Abstract
Phenol and its derivatives behave as mutagens, teratogens and carcinogens inducing adverse physiological effects and are considered environmental hazards. The present study focuses on high concentration phenol utilization by Aspergillus niger FP7 under various physicochemical parameters. The soil remediation potential of the culture for reducing phenol toxicity against Vigna radiata L. seed germination was also evaluated along with the extent of phenol utilization using high-performance liquid chromatography. Aspergillus niger FP7 showed phenol tolerance up to 1000 mg/l, beyond which there was a sharp reduction in phenol utilization. Supplementation of the mineral salt medium with glucose and peptone and application of a 100 rpm agitation rate enhanced phenol utilization (up to 88.3%). Phenol utilization efficiency decreased (up to 29.6%) when cadmium and mercury salts were present, but the same improved (59.4–75.5%) by the incorporation of cobalt, copper and zinc salts. Vigna radiata L. seeds sown in the non-augmented soil revealed a 3.27% germination index, and with fungal augmentation, the germination index improved (97.3%). The non-augmented soil demonstrated 3.1% phenol utilization, while for the augmented soil, the utilization was 79.3%. Based on the phytotoxicity study and chromatographic analysis, it could be inferred that Aspergillus niger FP7 significantly enhanced phenol utilization in soil. In the future, Aspergillus niger FP7 could be of potential use in bioremediation of sites polluted with high concentrations of phenol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phenol is a volatile aromatic compound, widely distributed as an environmental pollutant. Phenol along with its derivatives is commonly present in the effluents of many industrial processes: ceramic plants, coal conversion process, food industries, pharmaceuticals and oil refineries at a range from 1 to several hundred mg/l (Al Hashemi et al. 2015). Besides the high bioaccumulation rate along the food chain, phenolic compounds being lipophilic represent a serious threat against the natural environment and human health since they behave as mutagens, teratogens and carcinogens and induce adverse haematological and physiological effects (Zhou et al. 2017).
In pollution management, biological processes involving microorganisms and their metabolites facilitate a substitute to the existing physical/chemical technologies since the former is economical and environment friendly (Aracic et al. 2015). Numerous bacteria and fungi are known to utilize phenol aerobically as a source of energy and carbon (Pradeep et al. 2015). However, due to their capacity to grow under extreme conditions of nutrient deficiency, low pH, limited water supply etc. and being able to tolerate and utilize a range of xenobiotics, fungi may be preferred over bacteria in biodegradation of phenol and phenolic compounds (Yordanova et al. 2013). Filamentous fungi can mediate phenol degradation since they frequently inhabit wood where phenolic structures are abundant. Under such growth conditions, phenol degradation is mediated by enzymes (phenol hydroxylase and catechol 1,2-dioxygenase) that are commonly found in fungi (Sharma et al. 2016). Fungi such as Penicillium sp., Aspergillus sp., Fusarium sp., Graphium sp., Phanerochaete sp., Trametes sp. and Pleurotus sp. have the potential to degrade phenol and its derivatives (Carabajal et al. 2016).
Degradation of phenol by fungi under natural conditions is reported to be restricted by the high concentration of the contaminant, nutrient availability, adverse environmental conditions or non-availability of suitable microorganisms (Al-Khalid and El-Naas 2012). Although many phenol-utilizing fungi have been documented, available scientific literatures on physicochemical factors influencing utilization of phenol at higher concentrations are scanty. Keeping these points in the forefront, the present study explores utilization of high concentrations of phenol by fungi native to phenol-contaminated pharmaceutical effluent, analyses the factors influencing phenol utilization and checks the potency of the isolate to remediate phenol contamination in soil under laboratory conditions.
Materials and methods
Chemicals and reagents
Analytical grade chemicals and dehydrated media from HiMedia (Mumbai, India) were used.
Isolation of fungi
Pharmaceutical effluent (100 ml) was sampled from the Jigani Industrial Area (12.46° N, 77.38° E), Bangalore, India. In the laboratory, the effluent was stored under aseptic condition until used. To obtain pure fungal cultures, the sample was diluted (dilutions 10−1 to 10−7) and plated on potato dextrose agar (PDA). Plates were incubated (28 ± 2 °C, 5 days) and checked for the appearance of fungal colonies. Morphologically distinct cultures were inoculated on PDA slants and preserved at 4 °C.
Selection of potent fungal culture
The modified mineral salt medium of Bhattacharya et al. (2011) (composition g/l: (NH4)2SO4, 0.244; CaCl2·2H2O, 0.015; FeCl3·6H2O, 0.054; KH2PO4, 0.42; K2HPO4, 0.375; MgSO4·7H2O, 0.05; and NaCl, 0.015; pH 6.0 ± 0.2) was autoclaved. Sterile broth was then spiked with phenol (100 mg/l). A spore suspension (1 ml, 107 spores/ml) of the respective fungal isolate was inoculated in the sterile mineral salt medium taken in separate flasks. Uninoculated broth was considered ‘control’. The inoculated flasks and control were incubated (28 ± 2 °C, 5 days). Following incubation, a potent isolate was chosen based on the extent of phenol utilization.
Assessment of phenol utilization by the respective culture involved the 4-aminoantipyrine method (Campuzano et al. 2003). NH4OH solution (0.5 N, 70 μl) was mixed with the cell-free supernatant, and the pH was adjusted (7.9 ± 0.1) using 1 M phosphate buffer. Subsequently, 4-aminoantipyrine solution (30 μl) and K3Fe(CN)6 solution (30 μl) were added. After incubation in the dark (15 min), the absorbance of aliquots was monitored against the blank at 500 nm. The uninoculated media served as control. The percentage of phenol utilization was calculated as:
where Ci = initial phenol concentration and Cf = final concentration of phenol.
Molecular identification
The genomic DNA of the selected isolate was extracted using the Fungal Genomic DNA Spin-50 isolation kit (Chromous Biotech Pvt. Ltd., India). PCR amplification of the 18S rRNA gene sequence was performed with universal forward and reverse 18S rRNA primers (5′-GTAGTCATATGCTTGTCTC-3′ and 5′-GAAACCTTGTTACGACTT-3′, respectively). Genetic Analyzer (ABI 3500 XL, Applied Biosystems, USA) and the SeqScape software (version 5.2) were used for sequencing the PCR amplicon (Bhattacharya et al. 2020). Using BLASTn, the nucleotide sequence was aligned with other sequences available in the NCBI database (Altschul et al. 1990). The nucleotide sequence was submitted to GenBank (NCBI, USA) and provided an accession number.
Optimization of factors influencing phenol utilization
For estimating the phenol concentration that supports maximum utilization, the isolate was cultured with different phenol concentrations (200 to 2000 mg/l, with an increment of 200 mg/l). Phenol utilization was studied by evaluating the effect of co-substrates (1.0% w/v: glucose, fructose, maltose, sucrose or soluble starch), nitrogen supplements (1.0% w/v: peptone, beef extract, tryptone, yeast extract, (NH4)2SO4, NH4Cl or NH4NO3), heavy metal salts (0.01% w/v: CdCl2, CoCl2, CuSO4, HgCl2 or ZnSO4) and agitation rate (0, 50, 100, 150 or 200 rpm).
Phytotoxicity study
Vigna radiata L. seeds were procured from an outlet of the Horticultural Producers’ Cooperative Marketing and Processing Society, Bangalore, India. The seeds were authenticated by Dr. Sundara Rajan S. (retired professor and head, Post Graduate Department of Botany, St. Joseph’s College, Bangalore). The voucher specimen was deposited in the herbarium of the Department of Botany and Microbiology, JAIN (Deemed-to-be University), Bangalore, India, with the reference number JU/DBOMB/2015-1218/S30.
The phenol toxicity on germination of Vigna radiata L. seeds was assessed following the protocol of Krogmeier and Bremner (1989). V. radiata L. seeds were selected for analysing phenol toxicity because these are an important test species for evaluating the phytotoxicity of environmental pollutants. Besides being an erect or semi-erect legume with a well-developed root system (a trait valued in studying the effect of xenobiotics on plant growth), V. radiata L. often inhabits open wastelands, where it may come in contact with harmful aromatic compounds (Kannan and Upreti 2008).
The experimental conditions maintained were as follows: (1) control (garden soil moistened with 5 ml water + seeds), (2) non-augmented soil (garden soil moistened with 5 ml water containing 1000 mg/l phenol + seeds), (3) augmented soil (garden soil moistened with 5 ml water containing 1000 mg/l phenol + heavy metal salt solution containing heavy metal mix (0.01% w/v: CdCl2, CoCl2, CuSO4, HgCl2 or ZnSO4) + fungal spore suspension + seeds), and (4) augmented soil (garden soil moistened with 5 ml water containing 1000 mg/l phenol + selected co-substrate and nitrogen supplement at 1.0% w/v + fungal spore suspension + seeds). Prior to the experimentation, the garden soil was found to be devoid of any phenol contamination and constituted clay (10%), sand (55%), silt (35%), organic matter (24.1% dry weight), total organic carbon (5.6% dry weight) and pH 6.1.
Under each set of experimental conditions, garden soil (50 g) was taken in paper cups (5 cm rim diameter). Prior to sowing, all healthy V. radiata L. seeds were surface sterilized using HgCl2 (0.1% w/v). Subsequently, the seeds (5 seeds per cup) were sown in the cups. Paper cups to be seeded with the fungal culture received spore suspension of the culture (1 ml, 107 spores/ml). All the cups were maintained at 28 ± 2 °C in dark for 7 days. Sterile distilled water (5 ml) was regularly added to maintain the soil moisture. The germination index was calculated by the equation
where GP = number of germinated seeds expressed as a percentage of control values, La = root length average in the ‘test’ and Lc = root length average in the control (Wolski et al. 2012).
HPLC analysis
For residual phenol estimation, both the non-bioaugmented and bioaugmented soils (2 g each after 144 h incubation) were centrifuged (12,000 × g, 4 °C, 10 min) in methanol (5 ml) and the supernatant was subjected to filtration using a nitrocellulose membrane filter (0.25 μm). Eluates (20 μl) were injected into the HPLC system (Waters, USA, model number 2487 with a UV-Vis detector and a binary pump system, model number-1525). A Symmetry C-18 column (4.6 × 150 mm, 5 μm particle size) was used. The mobile phase was composed of acetonitrile:water (30:70 v/v). The flow rate was maintained at 1 ml/min. The UV-Vis detector was set at 270 nm. The percentage of utilization was calculated by recording the area under the peak of the ‘non-augmented’ and ‘augmented’ samples and comparing that with the area under the peak of the aqueous phenol solution (1000 mg/l) (Singh et al. 2009).
Statistical analysis
Experiments were conducted in triplicate (n = 3). The data was presented graphically (mean ± standard deviation of the mean (SD)). Microsoft Excel 2010 was used for data analysis. The analysis of percentage of phenol utilization was statistically evaluated by Student’s t-test (paired, 2-tailed); p-values ˂0.05 were considered statistically significant.
Results and discussion
Isolation and screening of potent phenol-utilizing fungi
Mineral salt medium spiked with phenol (100 mg/l) supported the isolation of ten phenol-utilizing fungi that were designated FP1 to FP10. Following incubation in mineral salt broth spiked with phenol, isolate FP7 demonstrated maximum phenol utilization (27.5%) and was chosen for the study.
Molecular identification
The PCR amplicon (~ 1.5 kbp) was obtained. Using BLASTn, the resulting nucleotide sequence (696 bp) when compared with 18S rRNA gene sequences in the NCBI database matched with a cluster containing Aspergillus sp. A similarity in sequence (96%) with Aspergillus niger strain TR-H was recorded. Hence, it was designated as Aspergillus niger FP7. This ribosomal sequence was provided an accession number (KR869888) by GenBank (NCBI, USA).
Effect of phenol concentration
The complete mineralization of phenol under oxygenic condition is generally a preference for wastewater treatment. Phenol being recognized as an inhibitory substrate at relatively low concentrations (100 mg/l) is an excellent model for kinetic studies related to the utilization of aromatic compounds (Pradeep et al. 2015). In the present study, the extent of phenol utilization by A. niger FP7 (after 5 days of incubation at 28 ± 2 °C) was inversely proportional to the increase in phenol concentration. Beyond 1000 mg/l of phenol, there was a sharp reduction in utilization; hence, this concentration was used for rest of the study (Fig. 1). The decrease in phenol utilization indicates that at high concentration, phenol presents an inhibitory effect on the metabolism of A. niger FP7.
At the cellular level, the lipophilic nature of phenol initiates its membrane-directed toxicity. This is correlated with the logarithm of its partition coefficient with water and n-octanol (log Pow of 1.46), thereby facilitating its high affinity for cellular membranes (Ruelle 2000). The inhibitory effect of phenol on microbial cells is governed by its molecular structure, where the non-polar part mediates transit across the cell membrane and a hydroxyl group (combined with a system of delocalized electrons) confers an acidic character, thereby destabilizing the cell membrane (Rao et al. 2010).
While evaluating the phenol biodegradation in an aerobic batch culture, phenol concentrations of 1000 mg/l was degraded by A. niger in more than 140 h to achieve a negligible biomass growth (Tebbouche et al. 2016). Earlier, the A. niger ATCC-16404 strain showed phenol tolerance up to 300 mg/l (Supriya and Neehar 2014). Other studies showed that within ˂ 2 weeks, three A. fumigatus strains could completely degrade phenol (500 mg/l) as a single carbon source (Gerginova et al. 2014).
Effect of co-substrates
The organopollutants’ utilization rate can be increased by involving co-metabolism: transformation of a non-growth substrate (one that cannot support cell division) in the presence of a growth substrate or another transferable compound. The growth substrate provides energy for microbial growth and maintenance, besides providing reducing equivalent/power (allowing utilization of non-growth substrates) (Bhattacharya et al. 2020).
Addition of co-substrates like glucose, starch, maltose, fructose or sucrose had a positive impact on phenol utilization by A. niger FP7 (incubated for 5 days at 28 ± 2 °C), wherein the monosaccharide glucose supported the highest utilization (44.5%). The data indicated that though A. niger FP7 utilized phenol as an energy and carbon source, the efficiency of phenol utilization was enhanced owing to co-metabolism (Fig. 2a). The impact of glucose as a co-metabolite for phenol uptake may be summarized in two points: glucose promoted better cellular growth and enhanced the utilization of the non-growth substrate like phenol. Simultaneously, it acted as a co-substrate in co-metabolism and might have induced the highest production of catabolic enzymes due to its simple structure (Prabu and Udayasoorian 2005).
Glucose, at a wide range of concentration, has been used as a carbon source for fungal growth concomitant with phenol biodegradation because it increased the tolerance of microbial cells to substrate inhibition (Loh and Tan 2000). The influence of glucose on phenol degradation was evaluated, indicating that a mixed substrate (phenol and glucose) facilitated better phenol degradation (Pradeep et al. 2015).
Effect of nitrogen supplements
Nitrogen is a key component in many compounds necessary for life, such as amino acids and nucleic acids. However, the effect of available nitrogen had shown mixed results on the ability of fungi to express catabolic enzymes. Many investigators had earlier shown the importance of nitrogen supplements to improve phenol utilization (Chandrasekaran et al. 2018).
In the present study, following incubation at 28 ± 2 °C for 5 days, A. niger FP7 showed varied levels of phenol utilization under the influence of different organic and inorganic nitrogen sources. Peptone was the most preferred nitrogen source, yielding the maximum utilization (56.6%). On the other hand, least utilization was obtained in the presence of (NH4)2SO4 and NH4NO3 (40.5%). The complete absence of a nitrogen source from the medium reduced the efficiency of phenol utilization (39%) (Fig. 2b).
Peptone being the water-soluble portion of enzymatically digested meat contains a high concentration of amino acids such as tryptophan required by fungi to form biomass and secrete oxidative enzymes (polyphenol oxidase etc.) responsible for phenol degradation (Viswanath et al. 2014). The production of polyphenol oxidase by Trametes pubescens was improved by peptone (Galhaup et al. 2002). Peptone was the best nitrogen source for polyphenol oxidase accumulation and degradation of phenol by Pleurotus ostreatus 98 and P. ostreatus 108, respectively (Mikiashvili et al. 2006).
Effect of heavy metals
Co-contamination of polluted sites with high concentrations of heavy metals may be a bottleneck for in situ biodegradation of xenobiotics. Heavy metals (chromium, copper, manganese, nickel and zinc) are naturally existent in the environment and act as micronutrients for cellular metabolism. However, certain heavy metals (cadmium, lead and mercury) devoid of any biological function are toxic to living organisms and aggregate in the environment due to anthropogenic activities (Ma et al. 2018).
In the present study, usage of heavy metals was undertaken to understand the phenol utilization capacity of A. niger FP7 in the presence of individual heavy metal. Phenol utilization was not affected by the existence of copper, cobalt and zinc salts. However, cadmium and mercury salts decreased the utilization efficiency drastically. The pattern of phenol utilization by A. niger FP7 (incubated for 5 days at 28 ± 2 °C) in the presence of heavy metal salts is illustrated in Fig. 3a.
Increase in phenol utilization by A. niger FP7 following the addition of copper salt may be attributed to increased polyphenol oxidase production as copper acts as a strong inducer of the enzyme in fungi (Patel and Gupte 2016). While characterizing polyphenol oxidase from Myrothecium verrucaria NF-05, the resultant enzyme activation in the presence of copper was due to type 2 copper-binding sites being filled (Zhao et al. 2012). Cadmium is a non-essential heavy metal whose presence severely affected A. niger FP7 probably due to induction of oxidative stress across the cell membrane. Parallel to the present result, when salts of cadmium, lead, mercury and silver were added (earlier than 12 days), degradation of phenol derivatives (due to polyphenol oxidase) declined (Baldrian and Gabriel 2002). Phenol utilization by A. niger FP7 severely decreased when mercuric salt was present, owing to the high affinity of mercury towards thiol groups in the active site of enzymes involved in phenol utilization, thereby resulting in their irreversible inactivation (Rubino 2015).
Effect of agitation
As microbial metabolism of phenol is generally an oxidative reaction, optimization of the agitation speed was required to correlate the role of aeration in phenol utilization. Agitation had a positive effect on A. niger FP7–mediated phenol degradation wherein the highest (88.3%) was reported for 100 rpm and the least (39.6%) for static culture when incubated for 5 days at 28 ± 2 °C (Fig. 3b).
Faster agitation of the broth culture of A. niger FP7 probably facilitated better hyphal formation due to better distribution of nutrients and temperature and reduced oxygen tension. However, under the highest agitation, phenol utilization decreased possibly due to the less contact time between the cells and phenol. Agitation increased not only phenol solubility but also the dissolved oxygen levels (an important step for aerobic degradation of phenol), wherein the dissolved oxygen proceeds in a series of chemical reactions that facilitate hydroxylation of phenol (Melo et al. 2005). When molecular oxygen was available in plenty, A. niger FP7 could enzymatically (possibly through polyphenol oxidases) incorporate oxygen more efficiently in the aromatic nucleus of phenol and bring about initial ring oxidation, a rate-limiting step in biodegradation of phenol (Gu et al. 2016).
A Saudi Arabian isolate of A. niger resulted in highest phenol degradation at 100 rpm, and a lower or higher speed decreased phenol degradation (Ibrahim and EL-Gamdi 2019). Other investigators used different shaking speeds ranging from 50 to 200 rpm for investigating phenol degradation and reported 125 rpm to favour the highest degradation of phenol by A. niger (Sharma and Gupta 2012).
Phytotoxicity study
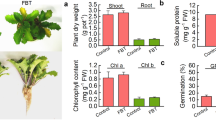
A phytotoxicity test was conducted with V. radiata L. seeds over a period of 7 days at 28 ± 2 °C. All the control seeds germinated well (100%) under the laboratory conditions and had an average root length of 3.66 cm. Seeds sown in non-augmented soil had an average root length of 0.12 cm and revealed a 3.27% germination index (Fig. 4).
Seeds sown in augmented soil (containing 0.01% w/v: CdCl2, CoCl2, CuSO4, HgCl2 or ZnSO4) had an average root length of 1.8 cm and revealed a 50.1% germination index. The extent of phytotoxicity recorded is due to the combined effect of phenol and heavy metals that probably resulted in denaturation of phytohormones and enzymes mediating seed germination. Literatures suggest that heavy metals like chromium, copper, manganese, nickel and zinc act as micronutrients for cellular metabolism and are naturally present in the environment (Ma et al. 2018). While the presence of copper salt might have improved polyphenol oxidase production (as copper acts as a strong inducer of the enzyme in fungi), the same might have been nullified to a certain extent by cadmium (due to induction of oxidative stress across the cell membrane) and mercury (owing to its high affinity towards thiol groups in the active site of enzymes involved in phenol utilization), thereby severely affecting A. niger FP7 metabolism (Rubino 2015; Patel and Gupte 2016). In corroboration to the present result, the study conducted by Baldrian and Gabriel (2002) reported that when salts of cadmium, lead, mercury and silver were added (earlier than 12 days), degradation of phenol derivatives (due to polyphenol oxidase) declined.
In the present study, seeds sown in augmented soil (with 1.0% w/v glucose and peptone) had an average root length of 3.56 cm and revealed a germination index of 97.3% (Fig. 4). This clearly indicated a promising reduction in germination inhibition which may be correlated with the degradation of phenol by the fungal isolate, thus reducing the phenol toxicity on seed germination. The positive effect of glucose as a co-metabolite for phenol uptake may be due to glucose promoting better cellular growth and enhancing the utilization of the non-growth substrate like phenol. Simultaneously, it acted as a co-substrate in co-metabolism and might have induced the highest production of catabolic enzymes (phenol oxidases) due to its simple structure (Prabu and Udayasoorian 2005). The amino acid tryptophan present in peptone is a requirement for fungi to synthesize biomass and oxidative enzymes (polyphenol oxidase etc.) responsible for phenol degradation (Viswanath et al. 2014). Addition of peptone greatly improved polyphenol oxidase production by Trametes pubescens (Galhaup et al. 2002). The highest accumulation of polyphenol oxidase supporting phenol degradation by P. ostreatus 98 and P. ostreatus 108 was recorded in the presence of peptone (Mikiashvili et al. 2006). The literature suggests that GI values ˃ 80% indicate less phytotoxicity, while values between 50 and 80% indicate moderate phytotoxicity and values ˂ 50% indicate high phytotoxicity (Osma et al. 2010).
Earlier, results of phytotoxicity showed that seed germination was 100% for wheat in the liquid mineral salt medium used as a control, 95% for seeds treated with the degradation products of phenol and 6.25% for 400 mg/l of phenol. No root lengthening was reported in the last case. Seeds in the control and in contact with phenol degradation products demonstrated similar root elongation, about 14 ± 7.1 mm. Therefore, GI values were 100% for both, while phenol (400 mg/l)–treated seeds showed a GI value of 0.4% (Wolski et al. 2012).
HPLC analysis
When tested for residual phenol, non-augmented soil demonstrated 3.1% phenol utilization while the same for augmented soil was 79.3%. Chromatograms for the HPLC analysis of phenol utilization under non-augmented and augmented conditions are illustrated in Fig. 5.
The capability of A. niger FP7 to utilize phenol (present at a highly toxic level) in augmented soil indicates the involvement of specific robust metabolic pathways for phenol ring cleavage. It is well established in the literature that filamentous fungi of the genus Aspergillus metabolizes phenol to a non-toxic intermediate by the 3-oxoadipate/ortho-pathway (aromatic ring monohydroxylation at the o-position). This catabolic mechanism involves four cytoplasmic enzymes, namely, phenol hydroxylase (catalyses the hydroxyl group attachment at the ortho-position of the phenol ring to liberate catechol), catechol 1,2-dioxygenase (incorporates molecular oxygen directly in the aromatic ring of catechol, liberating cis,cis-muconate), cis,cis-muconate lactonizing enzyme (transforms cis,cis-muconate to muconolactone) and 3-oxoadipate enol-lactone hydrolase (transforms 3-oxoadipate enol-lactone to 3-oxoadipate and water) (Krastanov et al. 2013). Such minimal phenol utilization in the non-augmented soil possibly resulted from the auto-oxidation of phenol, besides its adsorption on soil organic matter.
Conclusion
The present investigation reflects that A. niger FP7 displayed tolerance towards higher concentrations of phenol. Supplementation of the growth medium by co-substrates and nitrogen supplements enhanced the phenol utilization. The presence of cobalt, copper and zinc salts in the broth improved the utilization process. Post soil inoculation, A. niger FP7 retained its phenol utilization potential and greatly reduced the toxicity of phenol towards V. radiata L. seed germination. It is worth mentioning that in the future, A. niger FP7 could be of potential use in bioremediation of sites polluted with high concentrations of phenol.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Al Hashemi W, Maraqa MA, Rao MV, Hossain MM (2015) Characterization and removal of phenolic compounds from condensate-oil refinery wastewater. Desalin Water Treat 54(3):660–671. https://doi.org/10.1080/19443994.2014.884472
Al-Khalid T, El-Naas MH (2012) Aerobic biodegradation of phenols: a comprehensive review. Crit Rev Environ Sci Technol 42(16):1631–1690. https://doi.org/10.1080/10643389.2011.569872
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Aracic S, Manna S, Petrovski S, Wiltshire JL, Mann G, Franks AE (2015) Innovative biological approaches for monitoring and improving water quality. Front Microbiol 6:826. https://doi.org/10.3389/fmicb.2015.00826
Baldrian P, Gabriel J (2002) Copper and cadmium increase activity in Pleurotus ostreatus. FEMS Microbiol Lett 206(1):69–74. https://doi.org/10.1111/j.1574-6968.2002.tb10988.x
Bhattacharya S, Das A, Mangai G, Vignesh K, Sangeetha J (2011) Mycoremediation of Congo red dye by filamentous fungi. Braz J Microbiol 42(4):1526–1536. https://doi.org/10.1590/S1517-83822011000400040
Bhattacharya S, Das A, Srividya S, Prakruti PA, Priyanka N, Sushmitha B (2020) Prospects of Stenotrophomonas pavanii DB1 in diesel utilization and reduction of its phytotoxicity on Vigna radiata. Int J Environ Sci Technol 17:445–454. https://doi.org/10.1007/s13762-019-02302-w
Campuzano S, Serra B, Pedrero M, de Villena FJM, Pingarrón JM (2003) Amperometric flow-injection determination of phenolic compounds at self-assembled monolayer-based tyrosinase biosensors. Anal Chim Acta 494(1-2):187–197. https://doi.org/10.1016/S0003-2670(03)00919-X
Carabajal M, Perullini M, Jobbágy M, Ullrich R, Hofrichter M, Levin L (2016) Removal of phenol by immobilization of Trametes versicolor in silica-alginate-fungus biocomposites and loofa sponge. CLEAN 44(2):180–188. https://doi.org/10.1002/clen.201400366
Chandrasekaran S, Pugazhendi A, Banu RJ, Ismail IMI, Qari HA (2018) Biodegradation of phenol by a moderately halophilic bacterial consortium. Environ Prog Sustain 37(5):1587–1593. https://doi.org/10.1002/ep.12834
Galhaup C, Goller S, Peterbauer CK, Strauss J, Haltrich D (2002) Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology 148(Pt 7):2159–2169. https://doi.org/10.1099/00221287-148-7-2159
Gerginova M, Manasiev J, Yemendzhiev H, Terziyska A, Peneva N, Alexieva Z (2014) Biodegradation of phenol by Antarctic strains of Aspergillus fumigatus. Z Naturforschung C, J Biosci 68(9-10):384–393. https://doi.org/10.1515/znc-2013-9-1006
Gu Q, Wu Q, Zhang J, Guo W, Wu H, Sun M (2016) Community analysis and recovery of phenol-degrading bacteria from drinking water biofilters. Front Microbiol 7:495. https://doi.org/10.3389/fmicb.2016.00495
Ibrahim AG, EL-Gamdi LSYA (2019) Characterization of fungi that able to degrade phenol from different contaminated areas in Saudi Arabia. J Biol Sci 19:210-217. https://doi.org/10.3923/jbs.2019.210.217
Kannan A, Upreti RK (2008) Influence of distillery effluent on germination and growth of mung bean (Vigna radiata) seeds. J Hazard Mater 153:609–615. https://doi.org/10.1016/j.jhazmat.2007.09.004
Krastanov A, Alexieva Z, Yemendzhiev H (2013) Microbial degradation of phenol and phenolic derivatives. Eng Life Sci 13(1):76–87. https://doi.org/10.1002/elsc.201100227
Krogmeier MJ, Bremner JM (1989) Effects of phenolic acids on seed germination and seedling growth in soil. Biol Fertil Soils 8(2):116–122. https://doi.org/10.1007/BF00257754
Loh KC, Tan PP (2000) Effect of additional carbon sources on biodegradation of phenol. Bull Environ Contam Toxicol 64(6):756–763. https://doi.org/10.1007/s0012800068
Ma Y, Li X, Mao H, Wang B, Wang P (2018) Remediation of hydrocarbon–heavy metal co-contaminated soil by electrokinetics combined with biostimulation. Chem Eng J 353:410–418. https://doi.org/10.1016/j.cej.2018.07.131
Melo JS, Kholi S, Patwardhan AW, D’Souza SF (2005) Effect of oxygen transfer limitations in phenol biodegradation. Process Biochem 40(20):625–628. https://doi.org/10.1016/j.procbio.2004.01.049
Mikiashvili N, Wasser SP, Nevo E, Elisashvili V (2006) Effects of carbon and nitrogen sources on Pleurotus ostreatus ligninolytic enzyme activity. World J Microbiol Biotechnol 22(9):999–1002. https://doi.org/10.1007/s11274-006-9132-6
Osma JF, Toca-Herrera JL, Rodríguez-Couto S (2010) Transformation pathway of Remazol Brilliant Blue R by immobilised laccase. Bioresour Technol 101(22):8509–8514. https://doi.org/10.1016/j.biortech.2010.06.074
Patel H, Gupte A (2016) Optimization of different culture conditions for enhanced laccase production and its purification from Tricholoma giganteum AGHP. Bioresour Bioprocess 3:11. https://doi.org/10.1186/s40643-016-0088-6
Prabu PC, Udayasoorian C (2005) Phenol metabolism by white rot fungus Phanerochaete chrysosporium isolated from Indian paper mill effluent enriched soil samples. Asian J Plant Sci 4(1):56–59. https://doi.org/10.3923/ajps.2005.56.59
Pradeep NV, Anupama S, Navya K, Shalini HN, Idris M, Hampannavar US (2015) Biological removal of phenol from wastewaters: a mini review. Appl Water Sci 5(2):105–112. https://doi.org/10.1007/s13201-014-0176-8
Rao A, Zhang Y, Muend S, Rao R (2010) Mechanism of antifungal activity of terpenoid phenols resembles calcium stress and inhibition of the TOR pathway. Antimicrob Agents Chemother 54(12):5062–5069. https://doi.org/10.1128/AAC.01050-10
Rubino FM (2015) Toxicity of glutathione-binding metals: a review of targets and mechanisms. Toxics 3(1):20–62. https://doi.org/10.3390/toxics3010020
Ruelle P (2000) The n-octanol and n-hexane/water partition coefficient of environmentally relevant chemicals predicted from the mobile order and disorder (MOD) thermodynamics. Chemosphere 40(5):457–512. https://doi.org/10.1016/S0045-6535(99)00268-4
Sharma N, Gupta VC (2012) Batch biodegradation of paper and pulp effluent by Aspergillus niger. Int J Chem Eng Appl 3(3):182–186. https://doi.org/10.7763/IJCEA.2012.V3.183
Sharma R, Prakash O, Sonawane MS, Nimonkar Y, Golellu PB, Sharma R (2016) Diversity and distribution of phenol oxidase producing fungi from soda lake and description of Curvularia lonarensis. Front Microbiol 7:1847. https://doi.org/10.3389/fmicb.2016.01847
Singh S, Singh BR, Chandra R (2009) Biodegradation of phenol in batch culture by pure and mixed strains of Paenibacillus sp. and Bacillus cereus. Pol J Microbiol 58(4):319–325
Supriya CH, Neehar D (2014) Biodegradation of phenol by Aspergillus niger. IOSR J Pharm 4:11–17. https://doi.org/10.9790/3013-0407011017
Tebbouche L, Hank D, Zeboudj S, Namane A, Hellal A (2016) Evaluation of the phenol biodegradation by Aspergillus niger: application of full factorial design methodology. Desalin Water Treat 57(13):6124–6130. https://doi.org/10.1080/19443994.2015.1053991
Viswanath B, Rajesh B, Janardhan A, Kumar AP, Narasimha G (2014) Fungal laccases and their applications in bioremediation. Enzyme Res 2014:163242–163221. https://doi.org/10.1155/2014/163242
Wolski EA, Barrera V, Castellari C, González JF (2012) Biodegradation of phenol in static cultures by Penicillium chrysogenum ERK1: catalytic abilities and residual phytotoxicity. Rev Argent Microbiol 44(2):113–121
Yordanova G, Godjevargova T, Nenkova R, Ivanova D (2013) Biodegradation of phenol and phenolic derivatives by a mixture of immobilized cells of Aspergillus awamori and Trichosporon cutaneum. Biotechnol Biotechnol Equip 27(2):3681–3688. https://doi.org/10.5504/BBEQ.2013.0003
Zhao D, Zhang X, Cui D, Zhao M (2012) Characterisation of a novel white laccase from the deuteromycete fungus Myrothecium verrucaria NF-05 and its decolourisation of dyes. PLoS One 7(6):e38817. https://doi.org/10.1371/journal.pone.0038817
Zhou M, Zhang J, Sun C (2017) Occurrence, ecological and human health risks, and seasonal variations of phenolic compounds in surface water and sediment of a potential polluted river basin in China. Int J Environ Res Public Health 14(10):1140. https://doi.org/10.3390/ijerph1410114
Acknowledgements
We extend our sincere gratitude to the management of JAIN (Deemed-to-be University) for providing the research facilities.
Author information
Authors and Affiliations
Contributions
SB designed the study and interpreted the data. AD contributed in writing the manuscript. KK, NP and JS performed the experiments. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Gangrong Shi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bhattacharya, S., Das, A., Krishnan, K. et al. Co-substrate-mediated utilization of high concentration of phenol by Aspergillus niger FP7 and reduction of its phytotoxicity on Vigna radiata L.. Environ Sci Pollut Res 28, 64030–64038 (2021). https://doi.org/10.1007/s11356-021-13947-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13947-x