Abstract
Objective
To determine the incidence and MRI characteristics of the spectrum of posterolateral corner (PLC) injuries occurring in association with anterior cruciate ligament (ACL) rupture.
Materials and methods
We carried out a level IV, retrospective case series study. All patients clinically diagnosed with an ACL rupture between July 2015 and June 2016 who underwent MRI of the knee were included in the study. In addition to standard MRI knee reporting, emphasis was placed on identifying injury to the PLC and a description of involvement of these structures by two musculoskeletal radiologists. Association with PLC involvement was sought with concomitant injuries using correlation analysis and logistic regression.
Results
One hundred sixty-two patients with MRI following ACL rupture were evaluated. Thirty-two patients (19.7%) had an injury to at least one structure of the PLC, including the inferior popliteomeniscal fascicle (n = 28), arcuate ligament (n = 20), popliteus tendon (n = 20), superior popliteomeniscal fascicle (n = 18), lateral collateral ligament (n = 8), popliteofibular ligament (n = 7), biceps tendon (n = 4), iliotibial band (n = 3), and fabellofibular ligament (n = 1). Seventy-five percent of all patients with combined ACL and PLC injuries had bone contusions involving the lateral compartment of the knee. The presence of these contusions strongly correlated with superior popliteomeniscal fascicle lesions (p < 0.05). There was no correlation between injuries to other structures of the PLC and other intra-articular lesions.
Conclusion
Missed injuries of the PLC lead to considerable morbidity. The relevance of this study is to highlight that these injuries occur more frequently than previously described and that an appropriate index of suspicion, clinical examination, and MRI are all required to reduce the risk of missed diagnoses. The results of this study support previous suggestions that the rate of concomitant PLC injury in the ACL-deficient knee is under-reported. The rate of combined injuries in this series was 19.7%. The key message of this paper is that PLC injury is common in the presence of ACL injury and should be sought both clinically and radiologically.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Successful anterior cruciate ligament (ACL) reconstruction is defined by complete return of knee function without instability [1–3]. Numerous in vitro studies have shown that a deficiency of posterolateral corner (PLC) structures results in persistent knee instability and increased load on the grafts used for cruciate ligament reconstruction [4–9]. LaPrade et al. demonstrated that injuries to the PLC can result in a significant increase in the varus load on an ACL graft, thus increasing the risk for graft failure [6]. More recently, Bonanzinga et al. demonstrated that even in the presence of a partial lesion of the PLC, an isolated ACL reconstruction is not able to fully control rotational laxity of the knee [5].
These findings are consistent with data from clinical series with high rates of ACL graft rupture (ranging from 15 to 24%), being attributed to a lack of recognition of the PLC injury [6, 10, 11]. High rates (50–76%) of missed diagnoses of PLC injuries, even after a clinical review of patients by orthopedic surgeons, are reported [11]. Some authors have suggested that the correct diagnosis is frequently challenging owing to the anatomical complexity of this region and difficulty in interpreting MRI findings in the presence of diffuse post-traumatic soft-tissue edema [12–14]. To reduce the rate of missed diagnoses, it is important to have an appropriate index of suspicion for PLC injury [15–17]. This can only be guided by accurate data on its incidence in the ACL-injured knee.
The aim of this study was to determine the incidence and MRI characteristics of the spectrum of PLC injuries occurring in association with ACL rupture. The hypothesis was that the prevalence of PLC injury that occurs in the ACL-injured knee is higher than previously reported.
Materials and methods
Institutional Review Board approval was granted for this study. All patients with a clinical diagnosis of ACL rupture undergoing MRI evaluation of the knee between 1 July 2015 and 30 June 2016 at first assessment were included. Informed consent was obtained from patients before enrolment in the study.
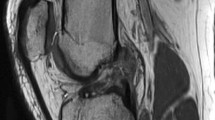
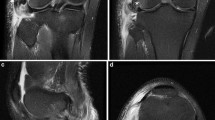
All patients presented with acute injuries and underwent MRI within 1 week of the initial clinical examination. In addition to standard MRI knee reporting practice, particular emphasis was placed on identifying injury to the PLC in its complexity (Fig. 1) and describing precisely the spectrum of involvement of these structures, as previously described [12, 16, 18–22]. Two independent radiologists with more than 10 years of experience in musculoskeletal radiology evaluated all cases. Previous radiological and anatomical descriptions were used as a basis for interpretation of the MR imaging of the PLC (Figs. 2, 3) [9, 11, 16, 17, 19, 20, 22–24].
MRI images demonstrating the normal posterolateral corner. a Sagittal view with arcuate ligament, capsule, and popliteomeniscal fascicles. b Coronal view of biceps femoris tendon. c Coronal view of lateral collateral ligament. d Axial view of the popliteus tendon. e Axial view of lateral collateral ligament. f Coronal view of popliteofibular ligament. g Sagittal view of popliteus tendon
MRI images demonstrating lesions in the posterolateral corner. a Sagittal view with arcuate ligament tear. b Sagittal view of popliteomeniscal fascicle tears. c Coronal view of lateral collateral ligament and popliteus tendon tear. d Coronal view of popliteofibular ligament tear. e Axial view of popliteus tendon tear. f Axial view of arcuate ligament tear
Magnetic resonance imaging was performed using a 1.5-T magnet with a wide-bore configuration (MAGNETOM Avanto, Siemens, Munich, Germany). Each scanning protocol commenced with a scout image in the axial, sagittal, and coronal planes. The axial images obtained were true axials and were derived directly from the scout image. In this image, injuries to the lateral meniscus, lateral collateral ligament (LCL), posterolateral joint capsule, and arcuate and fabellofibular ligaments were analyzed. The sagittal images were based on anatomical landmarks to individualize the sequence, ensuring proper visualization of the ACL and its two bundles for every patient. A plane was prescribed along the lateral femoral epicondyle at the level of the LCL, which is a constant anatomical landmark with little interpersonal variability. Evaluation of injuries (sprain or rupture) to the popliteus tendon, popliteomeniscal fascicles (superior and inferior), LCL, popliteofibular ligament (PFL), biceps tendon, and posterolateral joint capsule were made. An oblique sagittal sequence was also obtained. This plane was parallel to the ACL and allowed optimal visualization of both the anteromedial (AM) and posterolateral (PL) bundles. Routine coronal images for LCL, PFL, and biceps tendon evaluation and then special oblique–coronal images were obtained in the long axis of the ACL, starting at the intercondylar roof of Blumensaat’s line. The coronal–oblique sequence increases the sensitivity and specificity of diagnosing isolated AM or PL bundle injuries. It also helps to visualize the proximal insertion of the bundles for hemorrhage and rupture. A slice thickness of 3 mm can differentiate the two bundles as separate entities. In imaging these patients, it was important that the sagittal images covered the lateral-most portion of the knee joint so that the meniscocapsular junction was noted [19, 21].
The diagnosis of meniscal tears was performed using widely accepted criteria and reported according to their location [25, 26]. Injuries to the medial and LCLs were graded (0–3) according to Schweitzer et al., [27], and all injuries were considered significant for the purposes of this study according to Pacheco et al. [11]. For the PLC injuries, if the contour of the structures analyzed was irregular or if ligamentous edema existed, then the radiologists considered the structure to be abnormal. If only periligamentous edema existed, with identifiable, continuous low-signal intensity fibers, the ligament was considered intact [28]. Osseous injuries including bone contusions, cortical depression and trabecular fractures were located on the lateral and medial femoral condyle in addition to the lateral and medial tibial plateau. Injuries were classified as anterior, central, and posterior and were counted separately [29, 30].
All analyses were made with using the SPSS software (version 20.0, SPSS, Chicago, IL, USA) and the significance level was set at 5%. The Chi-squared test was used to evaluate the association between PLC and other injuries. Spearman’s rank correlation coefficient and logistic regression were also used to determine whether any variables were predictive of concomitant PLC injury in the ACL-deficient knee. Kappa (κ) values were measured to assess inter- and intra-reader agreement for determining the PLC lesions on MRI. The κ values were interpreted according to Landis and Koch recommendations [31].
Results
One hundred and sixty-two patients had a clinical diagnosis of ACL injury and subsequent MRI evaluation of the knee during the study period. MRI-proven combined ACL and PLC injuries occurred in 32 patients (19.7%). These patients were then further evaluated. Twenty-six patients (81.3%) were male. The mean of age was 32 years (±13). Right- and left-sided injuries occurred with equal frequency. Bone contusions occurred with the following frequencies: lateral tibial plateau 75%, lateral femoral condyle 75%, medial tibial plateau 46.9%, and medial femoral condyle 28.1%. Of the 32 patients, 46.9% had an MRI-proven medial meniscus lesion and 65.6% had a lesion of the lateral meniscus.
The distribution of the frequency of specific lesions involving the PLC is reported in Fig. 4. Table 1 demonstrates the correlation between concomitant injuries and the four most common structures of the PLC involved in this series (inferior and superior popliteomeniscal fascicle, popliteus tendon, and arcuate ligament). The presence of bone contusions was strongly correlated with superior popliteomeniscal fascicle lesions (p < 0.05). The other structures of the PLC could not be statistically analyzed for correlation with concomitant injuries owing to insufficient numbers. However, there was no correlation between other lesions of the PLC and other lesions after logistic regression analysis (p > 0.05). Intra-reader agreement was good for both readers (κ = 0.79 and 0.86). There was also good inter-reader agreement (κ = 0.79).
Discussion
In this MRI-based study, the main finding was that the incidence of PLC injury in the ACL-deficient knee was 19.7%. This is higher than the previously reported rates of 5–14.7% [11, 13, 14, 23, 24, 32, 33]. It is postulated that the relatively high rate of concomitant injuries to the PLC reported in the current series is a reflection of previous under-reporting and missed diagnoses, as alluded to by several authors [11, 13, 14, 23, 24, 32].
Although PLC injury is a well-known entity, it is surprisingly frequently missed, probably because of a poor understanding of the complex anatomy of this region [11, 19, 32, 34]. The major confusion about PLC anatomy found in the orthopedic literature lies with the structures that course from the fibular head and that attach to the popliteus complex and the joint capsule of the PL aspect of the knee. All these structures are difficult to identify separately in cadaveric and clinical evaluations. This anatomical complexity explains why most studies describe only the main structures of the PLC (LCL, PFL, and popliteus tendon) and this contributes to the relative under-diagnosis of injuries in previously reported studies compared with the current study, which evaluates all the structures within the PLC [15, 17, 19, 35]. In addition, in contrast to previous studies that evaluated patients with grade 3 PLC injury, the current study also included patients with grade 1 and 2 injuries. However, there are a number of additional reasons for the under-diagnosis of PLC injury. These include failure to perform appropriate clinical tests or using tests which have a low sensitivity [11, 14, 17, 18, 36–39] and the timing of imaging studies [11, 14, 17, 18, 36]. It is recognized that MRI performed within 3 weeks of the initial knee trauma is associated with better sensitivity and specificity in identifying PLC lesions than delayed imaging [11, 14, 18, 19, 21].
It is apparent that a greater awareness of the normal and abnormal MRI appearances of the structures of the PLC of the knee and of the patterns of injury often seen in patients with PLC rotatory instability will help clinicians suggest the diagnosis of PLC injury, even if it is not clinically detected [16, 19, 24]. The fact that the rate of combined ACL and PLC injuries in this series was almost 20%, supports the role of thorough clinical and MRI evaluation for PLC injury in all ACL-deficient knees [11, 18, 36]. Although many articles have reported the role of the PLC in rotatory stability, to our knowledge, this is the first to report a detailed radiological and anatomical characterization of the spectrum of injury in a large prospective series of ACL-injured knees [8, 33, 35, 37, 40, 41].
In this study, bone contusions occurred in 75% of patients in the lateral tibial plateau and lateral femoral condyle. The rate of bone contusions in patients with an ACL rupture but without a PLC injury was not evaluated, as the purpose of this series was to evaluate only the spectrum of PLC injuries occurring in association with ACL rupture. There was a statistically significant correlation between lateral bone contusions and superior popliteomeniscal fascicle lesions. However, this pattern of bone bruising is not specific to this injury, as other authors have also reported that lateral bone contusions are associated with lateral meniscal lesions, MCL tear, anterolateral ligament injury, high-grade (grade II and III) pivot shift, and that the severity of these injuries correlates with the degree of bone contusion [15, 30, 42]. Yoon et al. reported the same pattern, where the incidence of injuries to the menisci and the MCL were positively associated with the incidence of bone contusion [42]. In addition, Shaw et al. reported that lateral meniscus injuries were associated with a higher incidence of PLC injury (p > 0.05) [15]. These findings suggest that where any features of increased severity of knee injury might exist, the index of suspicion of injury to the PLC should be raised and a more careful clinical and radiological evaluation of those structures is warranted.
The main limitation of this study is the relatively small number of patients with PLC injury included; however, this reflects previous literature on this topic and includes all patients seen within a 12-month period in a busy tertiary referral specialist knee clinic. Despite the small numbers, the lack of similar studies characterizing the spectrum of PLC injuries in ACL-deficient knees means that this study is able to provide useful benchmark data to highlight the frequency and extent of these combined injuries. However, it is recognized that studies with larger numbers of patients may further elucidate the complex relationships of this lesion with ACL injuries and other lesions. A further important limitation is the absence of clinical follow-up of these patients; in particular, it is not known whether they required PLC reconstruction. The lack of these data means that it is not possible to correlate the radiological severity of PLC injury with clinically apparent instability. However, previous authors have suggested that the presence of tears of two or more structures of the PLC is a hallmark of high-grade injury and should direct the orthopedic surgeon to carefully examine the rotatory instability and evaluate if repair or reconstruction are necessary [7, 8, 16, 20, 22, 34, 37, 43]. Another limitation is that we did not record the precise mechanism of injury. It may be the case that the rate of injury to the PLC identified in this series is higher than in previous reports because of the heterogeneity with regard to mechanisms of injury. It is also possible that these findings were influenced by observer bias, as the radiologists were not blinded. Therefore, there was a risk of overcalling injuries, particularly when the focus of the study was to evaluate injuries to the PLC. However, the study design attempted to minimize bias by an independent evaluation of imaging by two highly experienced radiologists. Good inter- and intra-reader agreement provides some reassurance that the risk of the influence of observer bias on the key findings of this study was low.
The key message from this study is that PLC injury is more common than previously reported. This should result in an increased index of suspicion when evaluating the ACL-injured knee. Clearly, further studies will be required to clarify the long-term clinical relevance of MRI observations [8].
Conclusion
The incidence of PLC injury in the ACL-deficient knee has previously been under-reported. This series demonstrates that nearly 20% of patients with an ACL rupture have some injury to the PLC when evaluated by MRI. Further clinical and biomechanical studies are required to clarify the long-term clinical relevance of these injuries.
References
Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009;12(6):622–7.
Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91(10):2321–8.
Bourke HE, Salmon LJ, Waller A, Patterson V, Pinczewski LA. Survival of the anterior cruciate ligament graft and the contralateral ACL at a minimum of 15 years. Am J Sports Med. 2012;40(9):1985–92.
Amis AA, Bull AM, Lie DT. Biomechanics of rotational instability and anatomic anterior cruciate ligament reconstruction. Oper Tech Orthop. 2005;15(1):29–35.
Bonanzinga T, Signorelli C, Lopomo N, Grassi A, Neri MP, Filardo G, Zaffagnini S, Marcacci M. Biomechanical effect of posterolateral corner sectioning after ACL injury and reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2918–24.
LaPrade RF, Resig S, Wentorf F, Lewis JL. The effects of grade III posterolateral knee complex injuries on anterior cruciate ligament graft force. A biomechanical analysis. Am J Sports Med. 1999;27(4):469–75.
Mouton C, Theisen D, Pape D, Nuhrenborger C, Seil R. Static rotational knee laxity in anterior cruciate ligament injuries. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):652–62.
Thaunat M, Pioger C, Chatellard R, Conteduca J, Khaleel A, Sonnery-Cottet B. The arcuate ligament revisited: role of the posterolateral structures in providing static stability in the knee joint. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2121–7.
Plaweski S, Belvisi B, Moreau-Gaudry A. Reconstruction of the posterolateral corner after sequential sectioning restores knee kinematics. Orthop J Sports Med. 2015;3(2):2325967115570560.
Indelicato PA, Bittar ES. A perspective of lesions associated with ACL insufficiency of the knee. A review of 100 cases. Clin Orthop Relat Res 1985;(198):77–80.
Pacheco RJ, Ayre CA, Bollen SR. Posterolateral corner injuries of the knee: a serious injury commonly missed. J Bone Joint Surg Br. 2011;93(2):194–7.
Miller TT, Gladden P, Staron RB, Henry JH, Feldman F. Posterolateral stabilizers of the knee: anatomy and injuries assessed with MR imaging. AJR Am J Roentgenol. 1997;169(6):1641–7.
Recondo JA, Salvador E, Villanua JA, Barrera MC, Gervas C, Alustiza JM. Lateral stabilizing structures of the knee: functional anatomy and injuries assessed with MR imaging. Radiographics. 2000;20(Spec No):S91–102.
LaPrade RF, Wentorf FA, Fritts H, Gundry C, Hightower CD. A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy. 2007;23(12):1341–7.
Shaw KA, Dunoski BS, Mardis NJ, Pacicca DM. Combined posterolateral corner and acute anterior cruciate ligament injuries in an adolescent cohort: a magnetic resonance imaging analysis. Int Orthop. 2016;40(3):555–60.
Gimber LH, Hardy JC, Melville DM, Scalcione LR, Rowan A, Taljanovic MS. Normal magnetic resonance imaging anatomy of the capsular ligamentous supporting structures of the knee. Can Assoc Radiol J, 2016;67(4):356–67.
Chahla J, Moatshe G, Dean CS, LaPrade RF. Posterolateral corner of the knee: current concepts. Arch Bone Jt Surg. 2016;4(2):97–103.
Collins MS, Bond JR, Crush AB, Stuart MJ, King AH, Levy BA. MRI injury patterns in surgically confirmed and reconstructed posterolateral corner knee injuries. Knee Surg Sports Traumatol Arthrosc. 2015;23(10):2943–9.
Bolog N, Hodler J. MR imaging of the posterolateral corner of the knee. Skeletal Radiol. 2007;36(8):715–728.
Vinson EN, Major NM, Helms CA. The posterolateral corner of the knee. AJR Am J Roentgenol. 2008;190(2):449–58.
Vohra S, Arnold G, Doshi S, Marcantonio D. Normal MR imaging anatomy of the knee. Magn Reson Imaging Clin N Am. 2011;19(3):637–53.
Flandry F, Hommel G. Normal anatomy and biomechanics of the knee. Sports Med Arthrosc. 2011;19(2):82–92.
LaPrade RF, Terry GC. Injuries to the posterolateral aspect of the knee. Association of anatomic injury patterns with clinical instability. Am J Sports Med. 1997;25(4):433–8.
Tyler P, Datir A, Saifuddin A. Magnetic resonance imaging of anatomical variations in the knee. I. Ligamentous and musculotendinous. Skeletal Radiol. 2010;39(12):1161–73.
Oei EH, Nikken JJ, Verstijnen AC, Ginai AZ, Myriam Hunink MG. MR imaging of the menisci and cruciate ligaments: a systematic review. Radiology. 2003;226(3):837–48.
Sanders TG, Miller MD. A systematic approach to magnetic resonance imaging interpretation of sports medicine injuries of the knee. Am J Sports Med. 2005;33(1):131–48.
Schweitzer ME, Tran D, Deely DM, Hume EL. Medial collateral ligament injuries: evaluation of multiple signs, prevalence and location of associated bone bruises, and assessment with MR imaging. Radiology. 1995;194(3):825–9.
Helito CP, Helito PV, Bonadio MB, Pecora JR, Bordalo-Rodrigues M, Camanho GL, Demange MK. Correlation of magnetic resonance imaging with knee anterolateral ligament anatomy: a cadaveric study. Orthop J Sports Med. 2015;3(12):2325967115621024.
Van Dyck P, Clockaerts S, Vanhoenacker FM, Lambrecht V, Wouters K, De Smet E, Gielen JL, Parizel PM. Anterolateral ligament abnormalities in patients with acute anterior cruciate ligament rupture are associated with lateral meniscal and osseous injuries. Eur Radiol, 2016;26(10):3383–91.
Song GY, Zhang H, Wang QQ, Zhang J, Li Y, Feng H. Bone contusions after acute noncontact anterior cruciate ligament injury are associated with knee joint laxity, concomitant meniscal lesions, and anterolateral ligament abnormality. Arthroscopy, 2016;32(11):2331–41.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Beall DP, Googe JD, Moss JT, Ly JQ, Greer BJ, Stapp AM, Martin HD. Magnetic resonance imaging of the collateral ligaments and the anatomic quadrants of the knee. Radiol Clin N Am. 2007;45(6):983–1002.
Lee SH, Jung YB, Jung HJ, Song KS, Ko YB. Combined reconstruction for posterolateral rotatory instability with anterior cruciate ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 2010;18(9):1219–25.
George J, Saw KY, Ramlan AA, Packya N, Tan AH, Paul G. Radiological classification of meniscocapsular tears of the anterolateral portion of the lateral meniscus of the knee. Australas Radiol. 2000;44(1):19–22.
LaPrade RF, Wozniczka JK, Stellmaker MP, Wijdicks CA. Analysis of the static function of the popliteus tendon and evaluation of an anatomic reconstruction: the “fifth ligament” of the knee. Am J Sports Med. 2010;38(3):543–9.
Schweller EW, Ward PJ. Posterolateral corner knee injuries: review of anatomy and clinical evaluation. J Am Osteopath Assoc. 2015;115(12):725–31.
Zlotnicki JP, Naendrup JH, Ferrer GA, Debski RE. Basic biomechanic principles of knee instability. Curr Rev Musculoskelet Med. 2016;9(2):114–22
Jung YB, Lee YS, Jung HJ, Nam CH. Evaluation of posterolateral rotatory knee instability using the dial test according to tibial positioning. Arthroscopy. 2009;25(3):257–61.
Sekiya JK, Whiddon DR, Zehms CT, Miller MD. A clinically relevant assessment of posterior cruciate ligament and posterolateral corner injuries. Evaluation of isolated and combined deficiency. J Bone Joint Surg Am. 2008;90(8):1621–7.
Daggett M, Claes S, Helito CP, Imbert P, Monaco E, Lutz C, Sonnery-Cottet B. The role of the anterolateral structures and the ACL in controlling laxity of the intact and ACL-deficient knee: letter to the editor. Am J Sports Med. 2016;44(4):NP14–5.
Kim SJ, Choi DH, Hwang BY. The influence of posterolateral rotatory instability on ACL reconstruction: comparison between isolated ACL reconstruction and ACL reconstruction combined with posterolateral corner reconstruction. J Bone Joint Surg Am. 2012;94(3):253–9.
Yoon KH, Yoo JH, Kim KI. Bone contusion and associated meniscal and medial collateral ligament injury in patients with anterior cruciate ligament rupture. J Bone Joint Surg Am. 2011;93(16):1510–8.
Smith 3rd JP, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am J Sports Med. 2001;29(4):415–9.
Acknowledgements
The authors wish to thank the patients who participated in the study and the radiology centers (Ana Rita Soares, Guilherme Reis, and Sérgio Couto, Rogéria Rodrigues, and Alexia Abuhid) that carried out the assessment of images.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
B.S.-C. receives royalties from Arthrex Inc., is a paid consultant, receives research support, and has made presentations for Arthrex; M.T. is a paid consultant, receives research support, and has made presentations for Arthrex; A.S. is a paid consultant for Arthrex.
Additional information
Investigation performed at Hospital Madre Teresa, Belo Horizonte, Minas Gerais, Brazil, and Centre Orthopedic Santy, Lyon, France
Rights and permissions
About this article
Cite this article
Temponi, E.F., de Carvalho Júnior, L.H., Saithna, A. et al. Incidence and MRI characterization of the spectrum of posterolateral corner injuries occurring in association with ACL rupture. Skeletal Radiol 46, 1063–1070 (2017). https://doi.org/10.1007/s00256-017-2649-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-017-2649-y