Abstract
Bioremediation of areas co-contaminated with metals and polycyclic aromatic hydrocarbons (PAHs) by mushrooms has attracted considerable attention in recent years. In this study, Pleurotus eryngii was introduced for the removal of Mn and phenanthrene (Phe) from potato liquid medium (PDL) simultaneously. Effects of Tween 80 and saponin on P. eryngii growth together with Mn uptake as well as Phe removal were investigated. Although pollutants had a negative effect on mycelial morphology and growth, P. eryngii could still tolerate and remove Mn and Phe. Tween 80 increased removal of Mn and Phe through increase of P. eryngii growth, Phe solubility, pollutants bioavailability, and specific surface area of mycelium pellets, moreover, the activities of manganese peroxidase (MnP) and laccase, which played an important role on PAHs biodegradation. The maximal removal of Mn and Phe was achieved (92.17 and 93.85 % after 15 days incubation, respectively) with 0.6 g L−1 Tween 80. Treatments with saponin markedly inhibited P. eryngii growth (50.17–66.32 % lower relative to control) due to its fungistatic activity. Nevertheless, saponin could slightly enhance Phe removal through increasing solubility of Phe, and Phe removal rate varied from 80.53 to 87.06 % in saponin treatments. Joint stress of Mn and Phe induced a strong antioxidative response, and superoxide dismutase (SOD) activity decreased in surfactants-treated mycelium compared with control. Generally, Tween 80 was more suitable for strengthening mycoremediation by P. eryngii than saponin, and could be a promising alternative for the remediation of heavy metals and PAHs co-contaminated sites by mushrooms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contamination of heavy metals and polycyclic aromatic hydrocarbons (PAHs) in various ecosystems has become a widespread environmental problem, as the results of agricultural, industrial, and urban activities (Ghorbani and Eisazadeh 2012; Hong et al. 2010; Niu et al. 2013). Toxic metals pollution can cause severe environmental and human health problems because of their trophic transfer in organisms, biomagnification in food chains, and toxic effects on biota (Altomare et al. 1999; Goodyear and McNeill 1999). Manganese (Mn) is one of the most widely used metals in the world, which is also an essential element for the growth and development of humans, animals, and plants. However, excess manganese in water or food is dangerous for life being, which can induce health concerns, esthetic and economic problems, etc. (Diazveliz 2004; Santos-Burgoa et al. 2001). Due to PAHs’ known toxic, carcinogenic, and mutagenic properties, 16 PAHs has been listed as priority pollutants by the US Environmental Protection Agency (US-EPA) including phenanthrene (Phe) (Haritash and Kaushik 2009). Phe is one of the most abundant PAHs in aquatic environments (Bi-Xian et al. 2002; Chen et al. 2004) and able to accumulate in organisms due to its low molecular weight (Aksmann and Tukaj 2004).With the rapid urbanization and industrialization in China, many mixed contaminated sites are exposed (Yuan et al. 2010). Compared with individual pollutant, combinations frequently complicate the in situ remediation, and usually lead more serious toxicity (Mang et al. 2014; Wang and Brusseau 1995). Therefore, it is a major concern for researchers to develop eco-friendly and effective approaches to simultaneously remedy the co-contaminated sites. Bioremediation has been suggested to be an efficient, inexpensive, and environmentally safe cleaning method, which is a major process for the successful detoxication or removal of toxic pollutants from the environment (Pedetta et al. 2013; Tian et al. 2002).
Mushrooms, as macro-fungi, have been focused on the clean-up of areas contaminated with both PAHs and heavy metals because of their abilities to utilize a wide range of organic substrates, and produce various metal-chelating metabolites (Hadibarata and Kristanti 2014; Liu et al. 2015; Zhang et al. 2012b). Mushrooms have shown a distinct superiority to degrade PAHs due to its ability to secrete ligninolytic enzymes such as laccase and manganese peroxidase (MnP) (Thurston 1994; Wang and Ng 2006). And their certain advantages over bacteria in PAHs bioremediation have been extensively demonstrated (Novotny et al. 1999; Potin et al. 2004). Pleurotus eryngii (P. eryngii) is an edible mushroom with a high yield in the world, and it has been shown for the ability to degrade a variety of pollutants (Hadibarata et al. 2013). However, no studies have reported about the removal of heavy metals and PAHs in co-contaminated aquatic environment by P. eryngii, as well as its enzymes in the bioremediation process.
However, the low solubility of PAHs in water is a potential problem in biodegradation processes (Kumari et al. 2014). Surfactants seem to be available because they can enhance the solubility of PAHs in water, and consequently enhanced their bioavailability. Tween 80 is a non-ionic surfactant with less toxicity to microorganism than anionic and cationic surfactant (Collina et al. 2007), and has been widely used in PAHs bioremediation (Zhang and Zhu 2012). As to saponin, it is a representative plant-derived biosurfactant with greater environmental compatibility and higher biodegradability than synthetic surfactants (Cao et al. 2013). Less toxic surfactants Tween 80 and saponin have been chosen to elucidate the effects of surfactants on the biodegradation of Phe and uptake of Mn by P. eryngii. In previous works, saponin could effectively remove PAHs and heavy metals, such as Phe, cadmium, and zinc (Hong et al. 2002; Song et al. 2008). However, some researchers reported that saponin was detected with fungistatic activities (Hostettmann and Marston 2005; Osbourn et al. 1996). However, most antifungal studies of saponins have been carried out on yeast and other fungus, and a meager study has been done on P. eryngii. This encouraged us to study the effect of saponin on the mycoremediation processes by P. eryngii. To our best knowledge, no study has reported the surfactant-enhanced bioremediation for heavy metals and PAHs co-contaminated water by mushroom, especially by P. eryngii.
The objectives of this study were to investigate the capability of P. eryngii on removing Phe and Mn in the co-contaminated liquid medium. The specific objectives were to investigate: (1) the growth of P. eryngii and the removal of Phe and Mn by P. eryngii in co-contaminated liquid medium, (2) the production of enzymes during the bioremediation process, and (3) effects of surfactants on the uptake of Mn and Phe degradation in mixed solution. These results would provide informations not only for a better understanding about whether surfactants can affect mycoremediation of heavy metals and PAHs, but also for accessing the potential of surfactants in facilitating heavy metals and PAHs bioavailability during bioremediation of co-contaminated sites by heavy metals and PAHs.
Materials and methods
Chemicals and mycelium preparation
Phe was purchased from Kelong, Chemical Reagent Factory, Chengdu, China, with a purity of 99 %. Stock solution (20 mg mL−1) was prepared in acetone. Other chemicals were of analytical reagent grade and also obtained from Chengdu Kelong Corp. Stock solutions of Tween 80 and saponin were prepared in deionized water and sterilized through filtration by filter paper with a porosity of 0.2 mm.
The P. eryngii strain (ACCC 51388) was obtained from Sichuan Provincial Academy of Agricultural Sciences. The strain was cultured on potato dextrose agar (PDA) slants at 4 °C in the dark. The fungi were activated at 25 °C for 10 days prior to use.
Bioremediation and surfactants strengthen experiments
Batch experiments were performed to investigate the removal of Phe and Mn from co-contaminated potato liquid medium (PDL) with different solutions. First, clean PDL was mixed with different volumes of Mn stock solution to meet the required initial concentrations of Mn (0, 1, 2, 3, 4, 5, 6 mM). Then, Phe was added with an initial concentration of 20 mg L−1. At last, four agar plugs of mycelium punched out from the PDA culture plate were then transferred into the mixed contaminated liquid medium, respectively.
In the study, Phe concentrations were higher than the Phe pure-compound aqueous solubility limit, so the influence of surfactants on the degradation of Phe by P. eryngii could be evaluated. Surfactants were added from sterilized stock solutions, and the resulting initial concentration for each surfactant was 0.3, 0.6 g L−1 for Tween 80 and 2.0, 3.0 g L−1 for saponin, respectively. The control was carried out without surfactants, and an abiotic control without P. eryngii inoculum was performed to evaluate Mn and Phe depletion due to physicochemical processes.
All batch experiments were carried out in 250 mL Erlenmeyer flasks with 100 mL compound contamination solution and incubated in a gyratory shaker (SUKUN, SKY-211B) at 150 rpm in the dark at 28 °C. After 15 days, the mycelium was filtered by vacuum extraction (SHZ-D). One half of mycelium was oven dried at 80 °C to determine the dry weight and Mn content. The other half was quick-frozen in liquid nitrogen for detecting protein and enzymes. Each treatment was maintained in triplicates.
Scanning electron microscopy and X-ray microanalysis
Scanning electron microscopy (SEM) with energy dispersive X-ray microanalysis (EDX) was used to observe surface morphology features and metal distribution of the P. eryngii mycelium pellets cultured in different mixed solutions after 15 days. For SEM, samples were fixed in 2.5 % glutaraldehyde solution in 0.2 M of phosphate buffer, pH 7.0 at 4 °C for 24 h (Sun and Oelmüller 2010). Next, they were rinsed six times in the same buffer, post-fixed in 1 % buffered osmium tetroxide at room temperature for 1 h, and dehydrated by passing the samples in a series of ethyl alcohol (30, 50, 60, 70, 80, 90, and 100 %) for 15 min at each concentration (Silva et al. 2011). Samples were dried in a HCP-2 critical point dryer (Hitachi, Tokyo, Japan) using carbon dioxide as the transitional fluid, and coated with 2–3 nm of gold prior to analysis. Samples were performed by a high-resolution SEM equipped with an EDX analyzer (JSM-5900LV, Japan) and used an accelerating voltage of 5.0 kV.
Analysis of manganese
The liquid and mycelium pellets were separated by filtration to analyze the content of Mn. The solution was separated by centrifugation, then the total Mn concentration was analyzed by flame atomic absorption spectrometry (FAAS) according to Cao et al. (2012) with some modifications. The supernatant (2 mL) was digested with a mixture of concentrated HNO3 and 30 % H2O2 (5:2, v/v) using a microwave for 30 min, and finally diluted to 10 mL with deionized water. The removal rate of Mn by P. eryngii mycelium was calculated as Eq. (1):
where C i is the initial concentration of Mn in PDL and C x is the Mn concentration in PDL after harvest.
Following a 15-min elution period, the harvested mycelium pellets were suspended by desorption Tris-Mes buffer solution (2 mM Tris-Mes; 0.5 mM CaCl2, pH 7.2) to remove extracellular metal according to the method of Wang et al. (2013). Then, washing with deionized water, all the samples were then centrifuged at 4000 rpm for 5 min and collected for Mn analysis. The accumulation of Mn in the mycelium was also measured by FAAS, and determined via digestion with HCl/HNO3/HClO4 mixture (3:2:2, V/V/V) in a microwave for 30 min. After FAAS analysis, Mn accumulation by P. eryngii biomass was expressed in terms of Mn uptake per biomass (mg g−1) and calculated as followed:
Analysis of Phe
Residual Phe in PDL was analyzed according to procedure from Feng et al. (2012). Each solution sample was extracted with equal volumes of dichloromethane and submitted to ultrasonication for 20 min, followed by centrifugation at 4000 rpm for 10 min to separate aqueous and organic phases. After ultrasonication, centrifugation, and dewatering, the extracts of organic layer were concentrated in a rotary evaporator and moved into a sample vial with a final volume of 1.5 mL. After the mixture was filtered through a 0.22-mm filter, the concentration of Phe in the filtrate was analyzed by high-performance liquid chromatography (HPLC) fitted with a 4.6 × 250 mm reverse phase XDB-C18 column using methanol-water (80:20, v/v) as the mobile phase at a flow rate of 1 mL min−1 (Feng et al. 2014). The percentage of Phe removal from solution was calculated as Eq. (3):
where C 0 is the initial concentration of Phe in PDL and C t is the concentration of Phe in PDL after harvest.
Enzyme assay
After 15 days, the culture samples were harvested, homogenized at 6000 rpm, 4 °C for 15 min. The enzymatic activities of supernatant were analyzed after filtration. MnP was assayed by determination of absorbance of the extracted sample using UV spectrophotometer. Laccase activity was determined by ABTS (2,2-azino-di-[3-ethyl-benzothiazolin-sulphonate]) oxidation at 420 nm (Ɛ420 = 36, 000 L M−1 cm−1) (Bonugli-Santos et al. 2010). MnP activity was determined by oxidation of malonate and dimethoxyphenol in MnSO4 solution at 270 nm (Hadibarata et al. 2012). Activities were expressed as unit per liter (U L−1).
The fresh P. eryngii mycelium (1.0 g) was quickly frozen in liquid nitrogen and was grinded in a pre-cooled mortar, and then was extracted in 1 mL of 20 mM Tris–EDTA for 2 h at 4 °C. After that, the homogenate was centrifuged at 6000 rpm, 4 °C for 10 min, and the resulting supernatant was further centrifuged at 12,000 rpm at 4 °C for 1 h. Finally, the obtained supernatant was filtered through a 0.45-μm Millipore filter for measuring superoxide dismutase (SOD) activity with the method of Beaucham C and Fridovic I (1971) by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium (NBT).
All treatments and measurements were performed in triplicate. Results were given as the mean values ± standard deviation (SD). Statistical analysis were evaluated using SPSS package (version 21.0) with ANOVA, and means were compared using least significant differences (LSD) calculated at a significance level of P = 0.05. All figures were performed using Origin V8.5 software.
Results
Effects of Mn on the growth of P. eryngii and the removal of Phe and Mn without surfactants
Effects of Mn initial concentration on the biomass and the removal rate of Phe and Mn were shown in Table 1. Dry biomass of P. eryngii among the treatments decreased significantly (P ≤ 0.05) compared with control (G0, without contaminants), due to the toxicity of Phe and Mn. And biomass was only 54.48 % of control when Mn and Phe were 6 mM and 20 mg L−1, respectively (G8). Although mycelium growth was inhibited, P. eryngii could still survive and effectively remove Mn and Phe from co-contaminated PDL. It could be easily discovered that the initial Mn concentration in PDL distinctly influenced Mn accumulation and Phe degradation by P. eryngii. The degradation of Phe increased with the increase of Mn initial concentration when it is low, but in high dose of Mn (4 Mm), Phe degradation tended to decrease. In experiments without surfactants, the minimum removal rate of Mn was 45.96 % in G7, and the minimum degradation rate of Phe was 61.21 % in G1 after 15 days (Table. 1). The maximum removal rate of Mn (82.27 %) was achieved at 2 mM (G3), and the maximum Phe degradation rate (77.74 %) was achieved at 3 mM (G4). In sterilized control (G0) experiment, no significant changes in the amount of Mn and Phe occurred over time. Aiming to decrease the number of variable quantities, we selected two concentrations of Mn (2, 3 mM) which were used in all of the following experiments.
Effects of surfactants on growth of P. eryngii mycelium
The dry weight of P. eryngii mycelium with or without surfactant was presented in Fig. 1. Results showed that biomass of P. eryngii in treatments with Tween 80 increased relative to control without surfactants, and the increasing trend was observed with the increase of Tween 80 in treatments containing same pollutants. The effects of high-concentration Tween 80 were statistically significant (P < 0.05), and biomass was maximum (1.31 ± 0.124) in the treatment with 0.6 g L−1 Tween 80 when Mn and Phe were 3 mM and 20 mg L−1, respectively. On the contrary, experiments with two concentrations (2, 3 g L−1) of saponin both obviously suppressed P. eryngii growth relative to control without surfactants, And dry biomass was only 42.04 % of control when Mn, Phe, and saponin were 3 mM, 20 mg L−1, and 3 g L−1 in the PDL, respectively.
Influence of surfactants on growth of P. eryngii mycelium (dry weight) in Mn-Phe co-contaminated PDL. Erlenmeyer flask amended with: manganese (Mn), phenanthrene (Phe), Tween 80 (Tw), saponin (Sa), at different concentrations, relatively to the control (in the absence of surfactant). The initial concentration of Phe was 20 mg L−1. Error bars denote standard deviation of three replicates. Columns denoted by different letters indicated significance at P < 0.05 among different treatments
Effects of surfactants on Mn removal and Mn accumulation in P. eryngii
Effects of surfactants on Mn removal rate in abiotic control and in cultures inoculated with P. eryngii were shown in Fig. 2a. In the abiotic control experiment, no significant changes in the amount of Mn occurred over time. At the same Phe level, Mn removal efficiency by P. eryngii mycelium reduced with the increase of Mn concentration in PDL. With the addition of Tween 80, Mn removal rate increased remarkably relative to control, and Mn removal gradually increased with the increase of Tween 80 concentration. When Mn was 2 mM, the removal rate of Mn reached 84.9 % at 0.3 g L−1 of Tween 80, while Mn removal rate increased to 92.1 % at 0.6 g L−1 of Tween 80. As for saponin, it appeared to have no significant effect on Mn removal by P. eryngii mycelium compared with control without surfactant.
a Influence of surfactants on the Mn removal rate in abiotic control and in cultures inoculated with P. eryngii. b Effect of different surfactants on Mn accumulation in P. eryngii mycelium in the presence of different concentrations of Tween 80 (Tw) and saponin (Sa), relatively to the control in the absence of surfactant. The accumulation of Mn in P. eryngii mycelium washed with Tris-Mes (black square) and deionized water (white square). The initial concentration of Phe was 20 mg L−1. Error bars denote standard deviation of three replicates. Columns denoted by different letters indicated significance at P < 0.05 among different treatments
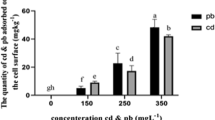
Mn accumulation in P. eryngii mycelium with or without surfactant was calculated and plotted in Fig. 2b. In general, Mn accumulation in mycelium increased with the increase of Mn amounts in liquid culture, and was influenced by different combination levels of surfactants. Particularly, treatments with saponin, Mn accumulation in mycelium (mg g−1) were much higher than other treatments. Mn accumulation in P. eryngii mycelium ranged from 6.80 ± 0.52 to 19.30 ± 1.32 mg g−1 in all treatments. It is noteworthy that the content of Mn in the mycelium was significantly reduced after washing by Tris-Mes solution than that in the untreated mycelium (Fig. 2b).
Effects of surfactants on removal rate of Phe
The effect of surfactants on the Phe removal rate in 15 days at various concentrations of mixed pollutants incubated with P. eryngii was examined (Fig. 3). For abiotic control, Phe was not significantly depleted without the incubation of P. eryngii (9.03 %), but Phe concentrations in other treatments decreased by 65.49–91.69 % at the end of the experiment. As shown in Fig. 3, Phe removal rate increased with the increase of Mn initial concentration. Furthermore, Phe was better degraded with surfactants than control without surfactants, and Tween 80 exerted a significant stimulatory effect Phe removal by P. eryngii mycelium, and the promoting effect was increased with the increase of Tween 80 initial concentration. In the presence of 0.6 g L−1 Tween 80, Phe removal rate reached 93.85 % after 15 days, 23.2 % higher compared with control, when Mn and Phe was 3 mM and 20 mg L−1, respectively. In treatments with 3 mM of Mn and 20 mg L−1 of Phe, the highest Phe removal rate (93.85 %) was obtained compared with that of other treatments (65.50–91.55 %). Comparison of Phe removal rate with or without saponin showed that saponin slightly enhanced Phe removal by P. eryngii mycelium (no significant differences were found). After 15 days of incubation, at least 80 % of Phe was degraded with addition of surfactants when initial Phe concentration was 20 mg L−1. Overall, the addition of Tween 80 and saponin enhanced degradation of Phe by P. eryngii mycelium, and Tween 80 yielding a higher Phe removal rate than saponin.
SEM and EDS studies
Effects of Mn, Phe, and Tween 80 on the P. eryngii mycelium were prominent on the mycelial ultrastructure as revealed by SEM (Fig. 4). The P. eryngii mycelium without any treatment revealed normal reticular mycelium surface with smooth and non-adhesive appearance (Fig. 4a). After exposure to Mn and Phe, P. eryngii mycelium morphology became sticky, shriveled, and distorted (Fig. 4b). Exposure of P. eryngii mycelium to Tween 80 also resulted in remarkable irregular, wizened, and microporous of hyphal elements (Fig. 4c). When P. eryngii mycelium was treated with Mn-Phe and Tween 80 simultaneously, the mycelium shriveled severely and appeared crumpled, adhesive, and twisted (Fig. 4d). These results suggested that treatments with Mn, Phe, or Tween 80 caused drastic changes in hyphal morphology, and the effect of Tween 80 was more pronounced than that of Mn-Phe. Elemental composition of pure and Mn-loaded biomass analyzed by SEM–EDS were shown in Fig. 5. And Fig. 5a showed that Mn ions were not found in the P. eryngii mycelium which was harvested in pure PDL. After culturing in PDL containing Mn, the weight percent of fungal biomass gives prominent manganese peak (1.88 % in weight) apart from C (40.15 %) and O (49.53 %) peaks.
Effects of surfactants on the laccase, MnP and SOD activity
Interactions between Mn-Phe co-contamination and surfactants on laccase and MnP activities were shown in Fig. 6a. Laccase activity was not detected in treatment without inoculation of P. eryngii, and laccase in the treatment without contaminants was only 6.36 U L−1. Positive co-effects by Mn and Phe were found on the exudation of laccase which led to an enhancement of laccase activity in solution samples, but laccase activity decreased with the increase of Mn concentration. In the presence of Tween 80, laccase activity was apparently promoted compared with control, and the promoting effect increased with the increase of Tween 80. Laccase in treatment (2 mM Mn and 20 mg L−1 Phe) and treatment (2 mM Mn, 20 mg L−1 Phe and 0.6 g L−1 Tween 80) were 566.02 and 996.25 U L−1, respectively. However, laccase activity significantly (P < 0.05) decreased with the existence of saponin and inhibiting effect in high level of saponin was stronger than that in low level. As shown in Fig. 6a, MnP activity showed similar trend with the laccase activity in experimental groups. The activity increased with the presence of Tween 80 but decreased with the existence of saponin. The activity in treatment (2 mM Mn, 20 mg L−1 Phe and 0.6 g L−1 Tween 80) was four times higher than that in treatment (2 mM Mn and 20 mg L−1 Phe), with a maximum activity at (740.27 U L−1), indicated the positive effect of high concentration of Tween 80 on MnP activity.
a Laccase and MnP activity in medium at 15 days during the bioremediation experiments in the presence of 20 mg L−1 Phe and different concentrations of Tween 80 (Tw) and saponin (Sa), relatively to the control in the absence of surfactant. b Effect of different surfactants on SOD activity of P. eryngii mycelium during the bioremediation of various concentrations of pollutants. The initial concentration of Phe was 20 mg L−1. Error bars denote standard deviation of three replicates. Columns denoted by different letters indicated significance at P < 0.05 among different treatments
The results presented in this study showed that antioxidant enzymes SOD had various responses to co-contaminants and surfactants. As shown in Fig. 6b, SOD activity significantly increased under joint stress of Mn and Phe in comparison with non-contaminations treatment (only 0.4 U mg−1) and reached maximum at 27.6 U mg−1 when 20 mg L−1 of Phe was mixed with 3 mM of Mn. The SOD activity increased at higher Mn concentration but in the presence of surfactants, a decrease in SOD activity was observed relative to control (without surfactants).
Discussion
Data showed that Mn-Phe co-contaminations had a negative effect on P. eryngii growth due to the toxicity of pollutants. Similarly to our study, Liu et al. (2015) suggested that heavy metals (Cu, Cd) and 2,4,5-trichlorophenol had obvious inhibition effect on mushroom growth. Previous studies also suggested that heavy metals could affect growth and metabolism of fungi (Guelfi et al. 2003), and PAHs had been shown to markedly affect the structure, integrity, and function of the membranes (Sikkema et al. 1995). Meanwhile, it had been reported that PAHs might affect the energy transduction across the biological membranes (Sikkema et al. 1994).
In the abiotic control experiment, no significant changes in the amount of Mn and Phe occurred over time, which indicated that the removal of Mn and Phe occurring in all of the following experiments was due to microbial action. Although mycelium growth was inhibited, P. eryngii demonstrated a remarkable tolerance to Mn-Phe stress under controlled conditions (since all P. eryngii survived). In addition, P. eryngii could still effectively remove Mn and Phe from co-contaminated PDL, and hence, it is a feasible choice for remediation. Similarly, many researchers reported that mushrooms could remove trace elements and PAHs, particularly in their mycelia (Cao et al. 2012; Hadibarata and Kristanti 2014). In addition, mushrooms can produce carbohydrates, amino acids, thiols, and all kinds of enzymes (Campos et al. 2009; Rangel-Castro et al. 2002), and these compounds can contribute to remove pollutants as well as lessen their toxicity (Singh et al. 2011). Previous studies showed that fungal siderophores might solubilize and sequester heavy metals such as iron, chromium, and lead (Renshaw et al. 2002). In addition, fungal immobilization processes can convert toxic metals in situ into insoluble and less toxic forms (Gadd 2000; Morley and Gadd 1995). Furthermore, exposure to Mn and Phe caused P. eryngii mycelium morphological alterations and increased its specific surface area, which could facilitate the biosorption of pollutants (Fig. 4). As a whole, P. eryngii could be a suitable candidate for mycoremediation of the Mn-Phe co-contaminated liquid medium.
However, the low aqueous solubility of PAHs is a potential problem in biodegradation processes (Prak and Pritchard 2002). Surfactants may solve the problem by enhancing the solubility of hydrophobic compounds (Li and Chen 2009; Sudarat et al. 1998). Many studies have been conducted to enhance the biodegradation of PAHs using surfactants, and consequently enhanced their biodegradation (Grimberg et al. 1996; Volkering et al. 1995). But surfactants may be toxic to microorganisms, and consequently, decrease degradation effect (Billingsley et al. 1999; Shiau et al. 1995). Biomass is an effective and sensitive indicator to study the effects of surfactants on P. eryngii metabolic growth. The addition of non-ionic surfactant Tween 80 showed a positive effect on P. eryngii growth, and the facilitating effect increased with the increase of Tween 80. Our result was in accordance with Zhou et al. (2007), who found Tween 80 could stimulate fungal growth (tested concentration ranges were from 100 to 700 mg L−1). On the contrary, experiments performed with saponin inhibited P. eryngii growth by 50.17–66.32 % relative to control, due to its fungistatic activity. Similarly, a number of studies have reported that many saponin or saponin-rich extracts from various plants showed antifungal activities (Escalante et al. 2002; Oleszek et al. 1990).
As shown in Fig. 2a, Tween 80 enhanced the removal rate of Mn, and in the presence of 0.6 g L−1 Tween 80, Mn removal reached a maximum (92.12 %) when Mn and Phe were 2 mM and 20 mg L−1, respectively. Treatment with Tween 80 could facilitate P. eryngii mycelium growth (Fig. 1), which might be the reason for the ability of mycelium to remove Mn more effectively from liquid medium spiked with Mn. SEM results showed that Tween 80 caused drastic changes in hyphal morphology which caused the increase of its specific surface area and the attachment point of pollutants (Fig. 4). These results indicated that these morphological alterations could facilitate the biosorption of pollutants, thus enhancing the removal efficiency. Moreover, surfactants can transfer metal ions from an aqueous to an organic phase by ion exchange, precipitation-dissolution, and counterion binding (Hiraide et al. 1997; Wang and Mulligan 2004), making these contaminants more available for remediation. Regarding saponin, it could complex with trace metal elements by the external carboxyl groups of saponin micelle which may enhance Mn removal. On the other hand, saponin markedly inhibited the growth of P. eryngii, and as a consequence, saponin exhibited no significant effect on Mn removal compared with control. These results indicated that the application of 0.6 g L−1 of Tween 80 was the most efficacious for strengthening Mn removal from co-contaminated solution by P. eryngii mycelium. The presence of prominent manganese peak appeared after adsorption (Fig. 5), and Tris-Mes solution reduced Mn accumulation in the mycelium (Fig. 2a), indicating a certain quantity of Mn ion was superficially bound to the surface of P. eryngii mycelium instead of completely entering the cells. Similarly, Rajkumar et al. (2010) suggested that extracellular materials can immobilize metals and prevent them entry into the cells.
There might be some mechanisms attributing to Phe degradation by P. eryngii mycelium in co-contaminated PDL with or without surfactants. As is well documented, many ligninolytic fungi can degrade PAHs because of the extracellular ligninolytic enzymatic system, such as laccase and MnP (Hofrichter 2002; Ruiz-Aguilar et al. 2002) The ligninolytic enzymes attack PAHs due to the structure of lignin polymers are similar to the aromatic molecular structure of PAHs (Acevedo et al. 2011; Baldrian 2006). Moreover, fungal metabolism may change the structure of Phe and make it more easy to metabolize, and these compounds may be used as a carbon source. Comparing the maximum removal rate of Phe in control solution without surfactants (2 mM Mn 66.99 %; 3 mM Mn 75.87 %) (Fig. 3), it was clear that Tween 80 and saponin played a positive role on Phe removal in mixed solution. Surfactants are a class of natural and synthetic chemicals that may result from a stimulation of dissolution rate or from surfactant-mediated dispersion, solubilization, or emulsification of poor soluble substrate (Noordman et al. 2002). In addition, surfactants are known to promote pollutants transferring into the water phase by decreasing the interfacial tension between water and hydrophobic pollutants (Juhasz et al. 1997), and the surfactant molecules may replace the hydrophilic molecular on the cell surface which could improve cell surface hydrophobicity (Zhao et al. 2011). These studies suggested that non-ionic surfactant Tween 80 and biosurfactant saponin can increase the solubility of Phe in the solution, and more Phe would be available to attack cell membrane, which might be the primary reason for the results showed above. Thus, the application of Tween 80 and saponin could largely improve the bioavailability and absorption of Phe by P. eryngii, as well as its biodegradation. Previous works have demonstrated that saponin could effectively remove PAHs, such as Phe and polychlorinated biphenyls (Song et al. 2008; Xia et al. 2009). The better P. eryngii mycelium growth and higher Phe-biodegradation observed in the presence of Tween 80 could also be due to that this surfactant was biodegradable and can be used as an additional carbon source for the microorganism (Franzetti et al. 2006). Furthermore, Tween 80 could be a ligninase inducer in agitated cultures that protects the enzymes from denaturing which may also contribute to the improvement of Phe degradation in experiments.
Mushroom attacks on PAHs appear to involve several enzymes such as MnP and Laccase. Our results indicated that higher laccase and MnP activity lead to a better removal of Mn and Phe. The addition of Tween 80 had a positive effect on the biodegradation of Phe in liquid medium, and may be associated with increases in MnP and laccase activity. Similar phenomenon has been observed in previous report, where the high removal of PAHs was prompted by a ligninolytic system (Acevedo et al. 2011). In the presence of saponin, the growth of P. eryngii significantly decreased due to its fungistatic activity; hence, laccase activity was inhibited. The toxicity of heavy metals in fungi could be the result of the generation of reactive oxygen species (ROS), which may cause wide-ranging damages to proteins, nucleic acids, and lipids, and even lead to cell death (Paula et al. 2009), so the tolerance of the fungi to heavy metals has been associated with its ability to clear away ROS (Stefan et al. 2005; Zhang et al. 2012a). Moreover, it has been reported that Phe can induce ROS and lead to oxidative stress (Hannam et al. 2004; Sun et al. 2006). Like plants, mushrooms are able to respond to elevated levels of ROS by activating their antioxidative defense systems. SOD is an efficient scavenger of ROS, destroys the free superoxide by converting it to peroxide and oxygen (Bai et al. 2003). Increase in the SOD activity may owe to the increased generation of ROS (Somashekaraiah et al. 1992), or increased expression of genes encoding SOD (Bowler et al. 1992). In this study, Mn and Phe could induce a strong antioxidative response in the mycelium of P. eryngii, and SOD activity significantly increased under joint stress of Mn and Phe in comparison with non-contaminations treatment (0.4 U mg−1). Moreover, SOD activity decreased in the treatments with surfactants relative to control, which may be due to the better removal efficiency of Mn and Phe. In general, SOD seem to play an important role in P. eryngii mycelium growth in lipid medium and meanwhile surfactants can partly alleviate the oxidative stress induced by joint stress of Mn and Phe, and thus decreased relevant enzymes activities.
In conclusion, our study obtained highlighted that P. eryngii was a suitable candidate for in situ mycoremediation of the Mn-Phe co-contaminated sites, and the biodegradation process could be enhanced by appropriate concentrations of Tween 80 and saponin. Furthermore, Tween 80 showed a better performance in promoting the mycoremediation effect than saponin, and could be a promising alternative candidate for the remediation of water co-contaminated with heavy metals and PAHs by P. eryngii.
References
Acevedo F, Pizzul L, Castillo MD, Cuevas R, Diez MC (2011) Degradation of polycyclic aromatic hydrocarbons by the Chilean white-rot fungus Anthracophyllum discolor. J Hazard Mater 185(1):212–219. doi:10.1016/j.jhazmat.2010.09.020
Aksmann A, Tukaj Z (2004) The effect of anthracene and phenanthrene on the growth, photosynthesis, and SOD activity of the green alga Scenedesmus armatus depends on the PAR irradiance and CO2 level. Arch Environ Contam Toxicol 47(2):177–184. doi:10.1007/s00244-004-2297-9
Altomare C, Norvell WA, Bjorkman T, Harman GE (1999) Solubilization of phosphates and micronutrients by the plant-growth-promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl Environ Microbiol 65(7):2926–2933
Bai Z, Harvey LM, McNeil B (2003) Oxidative stress in submerged cultures of fungi. Crit Rev Biotechnol 23(4):267–302
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30(2):215–242
Beaucham C, Fridovic I (1971) Superoxide Dismutase - Improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276-& doi:10.1016/0003-2697(71)90370-8
Bi-Xian M, Jia-Mo F, Guo-Ying S, Yue-Hui K, Zheng L, Gan Z, Yu-Shuan M, Zeng EY (2002) Chlorinated and polycyclic aromatic hydrocarbons in riverine and estuarine sediments from Pearl River Delta, China. Environ Pollut 117(3):457–474
Billingsley KA, Backus SM, Ward OP (1999) Effect of surfactant solubilization on biodegradation of polychlorinated bipbenyl congeners by Pseudomonas LB400. Appl Microbiol Biotechnol 52(2):255–260
Bonugli-Santos RC, Durrant LR, da Silva M, Sette LD (2010) Production of laccase, manganese peroxidase and lignin peroxidase by Brazilian marine-derived fungi. Enzym Microb Technol 46(1):32–37. doi:10.1016/j.enzmictec.2009.07.014
Bowler C, Mv M, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Biol 43(1):83–116
Campos JA, Tejera NA, Sanchez CJ (2009) Substrate role in the accumulation of heavy metals in sporocarps of wild fungi. Biometals 22(5):835–841. doi:10.1007/s10534-009-9230-7
Cao M, Hu Y, Sun Q, Wang L, Chen J, Lu X (2013) Enhanced desorption of PCB and trace metal elements (Pb and Cu) from contaminated soils by saponin and EDDS mixed solution. Environ Pollut 174:93–99. doi:10.1016/j.envpol.2012.11.015
Cao YR, Zhang XY, Deng JY, Zhao QQ, Xu H (2012) Lead and cadmium-induced oxidative stress impacting mycelial growth of Oudemansiella radicata in liquid medium alleviated by microbial siderophores. World J Microbiol Biotechnol 28(4):1727–1737. doi:10.1007/s11274-011-0983-0
Chen B, Xuan X, Zhu L, Wang J, Gao Y, Yang K, Shen X, Lou B (2004) Distributions of polycyclic aromatic hydrocarbons in surface waters, sediments and soils of Hangzhou City, China. Water Res 38(16):3558–3568
Collina E, Lasagni M, Pitea D, Franzetti A, Di Gennaro P, Bestetti G (2007) Bioremediation of diesel fuel contaminated soil: effect of non ionic surfactants and selected bacteria addition. Anal Chim 97(9):799–805. doi:10.1002/adic.200790065
Diazveliz G (2004) Behavioral effects of manganese injected in the rat substantia nigra are potentiated by dicumarol, a DT-diaphorase inhibitor. Pharmacol Biochem Be 77(2):245–251. doi:10.1016/j.pbb.2003.10.016
Escalante AM, Santecchia CB, López SN, Gattuso MA, Ravelo AG, Delle Monache F, Sierra MG, Zacchino SA (2002) Isolation of antifungal saponins from Phytolacca tetramera, an Argentinean species in critic risk. J Ethnopharmacol 82(1):29–34
Feng T, Lin H, Tang J, Feng Y (2014) Characterization of polycyclic aromatic hydrocarbons degradation and arsenate reduction by a versatile Pseudomonas isolate. Int Biodeterior Biodegradation 90:79–87. doi:10.1016/j.ibiod.2014.01.015
Feng T, Cui C, Dong F, Yy F, Yd L, Yang X (2012) Phenanthrene biodegradation by halophilic Martelella sp AD-3. J Appl Microbiol 113(4):779–789. doi:10.1111/j.1365-2672.2012.05386.x
Franzetti A, Di Gennaro P, Bevilacqua A, Papacchini M, Bestetti G (2006) Environmental features of two commercial surfactants widely used in soil remediation. Chemosphere 62(9):1474–1480. doi:10.1016/j.chemosphere.2005.06.009
Gadd GM (2000) Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr Opin Biotechnol 11(3):271–279. doi:10.1016/s0958-1669(00)00095-1
Ghorbani M, Eisazadeh H (2012) Fixed bed column study for Zn, Cu, Fe and Mn removal from wastewater using nanometer size polypyrrole coated on rice husk ash. Synth Met 162(15–16):1429–1433. doi:10.1016/j.synthmet.2012.05.018
Goodyear KL, McNeill S (1999) Bioaccumulation of heavy metals by aquatic macro-invertebrates of different feeding guilds: a review. Sci Total Environ 229(1–2):1–19. doi:10.1016/s0048-9697(99)00051-0
Grimberg SJ, Stringfellow WT, Aitken MD (1996) Quantifying the biodegradation of phenanthrene by Pseudomonas stutzeri P16 in the presence of a nonionic surfactant. Appl Environ Microb 62(7):2387–2392
Guelfi A, Azevedo RA, Lea PJ, Molina SMG (2003) Growth inhibition of the filamentous fungus Aspergillus nidulans by cadmium: an antioxidant enzyme approach. J Gen Appl Microbiol 49(2):63–73. doi:10.2323/jgam.49.63
Hadibarata T, Kristanti RA (2014) Potential of a white-rot fungus Pleurotus eryngii F032 for degradation and transformation of fluorene. Fungal Biol 118(2):222–227. doi:10.1016/j.funbio.2013.11.013
Hadibarata T, Teh ZC, Rubiyatno ZM, Khudhair AB, Yusoff ARM, Salim MR, Hidayat T (2013) Identification of naphthalene metabolism by white rot fungus Pleurotus eryngii. Bioprocess Biosyst Eng 36(10):1455–1461. doi:10.1007/s00449-013-0884-8
Hadibarata T, Yusoff ARM, Aris A, Kristanti RA (2012) Identification of naphthalene metabolism by white rot fungus Armillaria sp F022. J Environ Sci 24(4):728–732. doi:10.1016/s1001-0742(11)60843-7
Hannam ML, Bamber SD, Galloway TS, Moody AJ, Jones MB (2004) Effects of the model PAH phenanthrene on immune function and oxidative stress in the haemolymph of the temperate scallop Pecten maximus. J Phys Chem B 108(26):8970–8975
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169(1–3):1–15. doi:10.1016/j.jhazmat.2009.03.137
Hiraide M, Iwasawa J, Kawaguchi H (1997) Collection of trace heavy metals complexed with ammonium pyrrolidinedithiocarbamate on surfactant-coated alumina sorbents. Talanta 44(2):231–237. doi:10.1016/s0039-9140(96)02038-3
Hofrichter M (2002) Review: lignin conversion by manganese peroxidase (MnP). Enzym Microb Technol 30(4):454–466. doi:10.1016/s0141-0229(01)00528-2
Hong JW, Park JY, Gadd GM (2010) Pyrene degradation and copper and zinc uptake by Fusarium solani and Hypocrea lixii isolated from petrol station soil. J Appl Microbiol 108(6):2030–2040. doi:10.1111/j.1365-2672.2009.04613.x
Hong KJ, Tokunaga S, Kajiuchi T (2002) Evaluation of remediation process with plant-derived biosurfactant for recovery of heavy metals from contaminated soils. Chemosphere 49(4):379–387. doi:10.1016/s0045-6535(02)00321-1
Hostettmann K, Marston A (2005) Saponins. Cambridge University Press
Juhasz AL, Britz ML, Stanley GA (1997) Degradation of fluoranthene, pyrene, benz[a]anthracene and dibenz[a,h]anthracene by Burkholderia cepacia. J Appl Microbiol 83(2):189–198 doi:10.1046/j.1365-2672.1997.00220.x
Kumari B, Rajput S, Gaur P, Singh SN, Singh DP (2014) Biodegradation of pyrene and phenanthrene by bacterial consortium and evaluation of role of surfactant. Cell Mol Biol 60(5):22–28
Li JL, Chen BH (2009) Surfactant-mediated biodegradation of polycyclic aromatic hydrocarbons. Materials 2(1):76–94
Liu H, Guo S, Jiao K, Hou J, Xie H, Xu H (2015) Bioremediation of soils co-contaminated with heavy metals and 2,4,5-trichlorophenol by fruiting body of Clitocybe maxima. J Hazard Mater 294:121–127. doi:10.1016/j.jhazmat.2015.04.004
Mang L, Zhong-Zhi Z, Jing-Xiu W, Min Z, Yu-Xin X, Xue-Jiao W (2014) Interaction of heavy metals and pyrene on their fates in soil and tall fescue (Festuca arundinacea). Environ Sci Technol 48(2):1158–1165
Morley GF, Gadd GM (1995) Sorption of toxic metals by fungi and clay minerals. Mycol Res 99:1429–1438
Niu J, Dai Y, Guo H, Xu J, Shen Z (2013) Adsorption and transformation of PAHs from water by a laccase-loading spider-type reactor. J Hazard Mater 248-249:254–260. doi:10.1016/j.jhazmat.2013.01.017
Noordman WH, Wachter JHJ, de Boer GJ, Janssen DB (2002) The enhancement by surfactants of hexadecane degradation by Pseudomonas aeruginosa varies with substrate availability. J Biotechnol 94(2):195–212. doi:10.1016/s0168-1656(01)00405-9
Novotny C, Erbanova P, Sasek V, Kubatova A, Cajthaml T, Lang E, Krahl J, Zadrazil F (1999) Extracellular oxidative enzyme production and PAH removal in soil by exploratory mycelium of white rot fungi. Biodegradation 10(3):159–168. doi:10.1023/a:1008324111558
Oleszek W, Price KR, Colquhoun IJ, Jurzysta M, Ploszynski M, Fenwick GR (1990) Isolation and identification of alfalfa (Medicago sativa L.) root saponins: their activity in relation to a fungal bioassay. J Agric Food Chem 38(9):1810–1817. doi:10.1021/jf00099a006
Osbourn AE, Bowyer P, Daniels MJ (1996) Saponin detoxification by plant pathogenic fungi. In: Waller GR, Yamasaki K (eds) Saponins used in traditional and modern medicine, Adv Exp Med Biol, vol 404. Plenum Press Div Plenum Publishing Corp, New York, pp. 547–555
Paula B, Sílvia F, Elisa S, Valentim C, Bastos MDL (2009) Tolerance and stress response of Macrolepiota procera to nickel. J Agr Food Chem 57(15):7145–7152
Pedetta A, Pouyte K, Seitz MKH, Babay PA, Espinosa M, Costagliola M, Studdert CA, Peressutti SR (2013) Phenanthrene degradation and strategies to improve its bioavailability to microorganisms isolated from brackish sediments. Int Biodeterior Biodegradation 84:161–167. doi:10.1016/j.ibiod.2012.04.018
Potin O, Veignie E, Rafin C (2004) Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by Cladosporium sphaerospermum isolated from an aged PAH contaminated soil. FEMS Microbiol Ecol 51(1):71–78. doi:10.1016/j.femsec.2004.07.013
Prak D, Pritchard PH (2002) Solubilization of polycyclic aromatic hydrocarbon mixtures in micellar nonionic surfactant solutions. Water Res 36(14):3463–3472
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28(3):142–149. doi:10.1016/j.tibtech.2009.12.002
Rangel-Castro JI, Danell E, Pfeffer PE (2002) A C-13-NMR study of exudation and storage of carbohydrates and amino acids in the ectomycorrhizal edible mushroom Cantharellus cibarius. Mycologia 94(2):190–199. doi:10.2307/3761795
Renshaw JC, Robson GD, Trinci APJ, Wiebe MG, Livens FR, Collison D, Taylor RJ (2002) Fungal siderophores: structures, functions and applications. Mycol Res 106:1123–1142. doi:10.1017/s0953756202006548
Ruiz-Aguilar GML, Fernandez-Sanchez JM, Rodriguez-Vazquez R, Poggi-Varaldo H (2002) Degradation by white-rot fungi of high concentrations of PCB extracted from a contaminated soil. Adv Environ Res 6(4):559–568. doi:10.1016/s1093-0191(01)00102-2
Santos-Burgoa C, Rios C, Mercado LA, Arechiga-Serrano R, Cano-Valle F, Eden-Wynter RA, Texcalac-Sangrador JL, Villa-Barragan JP, Rodriguez-Agudelo Y, Montes S (2001) Exposure to manganese: health effects on the general population, a pilot study in Central Mexico. Environ Res 85(2):90–104. doi:10.1006/enrs.2000.4108
Shiau BJ, Sabatini DA, Harwell JH (1995) Properties of food grade (edible) surfactants affecting subsurface remediation of chlorinated solvents. Environ Sci Techno 29(12):2929–2935. doi:10.1021/es00012a007
Sikkema J,., Bont JA, De, Poolman B,. (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59(2):201–222
Sikkema J, Debont JAM, Poolman B (1994) Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem 269(11):8022–8028
Silva AR, Araújo JV, Braga FR, Benjamim LA, Souza DL, Carvalho RO (2011) Comparative analysis of destruction of the infective forms of Trichuris trichiura and Haemonchus contortus by nematophagous fungi Pochonia chlamydosporia; Duddingtonia flagrans and Monacrosporium thaumasium by scanning electron microscopy. Vet Microbiol 147(s 1–2):214–219
Singh AD, Vikineswary S, Abdullah N, Sekaran M (2011) Enzymes from spent mushroom substrate of Pleurotus sajor-caju for the decolourisation and detoxification of textile dyes. World J Microbiol Biotechnol 27(3):535–545. doi:10.1007/s11274-010-0487-3
Somashekaraiah B, Padmaja K, Prasad A (1992) Phytotoxicity of cadmium ions on germinating seedlings of mung bean (Phaseolus vulgaris): involvement of lipid peroxides in chlorphyll degradation. Physiol Plant 85(1):85–89
Song S, Zhu L, Zhou W (2008) Simultaneous removal of phenanthrene and cadmium from contaminated soils by saponin, a plant-derived biosurfactant. Environ Pollut 156(3):1368–1370. doi:10.1016/j.envpol.2008.06.018
Stefan F, Zoltán G, Borut P, Vekoslava S, Radmila M, Miklós P, Peter R, Martin B (2005) The oxidative stress response of the yeast Candida intermedia to copper, zinc, and selenium exposure. J Basic Microbiol 45(2):125–135
Sudarat B, Britz ML, Stanley GA (1998) Surfactant-enhanced biodegradation of high molecular weight polycyclic aromatic hydrocarbons by stenotrophomonas maltophilia. Biotechnol Bioeng 59(4):482–494
Sun C, Oelmüller R (2010) Technical note: Piriformospora indica hyphae and chlamydospores by scanning electron microscopy. Endocytobiosis & Cell Research
Sun Y, Yu H, Zhang J, Yin Y, Shi H, Wang X (2006) Bioaccumulation, depuration and oxidative stress in fish Carassius auratus under phenanthrene exposure. Chemosphere 63(8):1319–1327
Thurston CF (1994) The structure and function of fungal laccases. Microbiology 140(1):19–26
Tian L, Ma P, Zhong JJ (2002) Kinetics and key enzyme activities of phenanthrene degradation by Pseudomonas mendocina. Process Biochem 37(12):1431–1437. doi:10.1016/s0032-9592(02)00032-8
Volkering F, Breure AM, Andel JG, Van, Rulkens WH (1995) Influence of nonionic surfactants on bioavailability and biodegradation of polycyclic aromatic hydrocarbons. Appl Environ Microbiol 61(5):1699–1705
Wang H, Xu R, You L, Zhong G (2013) Characterization of Cu-tolerant bacteria and definition of their role in promotion of growth, Cu accumulation and reduction of Cu toxicity in Triticum aestivum L. Ecotoxicol Environ Saf 94(1):1–7
Wang HX, Ng TB (2006) Purification of a laccase from fruiting bodies of the mushroom Pleurotus eryngii. Appl Microbiol Biotechnol 69(5):521–525. doi:10.1007/s00253-005-0086-7
Wang SL, Mulligan CN (2004) An evaluation of surfactant foam technology in remediation of contaminated soil. Chemosphere 57(9):1079–1089. doi:10.1016/j.chemosphere.2004.08.019
Wang XJ, Brusseau ML (1995) Simultaneous complexation of organic compounds and heavy-metals by a modified cyclodextrin. Environ Sci Technol 29(10):2632–2635. doi:10.1021/es00010a026
Xia H, Chi X, Yan Z, Cheng W (2009) Enhancing plant uptake of polychlorinated biphenyls and cadmium using tea saponin. Bioresour Technol 100(20):4649–4653. doi:10.1016/j.biortech.2009.04.069
Yuan S, Wu X, Wan J, Long H, Lu X, Wu X, Chen J (2010) Enhanced washing of HCB and Zn from aged sediments by TX-100 and EDTA mixed solutions. Geoderma 156(3–4):119–125. doi:10.1016/j.geoderma.2010.02.006
Zhang D, Zhu L (2012) Effects of Tween 80 on the removal, sorption and biodegradation of pyrene by Klebsiella oxytoca PYR-1. Environ Pollut 164:169–174. doi:10.1016/j.envpol.2012.01.036
Zhang W, Hu Y, Cao Y, Huang F, Xu H (2012a) Tolerance of lead by the fruiting body of Oudemansiella radicata. Chemosphere 88(4):467–475
Zhang WW, Hu YJ, Cao YR, Huang FG, Xu H (2012b) Tolerance of lead by the fruiting body of Oudemansiella radicata. Chemosphere 88(4):467–475. doi:10.1016/j.chemosphere.2012.02.079
Zhao Z, Selvam A, Wong JW-C (2011) Effects of rhamnolipids on cell surface hydrophobicity of PAH degrading bacteria and the biodegradation of phenanthrene. Bioresour Technol 102(5):3999–4007. doi:10.1016/j.biortech.2010.11.088
Zhou J, Jiang W, Ding J, Zhang X, Gao S (2007) Effect of Tween 80 and beta-cyclodextrin on degradation of decabromodiphenyl ether (BDE-209) by white rot fungi. Chemosphere 70(2):172–177. doi:10.1016/j.chemosphere.2007.06.036
Acknowledgments
This study was financially supported by the NSFC (No. 41171253, No. J1103518), and the National High Technology Research and Development Program of China (No.2013AA06A210). The authors wish to thank Professor Dong Yu and Guanglei Cheng from Sichuan University for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Wu, M., Xu, Y., Ding, W. et al. Mycoremediation of manganese and phenanthrene by Pleurotus eryngii mycelium enhanced by Tween 80 and saponin. Appl Microbiol Biotechnol 100, 7249–7261 (2016). https://doi.org/10.1007/s00253-016-7551-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7551-3