Abstract
Punicic acid (PuA) is a conjugated linolenic acid (C18:3Δ9c,11t,13c) with a wide range of nutraceutic effects with the potential to reduce the incidence of a number of health disorders including diabetes, obesity, and cancer. It is the main component of seed oil from Punica granatum and Trichosanthes kirilowii. Previously, production of relatively high levels of this unusual fatty acid in the seed oil of transgenic Arabidopsis thaliana plant was accomplished by the use of A. thaliana fad3/fae1 mutant high in linoleic acid (18:2∆9c,12c) and by co-expression of P. granatum FATTY ACID CONJUGASE (PgFADX) with Δ12-DESATURASE (FAD2). In the current study, P. granatum cDNAs governing PuA production were introduced into the yeast Schizosaccharomyces pombe. Expression of PgFADX alone resulted in production of PuA at the level of 19.6% of total fatty acids. Co-expression PgFADX with PgFAD2, however, further enhanced PuA content to 25.1% of total fatty acids, the highest level reported to date for heterologous expression. Therefore, microbial systems can be considered as a potential alternative to plant sources for a source of PuA for nutraceutic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Punicic acid (PuA; C18:3Δ9c,11t,13c) belongs to the family of conjugated linolenic acids (CLNA) which naturally occur in the seed oils of plant species from several families including Cucurbitaceae, Punicaceae, Bignoniaceae, Rosaceae, Chrysobalanaceae, Lythraceae, Balsaminaceae, and Euphorbiaceae (Badami and Patil 1980; Rawat et al. 2012; Smith 1971). The major sources of PuA are seed oils of pomegranate (Punica granatum) [60–80% PuA (w/w)] and Chinese snake gourd (Trichosanthes kirilowii) [40% PuA (w/w)]; however, the availability of these oils is very limited (Joh et al. 1995; Koba et al. 2007; Takagi and Itabashi 1981).
Recently, it was reported that natural CLNA-enriched oils have nutraceutic properties with notable health benefits (for review, see Aruna et al. 2016). The antioxidant (Saha and Ghosh 2009), anti-diabetic (Arao et al. 2004; Koba et al. 2007), anti-cancer (Grossmann et al. 2010; Kohno et al. 2004; Suzuki et al. 2001; Tanaka et al. 2011; Wang et al. 2014), and anti-inflammatory (Boussetta et al. 2009; Costantini et al. 2014) activities of CLNA-enriched sources are currently under intensive investigation. In addition to health benefits, seed oils containing CLNA have other applications including the production of dyes, inks, coatings, resins, and cosmetics (Cahoon et al. 2006; Hornung et al. 2002; Mietkiewska et al. 2014).
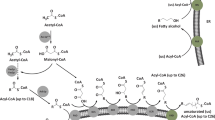
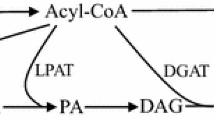
Limited natural sources of PuA combined with a high demand for oils enriched in PuA has led to a growing interest in the metabolic engineering of temperate plants to produce PuA in their seed oils (Mietkiewska et al. 2014). The fatty acid (FA) precursors oleic acid (OA; 18:1∆9c, hereafter 18:1) and linoleic acid (LA; 18:2∆9c,12c, hereafter 18:2) lead to PuA (Fig. 1a). This sequential conversion to PuA, however, takes place while OA is attached to phosphatidylcholine (Fig. 1b) (Mietkiewska et al. 2014). OA is converted to LA by the catalytic action of Δ12-fatty acid desaturase (FAD2), and subsequent conversion of LA to PuA is catalyzed by a divergent form of FAD2 often referred to as fatty acid conjugase (FADX) (Cahoon et al. 2006; Vanhercke et al. 2013). This enzyme catalyzes the conversion of the Δ12-double bond of LA into two conjugated double bonds at positions Δ11t and Δ13c (Hornung et al. 2002; Iwabuchi et al. 2003). The activity of native FADX required for the biosynthesis of PuA in P. granatum (PgFADX) and T. kirilowii (TkFADX) was initially characterized in the yeast Saccharomyces cerevisiae by Hornung et al. (2002) and Iwabuchi et al. (2003). In both studies, the heterologous production of PuA in yeast reached less than 2% of total FA. Recombinant PgFADX was also capable of catalyzing the formation of LA from OA indicating that the enzyme also had some FAD2 activity (Hornung et al. 2002; Iwabuchi et al. 2003) (Fig. 1b).
A few research groups have also been successful in the heterologous production of PuA in temperate plants. Upon over-expression of PgFADX or TkFADX in Arabidopsis thaliana, accumulation in seeds reached up to 4.4% (w/w) or 10% PuA (w/w), respectively (Iwabuchi et al. 2003). Over-expression of TkFADX in Brassica napus resulted in 2.5% accumulation of PuA in the seed oil (Koba et al. 2007). Combined expression of PgFADX and PgFAD2 in the A. thaliana fad3/fae1 mutant (high in LA), however, led to 21% accumulation of PuA of the total acyl lipids in the seed oil (Mietkiewska et al. 2014). Recently, the lignin promoter was shown to be very effective, relative to other plant promoters, in raising PuA levels in A. thaliana expressing PgFADX (Song et al. 2016). Limited accumulation of PuA in transgenic plant seeds indicates that additional pomegranate genes might be required to further enhance PuA content in temperate plants (Mietkiewska et al. 2014).
Microorganisms have shown great promise in production of value-added compounds (Garaiova et al. 2014; Holic et al. 2012; Ledesma-Amaro and Nicaud 2016; Meesapyodsuk et al. 2015). With appropriate gene combinations, it may be possible to metabolically engineer microorganisms to produce PuA.
Here, we report on metabolic engineering of PuA in Schizosaccharomyces pombe, a yeast with high OA content. The heterologous expression of PgFADX alone resulted in the production of PuA at the level of 19.6% of total FA. Co-expression of PgFADX and PgFAD2, however, resulted in a further increase in PuA content to 25.1% of total FA, the highest level reported to date for heterologous expression. Our investigation suggests that metabolically engineered yeast may potentially represent an alternate source of PuA to plant oils for nutraceutic applications of this conjugated FA.
Materials and methods
Materials
Media components were obtained from Becton Dickinson (USA) or BioLife (Italy). Fine chemicals were mostly from MP Biomedicals or Sigma-Aldrich (USA).
Strain, cultivation conditions, and yeast transformation
Wild-type S. pombe strain ED666 (h+ ade6-M210 ura4-D18 leu1-32) was obtained from Bioneer collection (USA). It was grown and maintained on the YES agar plates and liquid medium at 30 °C. Strain ED666 was used as a host strain for heterologous expression of codon optimized PgFAD2 cDNA (GenBank# KY996743) and/or codon-optimized PgFADX cDNA (GenBank# KY996742). S. pombe strains containing plasmids were grown in Edinburgh minimal medium (EMM) without supplements depending on the selective pressure required to maintain the plasmids. Solid plates were prepared with 2% agar. Growth in liquid media was performed at 30 °C with continuous shaking. Cell growth was estimated by measuring the OD at 600 nm (Spectrophotometer UV-2401 PC, Shimadzu, Japan). Optical density unit equal to 1 (OD = 1) corresponded approximately to 1 × 107 cells/mL. Growth in liquid media was monitored by measuring turbidity of the cells at OD600. For a detection of the growth in liquid media, strains grown in EMM overnight were inoculated into fresh EMM at OD600 = 0.2, and the growth curves were obtained by measuring the turbidity of the cells at 600 nm. Yeast transformation was performed using the lithium acetate method (Gietz et al. 1992).
Preparation of S. pombe expression plasmids of PgDADX and PgFAD2
Standard techniques of DNA manipulation used in this study are described in Sambrook and Russell (2001). PgFAD2 (GenBank# AY178447) and PgFADX (GenBank# AY178446) (Mietkiewska et al. 2014) were re-designed using yeast preference codon usage, and the cDNA were chemically synthesized by GenScript USA, Inc. (USA). The synthesized open reading frames of PgFAD2 (GenBank# KY996743) or PgFADX (GenBank# KY996742) were excised from Escherichia coli plasmid pUC57-Kan-PgFAD2 or pUC57-Kan-PgFADX, respectively, as NdeI–BamHI fragments by using artificially added restriction enzyme sites at the 5′ and 3′ ends of the cDNA, and they were inserted into the NdeI–BamHI multiple cloning sites of multicopy-type plasmids pREP1 (LEU2) or pREP2 (ura4+), respectively. PgFAD2 and PgFADX were expressed from plasmids under the control of the nmt1 promoter of S. pombe. Gene expression under the nmt1 promoter was repressed by 15 μM thiamine (5 μg/mL). The control strain contained the empty vectors pREP1 and pREP2.
Fatty acid analysis
Extraction of yeast lipids was performed as described by Mietkiewska et al. (2014) with minor modifications. Briefly, 25–35 mg of yeast cells was suspended in 1 mL of a mixture of chloroform and isopropanol (2:1, v/v) containing the antioxidant butylated hydroxytoluene at a final concentration of 0.01%. Cells were disrupted using the FastPrep disintegrator (MP Biomedicals) using glass beads (diameter 0.4 mm) at 3 × 40 s, at the highest speed (6.5 m/s), with 5 min cooling on ice between cycles. The homogenate was then dried under N2 gas and suspended in 1 mL of chloroform and isopropanol (2:1, v/v). An aliquot of 100 μL was transferred to a glass tube, dried under N2 gas, and transmethylated with 1 mL of 5% sodium methoxide (Na-OCH3) in methanol at 30 °C for 30 min. To stop the reaction, 1.5 mL of 0.9% (w/v) sodium chloride was added to the samples, and fatty acid methyl esters (FAME) were extracted with 1 mL of hexane as described by Iwabuchi et al. (2003). Samples were centrifuged at 1000×g for 5 min, and the hexane layers were transferred to glass vials. Analysis of FAME was performed by the injection of 1-μL aliquots to a gas chromatography (GC) apparatus (GC-2010 Plus, Shimadzu, Japan) equipped with a BPX70 capillary column (30 m × 0.25 mm × 0.25 μm, SGE Analytical Science, Australia) under temperature programming (165 °C hold for 4 min, 10 °C/min to 180 °C hold 5 min, and 10 °C/min to 230 °C hold 5 min) (Mietkiewska et al. 2011). Individual FAME were identified by comparison with the authentic standards of the C4–C24 FAME mixture (Supelco, USA). PuA methyl ester was identified by comparison with PuA (Larodan, Sweden) methylated by a method allowing free FA methylation (Stumpe et al. 2001) and by methylation of pomegranate oil (80 mg) (Bioshop, Biopurus). Quantification of individual FA was performed using the heptadecanoic acid methyl ester internal standard (Sigma-Aldrich, USA).
Results
Heterologous expression of PgFAD2 and PgFADX
cDNA-encoding PgFAD2 and PgFADX were previously identified as necessary factors for effective metabolic engineering of PuA biosynthesis in the model plant A. thaliana (Mietkiewska et al. 2014). To achieve the heterologous production of PuA in yeast, a S. pombe strain was transformed with the same cDNA under the control of the nmt1 promoter (repression by thiamine). The control strain contained the corresponding empty vectors.
Previous work on metabolic engineering of ricinoleic acid (12-OH 18:1∆9c) production in S. pombe cells showed that cultivation temperature highly affects the cell growth and the activity of the heterologously produced Claviceps purpurea oleate ∆12-hydroxylase enzyme (Holic et al. 2012). Therefore, first, we tested the growth of cells expressing PgFAD2 and/or PgFADX at various cultivation temperatures. Cells were spotted on agar plates with or without thiamine and incubated at 20, 30, and 37 °C. As shown in Fig. 2a, the control strain grew well in all tested temperatures. In the presence of thiamine (repressed condition), the growth of all strains was similar. In the absence of thiamine (induced condition), the growth of PgFADX-containing strain was affected at all tested temperatures. The growth of transformed yeast was strongly reduced when PgFADX was co-expressed together with PgFAD2, but PgFAD2 expression alone did not affect the growth. Since growth inhibition was not dependent on temperature, as in the case of heterologous expression of a cDNA-encoding C. purpurea oleate ∆12-hydroxylase (Holic et al. 2012), a standard growth condition of 30 °C was adopted. In addition, an earlier study showed that S. cerevisiae transformed with PgFADX exhibited more efficient production of PuA when cultured at 30 °C (Hornung et al. 2002). Next, the growth in liquid media was examined. Under either repressed or induced conditions, all strains showed similar growth (Fig. 2b, c, respectively).
Growth of S. pombe strains expressing P. granatum ∆-12 FATTY ACID DESATURASE (PgFAD2) and/or P. granatum FATTY ACID CONJUGASE (PgFADX) and the control strain. The control strain contained the corresponding empty vectors (pREP1 and pREP2). a Growth on EMM (Edinburgh minimal medium) agar plates at various temperatures. Cells were grown in EMM in the presence of thiamine overnight at 30 °C until an OD600 of about 6 was reached. Cells were washed in sterile water and 10 μL of 10-fold serial dilutions of the strains were spotted on EMM plates in the presence (repressed condition) or absence (induced condition) of 15 μM thiamine. The plates were incubated at 37, 30, and 20 °C, respectively, for 4 to 9 days as indicated depending on the growth at tested temperatures. Liquid growth of the strains in the b presence (repressed condition) of 15 μM thiamine or c absence (induced condition) of thiamine with starting OD600 of 0.2 after pre-culturing the cells in EMM in the presence of thiamine at 30 °C overnight. Cells were grown in EMM at 30 °C, and the cell turbidity was monitored spectrophotometrically (UV-2401 PC, Shimadzu, Japan)
Heterologous production of punicic acid
To confirm the activity of PgFAD2 and/or PgFADX in transformed S. pombe, the fatty acid methyl esters (FAME) from cells expressing PgFAD2 and PgFADX under induced conditions (without thiamine) were analyzed by GC (Fig. 3). In the case of PgFAD2 expression, production of LA was detected. In the case of PgFADX expression, peak for both LA and PuA was detected.
Gas chromatographic analysis of fatty acid methyl esters from the lipid extracts of S. pombe strains expressing P. granatum ∆-12 FATTY ACID DESATURASE (PgFAD2) and/or P. granatum FATTY ACID CONJUGASE (PgFADX) and the control strain. The control strain contained the corresponding empty vectors. Cells were grown at 30 °C for 3 days. The bottom panel indicates a gas chromatogram of punicic acid (PuA). Heptadecanoic acid methyl ester was used as an internal standard
It has been shown earlier that maximal expression driven by the nmt1 promoter after the thiamine wash is achieved after 18 h of cultivation (Maundrell 1990). Thus for the FA analysis, to release the thiamine repression, pre-culture cells were incubated overnight in media lacking thiamine as shown in Fig. 4a. Since the serial dilution test showed that the cell growth is affected upon heterologous co-expression of PgFADX with PgFAD2 (Fig. 2a), the culture cells for GC analysis were inoculated to high initial OD equal to 1. By this approach, only three generations were needed to reach the cell confluency. After 1 day of incubation, all cultures reached similar OD to the control except in transformed yeast co-expressing PgFADX with PgFAD2 in which growth was highly affected (Supplementary Table S1).
Fatty acid composition of S. pombe over-expressing P. granatum ∆-12 FATTY ACID DESATURASE (PgFAD2) and/or P. granatum FATTY ACID CONJUGASE (PgFADX) under induced conditions. a Diagram describing the culture process. Yeasts were pre-cultured in EMM in the absence of thiamine at 30 °C for 1 day (hatched box). The pre-cultured cells were inoculated in the same medium at OD600 of 1, and incubated at 30 °C for 10 days and sampled as indicated. b FA composition of the control, PgFAD2-expressing yeast, PgFADX-expressing yeast, or yeast co-expressing PgFAD2 with PgFADX at different incubation times. The values are the average of two independent experiments ± standard deviation. Abbreviations: EMM Edinburgh minimal medium; FA fatty acids; Others FA (16:0, 16:1, and 18:0); PuA punicic acid
FA analysis of yeast cells with heterologous PgFAD2 expression under induced conditions (absence of thiamine) revealed high production of LA (49.1%) at 30 °C after 1 day of incubation, and this high level of LA was maintained during 10 days of cultivation (Fig. 4b). Upon heterologous expression of PgFADX under induced conditions, PuA (8.5%) was detected at 30 °C after 1 day of incubation and the level of PuA was maintained during 10 days of cultivation (Fig. 4b). In the case of either PgFADX expression alone or PgFADX with PgFAD2 expression, the highest level of PuA production occurred within the first 3 days of cultivation. In addition, low production of LA (1.9%), the precursor of PuA, was detected in cells expressing PgFADX after 1 day of incubation and this level was maintained during 10 days of cultivation. Cells co-expressing PgFAD2 and PgFADX under induced conditions produced 20.6% LA and 9.8% PuA after 1 day of incubation, respectively (Fig. 4b). More extensive cultivation, beyond 1 day, resulted in further decreases in LA and PuA content (Fig. 4b).
Further enhancement of punicic acid synthesis and dynamics of accumulation
Increased PuA accumulation was achieved by change in the pre-culture conditions and by alteration of the initial culture OD. Pre-culture cells were cultivated in the presence of thiamine (repressed condition), inoculated to various initial OD into media lacking thiamine (induced condition), and incubated for 3 days as shown in Fig. 5a. In addition, FA analysis of cells expressing PgFADX (Fig. 5b) and cells co-expressing PgFAD2 with PgFADX (Fig. 5c) revealed that maximum accumulation of PuA occurred at the initial inoculation OD equal to 0.2. The highest accumulation of PuA (22.3%) was achieved by co-expression of PgFAD2 with PgFADX.
The extent of punicic acid (PuA) accumulation in S. pombe expressing P. granatum FATTY ACID CONJUGASE (PgFADX) or PgFADX with ∆-12 FATTY ACID DESATURASE (PgFAD2) is dependent on the initial OD of culture. a Diagram describing the culture process. Yeasts expressing PgFADX or yeasts co-expressing PgFAD2 with PgFADX were pre-cultured in EMM in the presence of thiamine at 30 °C for 1 day (white box). The pre-cultured cells were inoculated into the fresh EMM at various OD600 (from 0.05 to 2) and incubated at 30 °C for 3 days (hatched boxes). b FA composition of PgFADX-expressing yeast analyzed at day 3 after inoculation. c FA composition of yeast co-expressing PgFAD2 with PgFADX analyzed at day 3 after inoculation. The values are the average of three independent experiments ± standard deviation. Abbreviations: EMM Edinburgh minimal medium; FA fatty acids; Others FA (16:0, 16:1, and 18:0)
In order to further characterize the dynamics of PuA production in S. pombe expressing PgFADX, or co-expressing PgFAD2 with PgFADX, under more optimized conditions, PuA content of the cells was monitored for 10 days at 30 °C as shown in Fig. 6a. In the case of yeast expressing PgFADX alone, the level of PuA was steadily high from day 3 (18.7%) to day 6 (18.9%) with the maximal content of PuA (19.6%) occurring on day 4 (Fig. 6b). This level of PuA on day 4 corresponded to 38.71 μg PuA/mL of culture (Supplementary Table S2). A different dynamic in PuA accumulation was observed in the case of yeast co-expressing PgFAD2 with PgFADX. In this case, PuA content was only highest at day 2 (25.1%) and day 3 (24.3%) (Fig. 6c). This highest PuA content observed at day 2 corresponded to 34.33 μg PuA/mL of culture (Supplementary Table S2).
Punicic acid (PuA) content as a function of incubation time in S. pombe expressing P. granatum FATTY ACID CONJUGASE (PgFADX) or PgFADX with ∆-12 FATTY ACID DESATURASE (PgFAD2) under the induced condition (without thiamine). a Diagram describing the culture process. The PgFADX-expressing yeasts or yeasts co-expressing PgFAD2 with PgFADX were pre-cultured in EMM in the presence of thiamine at 30 °C for 1 day (white box). The pre-cultured cells were inoculated into the fresh EMM for an initial OD600 of 0.2 and incubated at 30 °C for 10 days (hatched boxes) and sampled as indicated. FA composition of b PgFADX-expressing yeast or c yeast co-expressing PgFAD2 with PgFADX and analyzed at indicated time points after the culture inoculation. The values are the average of three independent experiments ± standard deviation. Abbreviations: EMM Edinburgh minimal medium; FA fatty acids; Others FA (16:0, 16:1, and 18:0)
Discussion
The aim of this study was to produce PuA, a CLNA with health benefits, in S. pombe by heterologous expression of genes from P. granatum. A few groups have attempted to produce this unusual FA in transgenic A. thaliana (Iwabuchi et al. 2003; Mietkiewska et al. 2014) or B. napus plants (Koba et al. 2007). Only limited accumulation of PuA (4.4–10.2%) was observed in A. thaliana when compared with the levels of this CLNA (60–80%) in seeds from plants that naturally produced PuA (Iwabuchi et al. 2003). It is known that conjugated FAs are synthesized by a divergent form of FAD2 known as FADX, either from OA or from LA precursors esterified to the sn-2 position of PC (Cahoon et al. 2006; Vanhercke et al. 2013). Recently, our group reported on the production of PuA up to 11.3% of the total FA in the seed oil in transgenic A. thaliana over-expressing PgFADX in a fad3/fae1 mutant background containing a high level of LA (Mietkiewska et al. 2014). Further enhancement to 21.2% PuA content in the seed oil was achieved by co-expression of PgFAD2 with PgFADX because expression of the naturally occurring AtFAD2 was inhibited by transgenic expression of PgFADX (Mietkiewska et al. 2014).
In previous studies, S. cerevisiae was used to characterize the properties of microsomal recombinant PgFAD2 or PgFADX when the encoding cDNA was expressed under the control of a strong GAL10 promoter. Enzyme activity was very low, and only 0.2 and 0.8% of PuA of total FA was produced by growing cells at 30 °C for 3 days on 2% galactose without or with external LA supplementation, respectively (Iwabuchi et al. 2003). So far, the highest accumulation of PuA to 1.6% of total FA was achieved by growing yeast cells at 30 °C in media supplemented with 0.3 mM LA, the substrate for FADX (Hornung et al. 2002). This study also reported that PuA could only be detected after external supplementation of yeast growth media with LA (Hornung et al. 2002).
In the current study, we attempted to produce PuA in S. pombe instead of S. cerevisiae because of the high level of OA in this yeast. S. pombe strains with plasmids containing cDNA-encoding PgFAD2 and/or PgFADX were constructed under the control of a strong nmt1 promoter. Both cDNAs were codon optimized for yeast expression.
The heterologous expression of PgFADX driven by the nmt1 promoter resulted in a slight growth inhibition effect which was enhanced by co-expression of PgFAD2 and PgFADX (Fig. 2). This growth inhibition effect was also observed when we previously engineered S. pombe to produce ricinoleic acid (Holic et al. 2012). Preliminary flow cytometry data with asynchronous yeast culture co-expressing PgFAD2 with PgFADX showed that many cells were arrested in the S phase (data not shown). PuA production in our transformed yeast may have exerted a cytotoxic effect on the yeast to some extent. It should be noted, however, that combined expression of PgFAD2 and PgFADX actually led to lower levels of PuA after 4–10 days of induction when compared to the levels of this conjugated FA for expression of PgFADX alone (see Fig. 6b versus Fig. 6c). Therefore, there may have been other factors, in addition to possible PuA-mediated cell death, which contributed to the observed growth inhibition. The anti-cancer and cytotoxic properties of PuA, and pomegranate oil, however, have been documented (Costantini et al. 2014; Sharma et al. 2017; Shinohara et al. 2012; Yuan et al. 2014). In addition, conjugated FAs are known to inhibit DNA polymerases and DNA topoisomerases (Mizushina et al. 2004).
In the first attempt to produce PuA in S. pombe, the strategy of cell growth based on previous experiments with production of ricinoleic acid was adopted and modified. In this strategy, high accumulation of this hydroxy FA was achieved by inoculation of cells to high initial OD. In addition, this strategy reduced the carryover of thiamine (repressing the expression from the nmt1 promoter) by cultivation of the pre-culture in media lacking thiamine (Holic et al. 2012). The strategy used for engineering of ricinoleic acid production, however, was not applicable for engineering relatively high levels of PuA accumulation (Fig. 4b). Therefore, we optimized the pre-culture condition and tested the effect of various initial OD inoculations on final PuA production. In case of PuA accumulation, it was more effective to cultivate pre-culture cells in repressed condition and inoculate the cultured cells to an initial OD equal to 0.2. This approach greatly enhanced the production of PuA.
S. pombe appeared to be a much better host for production of PuA than S. cerevisiae. With introduction of recombinant PgFADX alone, substantial production of PuA to 19.6% of total FA was achieved. This is considerably greater than what was previously achieved when recombinant PgFADX was introduced into S. cerevisiae. The very low level of LA accumulation seen in our experiments suggests that PuA formation catalyzed by PgFADX kept pace with LA formation catalyzed by the same enzyme. Thus, it is possible that LA produced by the catalytic action of PgFADX is more readily used by PgFADX than a source of LA produced by the catalytic action of an independent FAD2. This would imply that substrate channeling may be operative in the conversion of OA to PuA catalyzed by PgFADX. Recently, substrate channeling was shown to be operative in plant recombinant FAD2 and ∆15-desaturase (FAD3) (sequentially catalyzing the conversion of 18:1-phosphatidylcholine to 18:3∆9cis,12cis,15cis-phosphatidycholine) which were shown to interact in the membrane (Lou et al. 2014).
Co-expression of PgFAD2 with PgFADX further increased PuA accumulation to 25.1% of the total FA in S. pombe. This exceeds the value of about 20% which was previously reported for the PuA content of the oil of A. thaliana co-expressing PgFAD2 with PgFADX (see Mietkiewska et al. 2014). In addition, in the current study, different dynamics of PuA accumulation in yeast over-expressing PgFADX when compared to yeast co-expressing PgFADX with PgFAD2 were observed. Elevated PuA content was maintained for a longer period in the case of PgFADX alone. In contrast, co-expression of PgFAD2 with PgFADX resulted in a higher percentage of PuA at an earlier incubation time. Although the LA precursor of PuA greatly increased with combined expression of PgFAD2 and PgFADX, the increase in the content of this precursor FA did not appear to be effective in sustaining relatively high levels of PuA beyond 3 days of incubation (Fig. 6c). Perhaps the introduction of recombinant PgFAD2 resulted in the production of a limited pool of phosphatidylcholine which was accessible to recombinant PgFADX.
The difficulty to engineer heterologous organisms with large amounts of an unusual FA is mainly caused by their toxicity in membrane phospholipids (Holic et al. 2012; Yazawa et al. 2013a) and lack of efficient channeling of unusual FA from phospholipid to triacylglycerol (Mietkiewska et al. 2014; Yazawa et al. 2013a; Yazawa et al. 2014). Recently, we demonstrated that the lipotoxicity of accumulated FA can be decreased by their excretion into the growth medium (Sec et al. 2015; Yazawa et al. 2013b). Since the conjugated system of double bonds makes PuA more unstable compared to other non-conjugated polyunsaturated FAs, more effective channeling of PuA into triacylglycerol may be a useful strategy in future work to further boost the PuA content of yeast. Indeed, the storage capacity of lipid droplets is a factor which we have shown to influence the toxicity of accumulated lipid in cells (Valachovic et al. 2016). It would be particularly useful to implement species or strains of yeast capable of accumulating relatively high levels of triacylglycerol for this type of investigation. The lipid content in S. pombe, similar to S. cerevisiae, is generally not high (5–10% of dry cell weight) (Holic et al. 2012; Kamisaka et al. 2006). Recent studies have shown, however, that by optimization of growth conditions and by modification of metabolic pathways, a much higher lipid content (> 20%) can in fact be achieved using S. cerevisiae (Kamisaka et al. 2016; Kamisaka et al. 2013; Kamisaka et al. 2007). Thus, it may also be possible to engineer S. pombe to accumulate substantially more lipids. Moreover, the high oil yeast, Yarrowia lipolytica, has already been used to produce a range of specialized FA (Ledesma-Amaro and Nicaud 2016).
In conclusion, the heterologous expression of PgFADX in S. pombe resulted in yeast cells accumulating PuA up to 19.6% of total FAs. Co-expression of PgFADX with PgFAD2 led to a further increase in PuA content up to 25.1% of total FAs, the highest level reported to date in a transgenic organism producing this FA.
References
Arao K, Wang YM, Inoue N, Hirata J, Cha JY, Nagao K, Yanagita T (2004) Dietary effect of pomegranate seed oil rich in 9cis, 11trans, 13cis conjugated linolenic acid on lipid metabolism in obese, hyperlipidemic OLETF rats. Lipids Health Dis 3:24
Aruna P, Venkataramanamma D, Singh AK, Singh RP (2016) Health benefits of punicic acid: a review. Compr Rev Food Sci Food Saf 15(1):16–27. https://doi.org/10.1111/1541-4337.12171
Badami RC, Patil KB (1980) Structure and occurrence of unusual fatty acids in minor seed oils. Prog Lipid Res 19(3):119–153. https://doi.org/10.1016/0163-7827(80)90002-8
Boussetta T, Raad H, Letteron P, Gougerot-Pocidalo MA, Marie JC, Driss F, El-Benna J (2009) Punicic acid a conjugated linolenic acid inhibits TNFalpha-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PLoS One 4(7):e6458. https://doi.org/10.1371/journal.pone.0006458
Cahoon EB, Dietrich CR, Meyer K, Damude HG, Dyer JM, Kinney AJ (2006) Conjugated fatty acids accumulate to high levels in phospholipids of metabolically engineered soybean and Arabidopsis seeds. Phytochemistry 67(12):1166–1176
Costantini S, Rusolo F, De Vito V, Moccia S, Picariello G, Capone F, Guerriero E, Castello G, Volpe MG (2014) Potential anti-inflammatory effects of the hydrophilic fraction of pomegranate (Punica granatum L.) seed oil on breast cancer cell lines. Molecules 19(6):8644–8660. https://doi.org/10.3390/molecules19068644
Garaiova M, Zambojova V, Simova Z, Griac P, Hapala I (2014) Squalene epoxidase as a target for manipulation of squalene levels in the yeast Saccharomyces cerevisiae. FEMS Yeast Res 14(2):310–323. https://doi.org/10.1111/1567-1364.12107
Gietz D, St Jean A, Woods RA, Schiestl RH (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20(6):1425
Grossmann ME, Mizuno NK, Schuster T, Cleary MP (2010) Punicic acid is an omega-5 fatty acid capable of inhibiting breast cancer proliferation. Int J Oncol 36(2):421–426
Holic R, Yazawa H, Kumagai H, Uemura H (2012) Engineered high content of ricinoleic acid in fission yeast Schizosaccharomyces pombe. Appl Microbiol Biotechnol 95(1):179–187. https://doi.org/10.1007/s00253-012-3959-6
Hornung E, Pernstich C, Feussner I (2002) Formation of conjugated Delta11Delta13-double bonds by Delta12-linoleic acid (1,4)-acyl-lipid-desaturase in pomegranate seeds. Eur J Biochem 269(19):4852–4859
Iwabuchi M, Kohno-Murase J, Imamura J (2003) Delta 12-oleate desaturase-related enzymes associated with formation of conjugated trans-delta 11, cis-delta 13 double bonds. J Biol Chem 278(7):4603–4610. https://doi.org/10.1074/jbc.M210748200
Joh Y-G, Kim S-J, Christie WW (1995) The structure of the triacylglycerols, containing punicic acid, in the seed oil of Trichosanthes kirilowii. J Am Oil Chem Soc 72(9):1037–1042. https://doi.org/10.1007/bf02660718
Kamisaka Y, Noda N, Tomita N, Kimura K, Kodaki T, Hosaka K (2006) Identification of genes affecting lipid content using transposon mutagenesis in Saccharomyces cerevisiae. Biosci Biotechnol Biochem 70(3):646–653
Kamisaka Y, Tomita N, Kimura K, Kainou K, Uemura H (2007) DGA1 (diacylglycerol acyltransferase gene) overexpression and leucine biosynthesis significantly increase lipid accumulation in the Deltasnf2 disruptant of Saccharomyces cerevisiae. Biochem J 408(1):61–68
Kamisaka Y, Kimura K, Uemura H, Yamaoka M (2013) Overexpression of the active diacylglycerol acyltransferase variant transforms Saccharomyces cerevisiae into an oleaginous yeast. Appl Microbiol Biotechnol 97(16):7345–7355. https://doi.org/10.1007/s00253-013-4915-9
Kamisaka Y, Kimura K, Uemura H, Ledesma-Amaro R (2016) Modulation of gluconeogenesis and lipid production in an engineered oleaginous Saccharomyces cerevisiae transformant. Appl Microbiol Biotechnol 100(18):8147–8157. https://doi.org/10.1007/s00253-016-7662-x
Koba K, Imamura J, Akashoshi A, Kohno-Murase J, Nishizono S, Iwabuchi M, Tanaka K, Sugano M (2007) Genetically modified rapeseed oil containing cis-9,trans-11,cis-13-octadecatrienoic acid affects body fat mass and lipid metabolism in mice. J Agric Food Chem 55(9):3741–3748. https://doi.org/10.1021/jf063264z
Kohno H, Suzuki R, Yasui Y, Hosokawa M, Miyashita K, Tanaka T (2004) Pomegranate seed oil rich in conjugated linolenic acid suppresses chemically induced colon carcinogenesis in rats. Cancer Sci 95(6):481–486
Ledesma-Amaro R, Nicaud JM (2016) Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog Lipid Res 61:40–50. https://doi.org/10.1016/j.plipres.2015.12.001
Lou Y, Schwender J, Shanklin J (2014) FAD2 and FAD3 desaturases form heterodimers that facilitate metabolic channeling in vivo. J Biol Chem 289(26):17996–18007. https://doi.org/10.1074/jbc.M114.572883
Maundrell K (1990) nmt1 of fission yeast. A highly transcribed gene completely repressed by thiamine. J Biol Chem 265(19):10857–10864
Meesapyodsuk D, Chen Y, Ng SH, Chen J, Qiu X (2015) Metabolic engineering of Pichia pastoris to produce ricinoleic acid, a hydroxy fatty acid of industrial importance. J Lipid Res 56(11):2102–2109. https://doi.org/10.1194/jlr.M060954
Mietkiewska E, Siloto RM, Dewald J, Shah S, Brindley DN, Weselake RJ (2011) Lipins from plants are phosphatidate phosphatases that restore lipid synthesis in a pah1Delta mutant strain of Saccharomyces cerevisiae. FEBS J 278(5):764–775. https://doi.org/10.1111/j.1742-4658.2010.07995.x
Mietkiewska E, Miles R, Wickramarathna A, Sahibollah AF, Greer MS, Chen G, Weselake RJ (2014) Combined transgenic expression of Punica granatum conjugase (FADX) and FAD2 desaturase in high linoleic acid Arabidopsis thaliana mutant leads to increased accumulation of punicic acid. Planta 240(3):575–583. https://doi.org/10.1007/s00425-014-2109-z
Mizushina Y, Tsuzuki T, Eitsuka T, Miyazawa T, Kobayashi K, Ikawa H, Kuriyama I, Yonezawa Y, Takemura M, Yoshida H, Sakaguchi K (2004) Inhibitory action of conjugated C18-fatty acids on DNA polymerases and DNA topoisomerases. Lipids 39(10):977–983
Rawat R, Yu X-H, Sweet M, Shanklin J (2012) Conjugated fatty acid synthesis: RESIDUES 111 AND 115 INFLUENCE PRODUCT PARTITIONING OF MOMORDICA CHARANTIA CONJUGASE. J Biol Chem 287(20):16230–16237. https://doi.org/10.1074/jbc.M111.325316
Saha SS, Ghosh M (2009) Comparative study of antioxidant activity of alpha-eleostearic acid and punicic acid against oxidative stress generated by sodium arsenite. Food Chem Toxicol 47(10):2551–2556. https://doi.org/10.1016/j.fct.2009.07.012
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Sec P, Garaiova M, Gajdos P, Certik M, Griac P, Hapala I, Holic R (2015) Baker’s yeast deficient in storage lipid synthesis uses cis-vaccenic acid to reduce unsaturated fatty acid toxicity. Lipids 50(7):621–630. https://doi.org/10.1007/s11745-015-4022-z
Sharma P, McClees SF, Afaq F (2017) Pomegranate for prevention and treatment of cancer: an update. Molecules 22(1):177. https://doi.org/10.3390/molecules22010177
Shinohara N, Tsuduki T, Ito J, Honma T, Kijima R, Sugawara S, Arai T, Yamasaki M, Ikezaki A, Yokoyama M, Nishiyama K, Nakagawa K, Miyazawa T, Ikeda I (2012) Jacaric acid, a linolenic acid isomer with a conjugated triene system, has a strong antitumor effect in vitro and in vivo. Biochim Biophys Acta 1821(7):980–988. https://doi.org/10.1016/j.bbalip.2012.04.001
Smith CR (1971) Occurrence of unusual fatty acids in plants. Prog Chem Fats Other Lipids 11:137–177. https://doi.org/10.1016/0079-6832(71)90005-X
Song Z, Mietkiewska E, Weselake RJ (2016) The linin promoter is highly effective in enhancing punicic acid production in Arabidopsis. Plant Cell Rep. https://doi.org/10.1007/s00299-016-2094-8
Stumpe M, Kandzia R, Gobel C, Rosahl S, Feussner I (2001) A pathogen-inducible divinyl ether synthase (CYP74D) from elicitor-treated potato suspension cells. FEBS Lett 507(3):371–376
Suzuki R, Noguchi R, Ota T, Abe M, Miyashita K, Kawada T (2001) Cytotoxic effect of conjugated trienoic fatty acids on mouse tumor and human monocytic leukemia cells. Lipids 36(5):477–482
Takagi T, Itabashi Y (1981) Occurrence of mixtures of geometrical isomers of conjugated octadecatrienoic acids in some seed oils: analysis by open-tubular gas liquid chromatography and high performance liquid chromatography. Lipids 16(7):546–551. https://doi.org/10.1007/bf02535054
Tanaka T, Hosokawa M, Yasui Y, Ishigamori R, Miyashita K (2011) Cancer chemopreventive ability of conjugated linolenic acids. Int J Mol Sci 12(11):7495–7509. https://doi.org/10.3390/ijms12117495
Valachovic M, Garaiova M, Holic R, Hapala I (2016) Squalene is lipotoxic to yeast cells defective in lipid droplet biogenesis. Biochem Biophys Res Commun 469(4):1123–1128. https://doi.org/10.1016/j.bbrc.2015.12.050
Vanhercke T, Wood CC, Stymne S, Singh SP, Green AG (2013) Metabolic engineering of plant oils and waxes for use as industrial feedstocks. Plant Biotechnol J 11(2):197–210. https://doi.org/10.1111/pbi.12023
Wang L, Li W, Lin M, Garcia M, Mulholland D, Lilly M, Martins-Green M (2014) Luteolin, ellagic acid and punicic acid are natural products that inhibit prostate cancer metastasis. Carcinogenesis 35(10):2321–2330. https://doi.org/10.1093/carcin/bgu145
Yazawa H, Holic R, Kumagai H, Uemura H (2013a) Toxicity of ricinoleic acid production in fission yeast Schizosaccharomyces pombe is suppressed by the overexpression of plg7, a phospholipase A2 of a platelet-activating factor (PAF) family homolog. Appl Microbiol Biotechnol 97(18):8193–8203. https://doi.org/10.1007/s00253-013-4987-6
Yazawa H, Kumagai H, Uemura H (2013b) Secretory production of ricinoleic acid in fission yeast Schizosaccharomyces pombe. Appl Microbiol Biotechnol 97(19):8663–8671. https://doi.org/10.1007/s00253-013-5060-1
Yazawa H, Ogiso M, Kumagai H, Uemura H (2014) Suppression of ricinoleic acid toxicity by ptl2 overexpression in fission yeast Schizosaccharomyces pombe. Appl Microbiol Biotechnol 98(22):9325–9337. https://doi.org/10.1007/s00253-014-6006-y
Yuan GF, Chen XE, Li D (2014) Conjugated linolenic acids and their bioactivities: a review. Food Funct 5(7):1360–1368. https://doi.org/10.1039/c4fo00037d
Acknowledgements
This work was supported by the Slovak Research and Development Agency under the contract No. APVV-0785-11 and APVV-15-0654 (RH) and a Natural Sciences and Engineering Research Council of Canada Discovery Grant (RJW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 121 kb)
Rights and permissions
About this article
Cite this article
Garaiova, M., Mietkiewska, E., Weselake, R.J. et al. Metabolic engineering of Schizosaccharomyces pombe to produce punicic acid, a conjugated fatty acid with nutraceutic properties. Appl Microbiol Biotechnol 101, 7913–7922 (2017). https://doi.org/10.1007/s00253-017-8498-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8498-8