Abstract
Purpose
Posterolateral corner structures functionally interact with the ACL. The aim of this study was to investigate the capability of an isolated ACL reconstruction control laxity parameters in a knee with combined ACL and PLC and the increase in terms of laxity produced by the resection of the PC in an ACL-deficient knee.
Method
An in vitro cadaveric study was performed on seven knees. The joints were analysed in the following conditions: intact, after ACL resection, after popliteus complex resection, after ACL reconstruction and after LCL. Testing laxity parameters were recorded with an intra-operative navigation system and defined as: AP displacement at 30° and 90° of flexion (AP30 and AP90) applying a 130 N load and IE at 30° and 90° of knee flexion with a 5 N load.
Results
Sectioning the ACL significantly increased the AP30 at 30° and 90° of knee flexion (p < 0.05). At 90° of knee flexion, the resection of the LCL determined a significant increase in terms of AP laxity (p < 0.05). At 90° has been found a significant difference for the IE laxity (p < 0.05) after PC resection. Sectioning the LCL produced a significant increase in IE laxity at 30° and 90° of knee flexion (p < 0.05).
Conclusion
Isolated ACL reconstruction is able to control the AP laxity with a combined complete lesion of the PLC at 30° of knee flexion, but not at higher angle of knee flexion. Considering the IE rotations, the reconstruction was not sufficient not even to control a partial lesion of the PLC. These findings suggest that additional surgical procedures should be considerate even when facing combined PLC lesion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anterior cruciate ligament (ACL) reconstruction is one of the most commonly performed procedures in orthopaedics for ACL injury [5]. Successful anterior cruciate ligament reconstruction depends on multiple factors. Recently, the importance of the secondary restraint of the knee has been highlighted as key factor in the failure of ACL reconstruction [1, 20]. Combined injuries to posterolateral corner (PLC) determine a pathological posterolateral laxity when an external rotational force is applied to the knee joint. Previous biomechanical studies have demonstrated that posterolateral corner structures functionally interact with the cruciates [13, 14, 19]. LaPrade et al. [14] have shown that the deficiency of posterolateral structures significantly increases the varus load on the ACL graft, determining an increased risk of failure. Therefore, in the combined injury setting, the consequence of missing a posterolateral lesion may affect the outcome of the anterior cruciate ligament reconstruction. Many authors have advocated unrecognized posterolateral laxity as a main cause of graft failures [2, 10, 12, 25]. The incidence of PLC injuries in ACL-deficient knee, ranging from 7.4 to 13.9 % [15], is probably under-reported. This is probably due to the fact that the correct diagnosis of a concomitant lesion occurring to the posterolateral region is often challenging due to its anatomical and biomechanical complexity. The two primary components of this complex structure are the lateral collateral ligament (LCL) and the popliteus complex (PC) [35]. The PC includes the popliteus muscle–tendon unit and all its connection to the fibula, tibia and meniscus. Other ligaments components of the PLC such as the arcuate ligament complex and the fabellofibular ligament have less biomechanical significance [3, 9, 11, 27, 30].
In the available literature, there is lack of data focusing on the capability of an isolated ACL reconstruction to control laxity parameters in PLC-deficient knee.
Therefore, the aim of this study was to investigate the capability of an isolated ACL reconstruction to restore the static laxity parameters in a knee with an ACL lesion combined to a partial and a complete PLC lesion. Further, the increase in terms of static laxity parameter was produced by the resection of the PC in an ACL-deficient knee.
Materials and methods
Eight knees from four fresh-frozen normal complete cadaveric specimens, age at time of death 80 ± 3 years old, were recruited in the study. All specimens were thawed from 24 to 48 h prior to testing. During the experimental procedure, the orthopaedic surgeon, who performed the surgical as well as the testing part of the study, controlled each joint to exclude soft tissue pathologies, arthritis, anatomical deformities or previous surgical interventions. One knee was excluded due to severe arthritis, and therefore seven knees were finally included. Skin and subcutaneous fat were removed to access the stabilizing structures. A parapatellar medial arthrotomy was performed to access the ACL. The iliotibial band was cut along its longitudinal fibres to access the LCL insertion on the fibula. The ligamentous and the tendinous structures were left intact.
Knee kinematics as well as anatomical data were acquired by a navigation system which consists of an optical localizer (Polaris, NDI, Waterloo, Ontario, Canada) and a custom-made software (MATLAB, The MathWorks Inc., Natick, Massachusetts, USA). During the test, the correct limb position was maintained by a testing rig where the foot of the tested limb was fastened.
In order to track the relative motion between the tibia and the femur, trackers provided by optical passive markers were mounted on both tibial and femoral bone by threaded Steinmann pins. The 3D root mean square (RMS) volumetric accuracy of the navigation system in the localization of a single passive marker is equal to 0.35 mm [7]. According to this latter value and choosing for a more conservative approach, all our results were rounded to the first decimal digit. In a previous in vivo study without load control, it has been also evaluated the reliability of the applied testing method. In particular, it has been found an intra-tester repeatability of about 1 mm for AP30/AP90 test, 1° for VV0/VV30 test stress test and 2° for IE30/IE90 test [18, 33].
The anatomical system of references, required to evaluate the knee joint kinematics, was identified through the acquisition of anatomical bony landmarks (medial and lateral malleolus, most medial and most lateral point of tibial plateaux, medial and lateral epicondyle) using a tracked probe. Moreover, the hip joint centre (HJC) necessary to complete the femoral system of reference was calculated through a pivoting motion [28].
An orthopaedic surgeon experienced with the navigation system performed the acquisition of the anatomical data and then a set of passive kinematics test for a total of four positions assessed while applying a manual controlled load. Static laxity evaluation was performed by the assessment of:
-
Anterior displacement at 30° and 90° of knee flexion (AP30 and AP90) applying a 130 N load;
-
Internal–external rotation range of motion at 30° and 90° of knee flexion (IE30 and IE90) applying a 5 Nm torque both for internal and external rotation;
The values of the applied load and torque were defined according to previously published manuscripts [4, 6, 31]. Each test was repeated three times to allow for confirmation of the intra-observer reliability of this methodology. Each test was performed with knee joint in five different conditions:
-
State I: Intact knee joint: only the trackers were fixed to the bones;
-
State II: A resection of the ACL;
-
State III: A resection of the popliteus complex (PC) at femoral attachment of the popliteus tendon and the fibular attachment of the popliteofibular ligament;
-
State IV: The ACL was reconstructed by a non-anatomical double-bundle technique [16];
-
State V: A resection of the lateral collateral ligament (LCL) at fibular attachment;
An analogical dynamometer (Yo-Zuri, Port St. Lucie, FL, USA; full scale = 24 kg/55 lbs) fixed to the knee joint by a screw and an analogical torquemeter (Stryker Howmedica Dall Miles Instruments, Limbach-Oberfrohna, Germany; full scale 20 Nm/180 lb-In) arranged to the testing rig were used to control the applied force and torque, respectively. The main axis of the dynamometer was maintained perpendicular to the tibial bone in alignment with the direction of the anterior–posterior displacement, while concerning the torquemeter it was maintained coplanar with the plane of rotation, i.e. perpendicular to the tibia.
Operating procedures
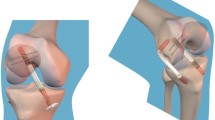
The non-anatomical double-bundle (NADB) [17, 34] reconstruction (Fig. 1) was performed using an autologous hamstring graft with the distal attachment left intact. An 8-mm reamer was used to drill the tibial tunnel with the knee flexed at 35° aiming at the posteromedial part of the ACL footprint.
The graft was passed through the tibial tunnel and throughout the posterior aspect of the capsule to reach the over-the-top position. Then, the distal portion of the graft was passed through a femoral tunnel, the joint and the same tibial tunnel. The femoral tunnel was previously drilled with a 7-mm reamer starting from the medial wall of the lateral condyle, approximately 5-mm anterior to the over-the-top position. The guide pin passes the femoral cortex with the knee flexed around 130°. In the NADB reconstruction, the distal origin of the tibial tunnel is decided by the surgeon to not conflict with the natural attachment of the hamstring graft.
The graft was pretensioned manually and then the knee cycled through a full range of motion 20 times. Then, the graft was fixed by two Richards barbed staple (Smith & Nephews, Richards Inc., Memphis, USA) at 90° of flexion on the femoral side and by one Richards barbed staple, in the medial aspect of the tibia just proximal to the distal insertion of the hamstring graft at 20° of knee flexion.
Statistical analysis
Intra-class correlation coefficient was evaluated in order to assess the test–retest repeatability for each of the five tested positions.
Paired Student’s t test was performed in order to assess any difference between the tested conditions. Statistical significance was set at p < 0.05. All the kinematics and statistical analysis were performed using unique MATLAB (MathWorks, Inc., Natick, MA, USA) function designed and implemented specifically for the present study.
Results
The analysis of the test repeatability was performed defining the ICC for each position obtained during the execution of the repeated tests. The results demonstrate an excellent correlation for each test. In particular, we found an ICC = 0.9 for all the static laxity parameters (AP30, AP90, IE30 and IE90).
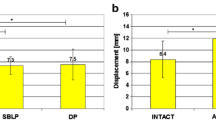
The specific static laxity analysis of the changes in displacement and rotation was reported (Table 1). In particular, we evaluated statistically significant differences in all ligaments conditions from the intact condition and from one ligament state and the previous one (*p < 0.05, Fig. 2).
In particular, concerning the AP30, we found a statistically significant difference between intact condition and after ACL resection (p = 0.03). No further significant increase in terms of laxity was produced by further cutting of the PC. The ACL reconstruction determined a significant reduction (p = 0.03) of the AP laxity while no significant increase in laxity was produced by the further LCL sectioning. The same statistically significant differences were confirmed during AP90 test except that for the final step (Fig. 2). Indeed, at 90° of knee flexion, the resection of the LCL determined a significant increase in terms of AP laxity (p = 0.04) in the ACL-reconstructed knee.
Considering the internal–external rotation at 30° of knee flexion (IE30), a significant increase was observed after the final resection of the LCL in the ACL-reconstructed knee when compared with the intact state (p = 0.0339). No other significant differences have been underlined during other steps of the present study.
At 90° of knee flexion, the PC resection significantly increases the IE90 rotation compared with the isolated ACL resection (p = 0.04). Analogously, the LCL resection increases the IE90 rotation value with respect to the previous step where the ACL reconstructive surgery was performed (p = 0.0056). When comparing the IE90 laxity values to the intact state, it has been found a statistically significant difference after PC resection (p = 0.09), after ACL surgery (p = 0.04) and after LCL resection (p = 0.0007), as well.
Discussion
The main finding of this study was that an isolated ACL reconstruction is able to control the AP laxity with a combined complete lesion of the PLC at 30° of knee flexion, but it fails at higher angle of knee flexion. Indeed, at 90° of flexion, a further lesion to the LCL determined a significant increase in the AP laxity. Considering the rotations, at 90° of knee flexion, PC resection determined a significant increase in laxity values that was not controlled by the isolated ACL reconstruction. Indeed, the ACL surgery did not reduce significantly the rotational laxity when compared with the previous step. Further, after the reconstruction step, the laxity value was still statistically different with respect to the intact knee state. Moreover, further sectioning of the LCL determined an additional significant increase. At 30° of flexion, the only significant difference was found when comparing the intact state with final step, meaning that ACL reconstruction was not able to control the rotations even at lower degrees of knee flexion when combined with a complete PLC lesion.
In the past decades, Nielsen et al. [21–24] performed a cutting study demonstrating the importance of posterolateral structures in resisting external rotation forces. In the same period, Gollehon et al. [8] were the first in investigating the static contribution of the PLC to the knee static laxity parameters in an ACL-deficient knee. In a more complex cutting study, they analysed this combined injury set-up by sectioning the LCL and the so named ‘deep ligament complex’ which included the PC, the arcuate ligament and the posterolateral part of the joint capsule; they varied the sequence of sectioning of the lateral structures and finally, as last step, an ACL cut was performed in four specimens. With all the resections performed, they reported an increase in terms of AP laxity at 30° and 60° degrees of knee flexion when an internal tibial torque was applied. Further, in their study, isolated sectioning of LCL determined a significant increase in external rotation at 30° and 90° of knee flexion, while isolated sectioning of the deep structures determined a significant increase only at 90°. These data are in accordance with the current paper where the popliteus complex sectioning determined a significant increase only at 90°, while the torn LCL determined a significant increase in rotational laxity compared with the intact state at both angles of knee flexion.
In a similar study, Veltri et al. [29] analysed a subgroup of five knees undergoing sequential cutting of the PLC, including LCL, tibial attachment of the popliteus muscle–tendon unit and popliteofibular ligament, and of the ACL. Concerning the AP laxity, no significant increase in laxity was determined by the sectioning of the PCL. When the ACL was cut as well they found a significant increase in anterior translation at all angles of knee flexion with a maximal increase at 30° of flexion. Moreover, they found that PLC sectioning determined a significant increase in terms of external rotation especially at 45° of knee flexion, but further sectioning of the ACL resulted reduced external rotation at 0° and 30°. They suggested that the decrease was due to a lateral shift of the axis of tibial rotation produced by the sectioning of the ACL and a subsequent internal and anterior subluxation of the tibial plateau. These results differed from that of Wroble et al. [32] who detected an increase in external rotation in the same experimental setting. Veltri et al. [29] attribute this contrast to different experimental set-up concerning torque application and variability in knees features. In the current paper, primary external rotation and primary internal rotation were not analysed separately, but the whole range of range of rotation was assessed. The rationale of this decision lays in the knowledge that ligament lesion and reconstruction alter the joint kinematics [14], and this affects the rotation path making hard to identify the neutral starting position [18].
More recently, Zantop et al. [35], on ten cadaver knees, investigated the effect of subsequent sectioning of ACL, LCL ad PC under combined rotational load using a robot testing system. They reported a significant increase in anterior displacement after sectioning the LCL in a ACL-deficient knee at 0° and 30° of knee flexion, while the subsequent cut of the PC determined a further significant increase just at 90° of flexion. In the current study, the sectioning of the PC determined a significant increase in terms of laxity at both 30° and 90° of flexion in an ACL-deficient knee, while the deficiency of the LCL produced a significant increase only at 90° of flexion. The authors believe that the rationale of this difference lies in the difference of the study design. The different sequence of sectioning and the concomitant reconstruction compromised the possibility to deeply analyse the difference in kinematical behaviour among these two studies. The order of sectioning was defined to analyse the efficacy of an isolated ACL reconstruction with a concomitant partial and complete lesion of the PCL. Moreover, to the author’s knowledge, this is the first in vitro study evaluating the possibility of an isolated ACL reconstruction to control laxity parameters in PLC-deficient knee. The only available biomechanical data on a PLC lesion and ACL-reconstructed knee were focused on the variation of force in the graft after sequential sectioning of the posterolateral structures. LaPrade et al. [14] demonstrated a significant increase in force on the graft even with partial PLC. They concluded that repair or reconstruction should be considerate in knees with combined ACL and PLC injury.
Therefore, the results of the current study further empathize the importance of surgical treatment of the PLC when facing this combined injury scenario in everyday clinical practice.
The authors noted some limitations. First, the kinematic evaluation was performed by means of a navigation system in place of a robot testing system. The robot is capable of higher kinematic measurement [16, 18, 26, 35]; however, the navigation system allows for an experimental set-up closer to the in vivo one. Second, the size of the samples was small and the age of the cadavers was not similar to a normal ligament injured knee, but this is common problem of the in vitro studies due to the paucity of human donors. Third, as obvious in an in vitro set-up, the active muscle function was not allowed, and this may have jeopardized the active stabilizing function of the popliteus muscle–tendon unit.
Conclusion
In conclusion, the current study shows that an isolated ACL reconstruction is not able to fully control the static laxity parameters with a concomitant complete lesion of the PCL. Considering the IE rotations, the reconstruction was not sufficient not even to control a partial lesion of the PLC involving just the popliteus complex. These findings suggest that additional surgical procedures should be considerate even when facing partial combined PLC lesion. The authors advocate deep laxity assessment in order to customize the surgical procedure to the patient specific features. Moreover, there is a need to validate a new and more specific arthrometer able to asses laxity in an in vivo set-up preserving the active muscle function.
References
Bonanzinga T, Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, Neri MP, Marcacci M (2014) Management of combined anterior cruciate ligament-posterolateral corner tears: a systematic review. Am J Sports Med 42(6):1496–1503
Corten K, Bellemans J (2008) Cartilage damage determines intermediate outcome in the late multiple ligament and posterolateral corner-reconstructed knee: a 5- to 10-year follow-up study. Am J Sports Med 36(2):267–275
Covey DC (2001) Current concepts review: injuries of the posterolateral corner of the knee. J Bone Joint Surg 83:106–118
Driscoll MD, Isabell GP Jr, Conditt MA, Ismaily SK, Jupiter DC, Noble PC, Lowe WR (2012) Comparison of 2 femoral tunnel locations in anatomic single-bundle anterior cruciate ligament reconstruction: a biomechanical study. Arthroscopy 28(10):1481–1489
Ferretti A, Monaco E, Giannetti S, Caperna L, Luzon D, Conteduca F (2011) A medium to long-term follow-up of ACL reconstruction using double gracilis and semitendinosus grafts. Knee Surg Sports Traumatol Arthrosc 19(3):473–478
Gadikota HR, Seon JK, Kozanek M, Oh LS, Gill TJ, Montgomery KD, Li G (2009) Biomechanical comparison of single-tunnel-double-bundle and single-bundle anterior cruciate ligament reconstructions. Am J Sports Med 37(5):962–969
http://www.ndigital.com/medical/polarisfamily-techspecs.php. Date of display: July 1st, 2015
Gollehon DL, Torzilli PA, Warren RF (1987) The role of the posterolateral and cruciate ligaments in the stability of the human knee. A biomechanical study. J Bone Joint Surg Am 69:233–242
Kanamori A, Sakane M, Zeminski J, Rudy TW, Woo SL (2000) In-situ force in the medial and lateral structures of intact and ACL-deficient knees. J Orthop Sci 5:567–571
Kannus P (1989) Nonoperative treatment of Grade II and III sprains of the lateral ligament compartment of the knee. Am J Sports Med 17:83–88
Kim YC, Chung IH, Yoo WK, Shu JS, Kim SJ, Park CI (1997) Anatomic and magnetic resonance imaging of the posterolateral structures of the knee. Clin Anat 10:397–404
LaPrade RF, Hamilton CD, Engebretsen L (1997) Treatment of acute and chronic combined anterior cruciate ligament and posterolateral knee ligament injuries. Sports Med Arthrosc Rev 5:91–99
LaPrade RF, Johansen S, Wentorf FA, Engebretsen L, Esterberg JL, Tso A (2004) An analysis of an anatomical posterolateral knee reconstruction: an in vitro biomechanical study and development of a surgical technique. Am J Sports Med 32(6):1405–1414
LaPrade RF, Resig S, Wentorf F, Lewis JL (1999) The effects of grade III posterolateral knee complex injuries on anterior cruciate ligament graft force. A biomechanical analysis. Am J Sports Med 27:469–475
LaPrade RF, Wentorf FA, Fritts H, Gundry C, Hightower CD (2007) A prospective magnetic resonance imaging study of the incidence of posterolateral and multiple ligament injuries in acute knee injuries presenting with a hemarthrosis. Arthroscopy 23(12):1341–1347
Lenschow S, Zantop T, Weimann A, Lemburg T, Raschke M, Strobel M, Petersen W (2006) Joint kinematics and in situ forces after single bundle PCL reconstruction: a graft placed at the center of the femoral attachment does not restore normal posterior laxity. Arch Orthop Trauma Surg 126:253–259
Marcacci M, Molgora AP, Zaffagnini S, Vascellari A, Iacono F, Presti ML (2003) Anatomic double-bundle anterior cruciate ligament reconstruction with hamstrings. Arthroscopy 19(5):540–546
Martelli S, Lopomo N, Bignozzi S, Zaffagnini S, Visani A (2007) Validation of a new protocol for navigated intraoperative assessment of knee kinematics. Comput Biol Med 37:872–878
Markolf KL, Graves BR, Sigward SM, Jackson SR, McAllister DR (2007) How well do anatomical reconstructions of the posterolateral corner restore varus stability to the posterior cruciate ligament-reconstructed knee? Am J Sports Med 35(7):1117–1122
Ménétrey J, Duthon VB, Laumonier T, Fritschy D. “Biological failure” of the anterior cruciate ligament graft (2008) Knee Surg Sports Traumatol Arthrosc 16(3):224–231
Nielsen S, Helmig P (1986) Posterior instability of the knee joint. An experimental study. Arch Orthop Trauma Surg 105:121–125
Nielsen S, Helmig P (1986) The static stabilizing function of the popliteal tendon in the knee. An experimental study. Arch Orthop Trauma Surg 104:357–362
Nielsen S, Ovesen J, Rasmussen O (1985) The posterior cruciate ligament and rotatory knee instability. An experimental study. Arch Orthop Trauma Surg 104:53–56
Nielsen S, Rasmussen O, Ovesen J, Andersen K (1984) Rotatory instability of cadaver knees after transection of collateral ligaments and capsule. Arch Orthop Trauma Surg 103:165–169
O’Brien SJ, Warren RF, Pavlov H et al (1991) Reconstruction of the chronically insufficient anterior cruciate ligament with the central third of the patellar ligament. J Bone Joint Surg 73A:278–286
Petersen W, Lenschow S, Weimann A, Strobel MJ, Raschke MJ, Zantop T (2006) Importance of femoral tunnel placement in double-bundle posterior cruciate ligament reconstruction: biomechanical analysis using a robotic/universal force-moment sensor testing system. Am J Sports Med 34:456–463
Seering WP, Piziali RL, Nagel DA, Schurman DJ (1980) The function of the primary ligaments of the knee in varus–valgus and axial rotation. J Biomech 13:785–794
Siston RA, Delp SL (2006) Evaluation of a new algorithm to determine the hip joint center. J Biomech 39:125–130
Veltri DM, Deng XH, Torzilli PA, Warren RF, Maynard MJ (1995) The role of the cruciate and posterolateral ligaments in stability of the knee. A biomechanical study. Am J Sports Med 23:436–443
Vogrin TM, Hoher J, Aroen A, Woo SL, Harner CD (2000) Effects of sectioning the posterolateral structures on knee kinematics and in situ forces in the posterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 8:93–98
Wang JH, Kato Y, Ingham SJ, Maeyama A, Linde-Rosen M, Smolinski P, Fu FH (2012) Measurement of the end-to-end distances between the femoral and tibial insertion sites of the anterior cruciate ligament during knee flexion and with rotational torque. Arthroscopy 28(10):1524–1532
Wroble RR, Grood ES, Cummings JS, Henderson JM, Noyes FR (1993) The role of the lateral extraarticular restraints in the anterior cruciate ligament-deficient knee. Am J Sports Med 21:257–263
Zaffagnini S, Bignozzi S, Martelli S, Imakiire N, Lopomo N, Marcacci M (2006) New intraoperative protocol for kinematic evaluation of ACL reconstruction: preliminary results. Knee Surg Sports Traumatol Arthrosc 14:811–816
Zaffagnini S, Marcheggiani Muccioli GM, Signorelli C, Lopomo N, Grassi A, Bonanzinga T, Nitri M, Marcacci M (2014) Anatomic and nonanatomic double-bundle anterior cruciate ligament reconstruction: an in vivo kinematic analysis. Am J Sports Med 42(3):708–715
Zantop T, Schumacher T, Diermann N, Schanz S, Raschke MJ, Petersen W (2007) Anterolateral rotational knee instability: role of posterolateral structures. Winner of the AGA-DonJoy Award 2006. Arch Orthop Trauma Surg 127(9):743–752
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonanzinga, T., Signorelli, C., Lopomo, N. et al. Biomechanical effect of posterolateral corner sectioning after ACL injury and reconstruction. Knee Surg Sports Traumatol Arthrosc 23, 2918–2924 (2015). https://doi.org/10.1007/s00167-015-3696-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3696-3