) for high-grade meningiomas, respectively. During long survival, it was not possible for us to determine cause of death; however, in the group of low-grade cases, 11 of 51 (22%) deceased patients had a local recurrence; in high-grade cases, 11 of 15 (73%) deceased patients most likely died because of local tumor progression.
This number of events limited the number of factors that could be analyzed in MVA, and as PET-imaging was not significant in univariate analyses (UVA) for high-grade cases, we performed MVA only for low-grade cases.
MVA for OS of low-grade meningiomas could be calculated with five factors. PET-imaging was no longer significant; only the age, the time between resection and RT, and the PTV remained significant factors. LC could be calculated with two factors, and PET-imaging remained significant with p = 0.012 as well as PTV with p < 0.001 (see Supplement Table 1 for all MVA).
Discussion
PET-imaging has been evaluated for treatment planning of meningiomas by several institutions. The present manuscript firstly describes a significant impact of PET-imaging on outcome after high-precision RT for meningiomas. Most prominently, the integration of PET-imaging into RT treatment planning significantly improves LC, which is the key endpoint in low-grade meningioma treatment.
Since meningioma histologies are intricate and low- and high-grade lesions are associated with different growth and aggressiveness patterns, evaluation of results was discriminated according to histology [2].
Low-grade meningiomas are slow-growing lesions; long-term LC with preservation of normal functioning is of utmost importance. The efficacy of high-precision RT has been shown previously, and a potential impact of PET into target volume definition has to date only been evaluated in smaller patient cohorts. The present study includes 332 patients with 339 meningiomas treated with primary RT. PET-imaging was performed with 11C-MET and 18F-FET tracer until 2011, and after that with 68Ga-DOTA as a tracer. Importantly, there is no difference in terms of LC between both tracers available for meningioma detection; however, for low-grade meningiomas, it could be clearly shown that the addition of PET-imaging for target volume definition led to a significantly enhanced LC compared with treatment planning with MRI and CT alone. Since low-grade meningiomas are generally associated with long-term survival and good overall prognosis, preservation of LC is key. Therefore, improvement of target volume delineation is a first essential step in the radiation oncology treatment planning process. Previously, we had shown that integration of PET-imaging significantly enhances target volume definition. More precisely, meningiomas with bony infiltration are generally seen best on CT-imaging, and integration of PET-imaging led to improved detection of meningioma tissue and to a subsequent enlargement of volumes. In meningiomas infiltrating into soft tissue or after extensive surgical resection, PET-imaging leads to better discrimination between tissue altered by surgical intervention or other soft tissue areas affected by edema. Therefore, target volumes in those lesions are significantly smaller when PET-imaging is added [10, 14]. The integration of PET-imaging into RT planning has been shown by several groups; however, to date no meaningful impact on outcome has been shown [10, 13, 14, 16,17,18]. In the present study, we show clearly that modification of target volumes based on PET-imaging significantly improves LC as a key outcome measure in low-grade meningiomas.
PET-imaging also impacted overall survival; however, the benefit remained only significant in univariate analyses. Since low-grade meningiomas are predominantly benign lesions, however, survival is a subordinated outcome measure in those patients. Mostly, age or intercurrent diseases lead to death, especially in patients with low-grade meningiomas; therefore, OS is comprehensibly unaffected by improved meningioma detection.
For high-grade meningiomas, no impact was found on both OS and LC. This correlates with the review findings by Galldiks et al. [19]. Target volume delineation of high-grade meningiomas remains more challenging. Firstly, the target volumes include macroscopic lesions but also infiltration zones to account for microscopic cell spread. To date, this infiltration zone is difficult to identify on imaging and therefore is generally based on established safety margins into alleged healthy tissue at risk for infiltration. Compared with low-grade meningiomas, the biology and growth patterns of high-grade meningiomas are generally more heterogeneous, and the values of FET or DOTATOC-PET are not as established as for low-grade meningiomas. For high-grade meningiomas, the infiltrative growth is a challenge for the radiation oncologist; the target volume for radiotherapy includes any visible tumor plus a safety margin accounting for this microscopic spread which ranges from 1 to 2 cm depending on personal experience and morphology of the tumor, as well as underlying histopathology [20, 21]. Since integration of improved mainly optimization of GTV, which is the relevant target on low-grade meningiomas, the lacking benefit in high-grades can be easily explained by the necessity of larger safety margins; there, PET-imaging might contribute to diagnosis of the tumor, however not to definition of high-precision target volume delineation.
When possible, microsurgery and radical removal of meningiomas remains the gold standard in treatment of meningiomas [1, 22]. Depending on the anatomical location of the meningioma, complete removal can be difficult to achieve while preserving normal nerve functioning and quality of life (QOL). Interdisciplinary discussion of each case is essential; in some cases, partial function-preserving resection followed by high-precision RT of the planned tumor remnant can be an alternative [23,24,25].
Before any treatment decision, elaborate imaging including CT, MRI, and ideally PET helps identify exact tumor extensions as well as any additional lesions present. For that purpose, the value of PET-imaging on the detection of meningiomas as well as on the precision of target volume definition has been shown previously [3, 4, 7, 9,10,11, 14,15,16, 26,27,28]. Afshar-Oromieh et al. [7] evaluated 134 patients in which 190 meningiomas could be detected by PET as compared with 171 by MRI, and CT alone. The study showed that the addition of 68Ga-DOTA-PET-imaging increased the detection rate and, in some cases, distant lesions were identified not seen clearly on CT and/or MRI.
Graf et al. [11] evaluated 16 patients with intracranial meningioma extension, visible on MRI ± CT (MRI/CT) or PET, and were evaluated further. They showed nicely that the mean volume was larger when delineated on PET than on MRI/CT only; however, intracranial invasion below the skull base was better detected with MRI/CT/PET than with PET-imaging alone. The impact on target volume delineation is largest in infiltration zones: In areas of bony infiltration, lesions seem lager on CT/MRT than shown on PET-imaging, whereas soft tissue infiltration can be better discriminated from other changes, such as postoperative, and target volumes become smaller by the addition of PET-imaging [10, 29]. Further analyses should also include the location of the lesion. PET imaging might be more useful in skull base lesions as opposed to convexity lesions where the tumor margins are expected to be relatively well delineated. The reduction of treatment volume also has an impact on dose to healthy tissue during RT: while the benefit of PET-imaging is marginal in proton therapy, the impact on dose reduction of healthy tissue is highly significant for patients treated with photons, such as with fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT) [14].
To date, the best tracer for meningioma imaging is most likely to be 68Ga-DOTA and most reports on PET-imaging focus on this tracer [14, 19, 30]. An alternative tracer with alleged equieffectiveness seems to be 11C-MET [16]. In the present analyses, we could not identify any differences regarding outcome when comparing tracers. However, the most commonly used tracer and the one with the most clinical experience remains to be 68Ga-DOTA. In the past, some institutions discussed 18F-FET for meningiomas. Comparative analysis, however, showed significant discrepancies between 68Ga-DOTA and 18F-FET-PET-imaging, and correction with macroscopic meningioma lesions as seen on MRI and CT was significantly higher with 68Ga-DOTA [9].
Regarding the prognostic factors analyzed, we found, in the multivariate evaluation female gender, smaller PTV, and a shorter time between surgery and RT to benefit OS; for LC PET imaging and a smaller PTV showed a significant impact. In our study, 68Ga-DOTA, as well as 11C-MET tracers, had been used for treatment planning. The results demonstrate no difference between both tracers applied, supporting the hypothesis that both, independently of their different biological target, have eligibility in patients with meningiomas. For the first time, we confirm that the integration of PET-imaging into meningioma RT planning improved LC significantly. Especially in low-grade meningiomas, LC is the most important outcome factor. Our analysis of potentially relevant prognostic factors revealed that, in the multivariate evaluation, smaller volume size had a more favorable outcome. For complete analysis, we also evaluated OS while keeping in mind that this endpoint is most likely not as relevant especially in low-grade histology. There, female gender, smaller PTV, and a shorter time between surgery and RT were associated with beneficial OS in multivariate analysis. While volumes as well as time issues are known prognostic factors in oncology, there is no other study having shown better outcome in female patients; perhaps, due to the fact that the female cohort is substantially larger than the male cohort, this may influence the difference in outcome; nevertheless, multivariate analysis might have evened out this difference in numbers.
The data from the present prospectively documented patients treated homogeneously within one institution clearly shows a benefit for PET-imaging in radiation treatment planning of low-grade meningiomas. One limitation might be that three PET-tracers have been evaluated, and for each tracer, the patient numbers are therefore comparably low. However, following general scientific discussions on which PET-tracer to use, there is an agreement that all of the above-used tracers have a role for meningioma imaging. Therefore, we think it is justified to pool all three tracer groups into one analysis which then clearly shows the impact of additional PET-imaging. Therefore, based on the results of the current study, we suggest that PET-imaging should be included in treatment guidelines for low-grade meningiomas, and patients should not be treated without additional PET-imaging for target volume definition. For high-grade meningiomas further analyses are necessary. Our small cohort of 60 cases is a limiting factor. To re-evaluate our results from the present study, we already initiated a multicenter study to gather a larger patient cohort of high-grade meningioma cases.
Conclusion
Our study firstly described a significant impact of PET-imaging on outcomes after high-precision RT for meningiomas. For low-grade meningiomas, it could be clearly shown that the addition of PET-imaging for target volume definition led to a significantly enhanced LC compared with treatment planning with MRI and CT alone. Thus, PET-imaging improves the detection of tumor cells and helps distinguish between healthy tissue and meningioma tissue, especially during the treatment planning process. Despite our results for high-grade cases, we plan a multicenter evaluation with a larger patient cohort to validate the results of both low- and high-grade cases. Further studies are intended to determine dosimetric predictors in addition to those prognostic factors identified in this analysis to develop a weighted scoring system for prognostic assessment for meningiomas.
References
Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–91.
Combs SE, Ganswindt U, Foote RL, Kondziolka D, Tonn JC. State-of-the-art treatment alternatives for base of skull meningiomas: complementing and controversial indications for neurosurgery, stereotactic and robotic based radiosurgery or modern fractionated radiation techniques. Radiat Oncol. 2012;7(1):226.
Rogers L, Zhang P, Vogelbaum MA, Perry A, Ashby LS, Modi JM, et al. Intermediate-risk meningioma: initial outcomes from NRG Oncology RTOG 0539. J Neurosurg. 2018;129(1):35–47.
Weber DC, Ares C, Villa S, Peerdeman SM, Renard L, Baumert BG, et al. Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: a phase-II parallel non-randomized and observation study (EORTC 22042-26042). Radiother Oncol. 2018;128(2):260–5.
Wang C, Kaprealian TB, Suh JH, Kubicky CD, Ciporen JN, Chen Y, et al. Overall survival benefit associated with adjuvant radiotherapy in WHO grade II meningioma. Neuro-oncology. 2017;19(9):1263–70.
Kessel KA, Fischer H, Oechnser M, Zimmer C, Meyer B, Combs SE. High-precision radiotherapy for meningiomas : Long-term results and patient-reported outcome (PRO). Strahlenther Onkol. 2017;193(11):921–30.
Afshar-Oromieh A, Giesel FL, Linhart HG, Haberkorn U, Haufe S, Combs SE, et al. Detection of cranial meningiomas: comparison of (6)(8)Ga-DOTATOC PET/CT and contrast-enhanced MRI. Eur J Nucl Med Mol Imaging. 2012;39(9):1409–15.
Rachinger W, Stoecklein VM, Terpolilli NA, Haug AR, Ertl L, Poschl J, et al. Increased 68Ga-DOTATATE uptake in PET imaging discriminates meningioma and tumor-free tissue. J Nucl Med. 2015;56(3):347–53.
Dittmar JO, Kratochwil C, Dittmar A, Welzel T, Habermehl D, Rieken S, et al. First intraindividual comparison of contrast-enhanced MRI, FET- and DOTATOC- PET in patients with intracranial meningiomas. Radiat Oncol. 2017;12(1):169.
Combs SE, Welzel T, Habermehl D, Rieken S, Dittmar JO, Kessel K, et al. Prospective evaluation of early treatment outcome in patients with meningiomas treated with particle therapy based on target volume definition with MRI and 68Ga-DOTATOC-PET. Acta Oncol. 2013;52(3):514–20.
Graf R, Nyuyki F, Steffen IG, Michel R, Fahdt D, Wust P, et al. Contribution of 68Ga-DOTATOC PET/CT to target volume delineation of skull base meningiomas treated with stereotactic radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(1):68–73.
Grosu AL, Weber WA, Astner ST, Adam M, Krause BJ, Schwaiger M, et al. 11C-methionine PET improves the target volume delineation of meningiomas treated with stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(2):339–44.
Milker-Zabel S, Zabel-du Bois A, Henze M, Huber P, Schulz-Ertner D, Hoess A, et al. Improved target volume definition for fractionated stereotactic radiotherapy in patients with intracranial meningiomas by correlation of CT, MRI, and [68Ga]-DOTATOC-PET. Int J Radiat Oncol Biol Phys. 2006;65(1):222–7.
Stade F, Dittmar JO, Jakel O, Kratochwil C, Haberkorn U, Debus J, et al. Influence of (68)Ga-DOTATOC on sparing of normal tissue for radiation therapy of skull base meningioma: differential impact of photon and proton radiotherapy. Radiat Oncol. 2018;13(1):58.
Afshar-Oromieh A, Wolf MB, Kratochwil C, Giesel FL, Combs SE, Dimitrakopoulou-Strauss A, et al. Comparison of (6)(8)Ga-DOTATOC-PET/CT and PET/MRI hybrid systems in patients with cranial meningioma: initial results. Neuro-Oncology. 2015;17(2):312–9.
Astner ST, Dobrei-Ciuchendea M, Essler M, Bundschuh RA, Sai H, Schwaiger M, et al. Effect of 11C-methionine-positron emission tomography on gross tumor volume delineation in stereotactic radiotherapy of skull base meningiomas. Int J Radiat Oncol Biol Phys. 2008;72(4):1161–7.
Graf R, Plotkin M, Steffen IG, Wurm R, Wust P, Brenner W, et al. Magnetic resonance imaging, computed tomography, and 68Ga-DOTATOC positron emission tomography for imaging skull base meningiomas with infracranial extension treated with stereotactic radiotherapy--a case series. Head Face Med. 2012;8(1):1.
Acker G, Kluge A, Lukas M, Conti A, Pasemann D, Meinert F, et al. Impact of 68Ga-DOTATOC PET/MRI on robotic radiosurgery treatment planning in meningioma patients: first experiences in a single institution. Neurosurg Focus. 2019;46(6):E9.
Galldiks N, Albert NL, Sommerauer M, Grosu AL, Ganswindt U, Law I, et al. PET imaging in patients with meningioma-report of the RANO/PET Group. Neuro-Oncology. 2017;19(12):1576–87.
Adeberg S, Hartmann C, Welzel T, Rieken S, Habermehl D, von Deimling A, et al. Long-term outcome after radiotherapy in patients with atypical and malignant meningiomas--clinical results in 85 patients treated in a single institution leading to optimized guidelines for early radiation therapy. Int J Radiat Oncol Biol Phys. 2012;83(3):859–64.
Rosenberg LA, Prayson RA, Lee J, Reddy C, Chao ST, Barnett GH, et al. Long-term experience with World Health Organization grade III (malignant) meningiomas at a single institution. Int J Radiat Oncol Biol Phys. 2009;74(2):427–32.
Frostell A, Hakim R, Dodoo E, Sinclair G, Ohlsson M, Forander P, et al. Adjuvant stereotactic radiosurgery reduces need for retreatments in patients with meningioma residuals. World Neurosurg. 2016;88:475–82.
Terpolilli NA, Ueberschaer M, Niyazi M, Hintschich C, Egensperger R, Muacevic A, et al. Long-term outcome in orbital meningiomas: progression-free survival after targeted resection combined with early or postponed postoperative radiotherapy. J Neurosurg. 2019;1–11. https://doi.org/10.3171/2019.3.JNS181760
Zanoletti E, Mazzoni A, Martini A, Abbritti RV, Albertini R, Alexandre E, et al. Surgery of the lateral skull base: a 50-year endeavour. Acta Otorhinolaryngol Ital. 2019;39(SUPPL. 1):S1–S146.
Kim JW, Jung HW, Kim YH, Park CK, Chung HT, Paek SH, et al. Petroclival meningiomas: long-term outcomes of multimodal treatments and management strategies based on 30 years of experience at a single institution. J Neurosurg. 2019;1–8. https://doi.org/10.3171/2019.2.JNS182604
Thorwarth D, Müller A-C, Pfannenberg C, Beyer T. Combined PET/MR imaging using 68Ga-DOTATOC for radiotherapy treatment planning in meningioma patients. In: Theranostics, Gallium-68, and other radionuclides, vol. 194. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012. p. 425–39.
Henze M, Schuhmacher J, Hipp P, Kowalski J, Becker DW, Doll J, et al. PET imaging of somatostatin receptors using [68GA]DOTA-D-Phe1-Tyr3-octreotide: first results in patients with meningiomas. J Nucl Med. 2001;42(7):1053–6.
Nyuyki F, Plotkin M, Graf R, Michel R, Steffen I, Denecke T, et al. Potential impact of (68)Ga-DOTATOC PET/CT on stereotactic radiotherapy planning of meningiomas. Eur J Nucl Med Mol Imaging. 2010;37(2):310–8.
Kunz WG, Jungblut LM, Kazmierczak PM, Vettermann FJ, Bollenbacher A, Tonn JC, et al. Improved detection of transosseous meningiomas using (68)Ga-DOTATATE PET/CT compared with contrast-enhanced MRI. J Nucl Med. 2017;58(10):1580–7.
Sommerauer M, Burkhardt JK, Frontzek K, Rushing E, Buck A, Krayenbuehl N, et al. 68Gallium-DOTATATE PET in meningioma: a reliable predictor of tumor growth rate? Neuro-Oncology. 2016;18(7):1021–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The local ethics committee of the Medical Faculty of the Technical University Munich (TUM) approved the study, vote number 434/15. This study is registered under the open science framework: DOI 10.17605/OSF.IO/RYX9D.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology – Brain
Electronic supplementary material
ESM 1
(DOCX 43 kb)
Rights and permissions
About this article
Cite this article
Kessel, K.A., Weber, W., Yakushev, I. et al. Integration of PET-imaging into radiotherapy treatment planning for low-grade meningiomas improves outcome. Eur J Nucl Med Mol Imaging 47, 1391–1399 (2020). https://doi.org/10.1007/s00259-019-04591-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-019-04591-2