Abstract
To date, few studies have focused on reducing the toxic by-product acetate during 1,3-propanediol production by Klebsiella pneumoniae. In this study, the effects of deleting the poxB, pta, and ackA genes, which are involved in the two main acetate synthesis pathways, on cell growth and 1,3-propanediol production were investigated. Although acetate synthesis via pyruvate oxidase (PoxB, encoded by poxB) generally seems unnecessary and wasteful, PoxB was shown to play an important role in K. pneumoniae. Deletion of poxB severely inhibited cell growth, and the poxB mutant exhibited an anomalously high accumulation of acetate in aerobic cultures and failed to produce an endogenous supply of carbon dioxide (CO2) in anaerobic cultures. It is interesting that both the aerobic and anaerobic growth defects of the poxB mutant were corrected by further deleting pta and ackA, which blocked the other main acetate synthesis pathway. The poxB-pta-ackA mutant excreted less acetate and showed an excellent ability to produce 1,3-propandiol. The final 1,3-propanediol yield and concentration in a 2-L fed-batch fermentation reached 0.66 (mol/mol) and 76.8 g/L, respectively, which were 16 and 15 % greater, respectively, than those of the parent strain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
1,3-Propanediol (1,3-PD) is a valuable and important chemical that is widely used in polyester, cosmetic, and pharmaceutical industries (Durgapal et al. 2014; Szymanowska-Powalowska and Kubiak 2015). Because of the large amount of low-cost raw glycerol produced during biodiesel production, the biological production of 1,3-PD from glycerol has attracted significant interest recently (Khan et al. 2013; Szymanowska-Powalowska and Bialas 2014). Klebsiella pneumoniae, a facultative anaerobe, has the ability to produce high yields of 1,3-PD from glycerol (Celinska 2012; Yen et al. 2014).
The metabolic pathway responsible for the microbial production of 1,3-PD from glycerol has been well studied in K. pneumoniae (Kumar et al. 2012; Seo et al. 2009; Skraly et al. 1998; Zhuge et al. 2010). Generally, glycerol is converted through reductive and oxidative pathways (Fig. 1), the reductive branch leading to 1,3-PD production, and the oxidative branch providing the reducing power for 1,3-PD synthesis; during the fermentation process, the formation of by-products derived from oxidative branch, including acetate, lactate, and 2,3-butanediol (2,3-BD), leads to the decreases of carbon flux and reducing power towards 1,3-PD synthesis (Huang et al. 2012; Petrov and Petrova 2009; Tang et al. 2009). To improve the production of 1,3-PD in K. pneumoniae, various efforts have been made to delete the genes responsible for the formation of these by-products. It was found that lactate formation was blocked by deleting the ldhA gene (Xu et al. 2009; Zheng et al. 2008), and that 2,3-BD formation was also completely abolished by deleting the genes responsible for its synthesis (Cui et al. 2014). Because blocking 2,3-BD formation results in an extreme growth defect and a decrease in 1,3-PD production (Cui et al. 2014; Zhu et al. 2015), many high 1,3-PD producers are based on lactate-deficient (ldhA-deleted) K. pneumoniae strains, although the deletion of ldhA is actually more beneficial for 2,3-BD production than 1,3-PD production (Durgapal et al. 2014; Kumar et al. 2013; Xu et al. 2009).
Metabolic pathways of glycerol metabolism in K. pneumoniae. GLY glycerol, PYR pyruvate, PEP phosphoenolpyruvate, 3-HPA 3-hydroxypropinaldehyde, 1,3-PD 1,3-propanediol, 2,3-BD 2,3-butanediol, LAC lactate, ACE acetate, SUC succinate, α-KG α-ketoglutarate, TCA tricarboxylic acids cycle, Acetyl-P acetylphosphate, pta encoding phosphotransacetylase, ackA encoding acetate kinase, poxB encoding pyruvate oxidase, ldhA encoding lactate dehydrogenase, acs encoding acetyl-CoA synthetase
Compared with lactate and 2,3-BD, few reports have focused on reducing the other main by-product, acetate, which is commonly considered to be more toxic than lactate and 2,3-BD (Celinska 2010). Recently, Lee et al. (2014) reported that deleting the pta gene decreased acetate formation in a both 2,3-butanediol- and lactate-deficient K. pneumoniae strain. In Escherichia coli, acetate is synthesized by two major routes: the first occurs via the actions of phosphotransacetylase (Pta, encoded by the pta gene) and acetate kinase (AckA, encoded by the ackA gene), which convert acetyl-CoA to acetate and one molecule of adenosine triphosphate (ATP); the other route is via pyruvate oxidase (PoxB, encoded by the poxB gene), which oxidizes pyruvate to generate acetate and one molecule of CO2 (Ashok et al. 2011). Generally, the Pta-AckA pathway is the primary one, while deletion of poxB seems to affect central carbon metabolism, such as the tricarboxylic acid (TCA) cycle. Deleting both pathways results in decreased cell growth and acetate formation in E. coli (De Mey et al. 2007; Li et al. 2012). On the other hand, acetate can be assimilated by E. coli, mainly through acetyl-CoA synthetase (ACS, encoded by the acs gene) to generate acetyl-CoA. The recycling of acetate by ACS preserves the pool of free reduced CoA (CoA-SH) and mitigates acetate overflow in aerobic E. coli cultures (Dittrich et al. 2005; Peebo et al. 2014). Because of its close genetic relatedness to E. coli, K. pneumoniae is expected to have the same regulatory mechanism for acetate synthesis. However, to date, no detailed study has focused on the acetate synthesis pathway, especially, its effect on 1,3-PD production and physiology of K. pneumoniae.
In this study, the effects of blocking the two acetate synthesis pathways, PoxB and Pta-AckA, on cell growth and 1,3-PD production in K. pneumoniae were investigated under different culture conditions. It was shown that deletion of poxB severely inhibited cell growth. Interestingly, the growth defect of the poxB mutant was alleviated by further deleting the Pta-AckA acetate synthesis pathway. With comprehensive analysis, the roles of PoxB and Pta-AckA in K. pneumoniae were represented. And, as a result, a strategy for efficient production of 1,3-PD by directly blocking acetate synthesis pathways was proposed.
Materials and methods
Strains, plasmids, primers, media, and culture conditions
Strains, plasmids, and primers are listed in Table 1. The lactate-deficient K. pneumoniae strain KG2 (Zhu et al. 2015), which was derived from K. pneumoniae strain KG (CCTCC M2014574) by deleting the ldhA gene, is a high 1,3-PD producer, and it was used as the parent strain in this study.
Batch culture in 250-mL shake flask containing 50 mL of medium with 5 % inoculum for the assessment of mutants was performed at 37 °C for 24 h with shaking at 200 rpm under aerobic and anaerobic conditions. Pre-culture was cultivated in 10-mL test tube with 5 mL Luria-Bertani broth (LB) (37 °C, 18 h). The shake flask medium (pH 7.0) contained 60 g/L glycerol (or glucose), 1 g/L KCl, 2.2 g/L KH2PO4·2H2O, 4 g/L (NH4)2SO4, 0.4 g/L MgSO4·7H2O, and 2 g/L yeast extract. To test the effect of CO2 on bacterial growth, 1 g/L NaHCO3 was added to the medium. For aerobic cultures, flasks were covered with eight layers of gauze to permit air penetration; for anaerobic cultures, the air in the flasks was replaced with nitrogen gas before cultivation, and then, the flasks were plugged with a gas-impermeable rubber stopper.
Flask-scale batch fermentation for 1,3-PD production was performed micro-aerobically at 37 °C for 24 h with shaking at 50 rpm; the medium and other procedure were the same as those for aerobic flask culture mentioned above. Fed-batch fermentation for 1,3-PD production was performed in a 5-L stirred reactor (Shanghai Bailun Bio-technology Co., Ltd., Shanghai, China) with a working volume of 2 L. The reactor was inoculated with 5 % (v/v) of an overnight culture, and then, the bacteria were incubated under micro-aerobic conditions at 37 °C for 30 h. The aeration rate was 0.1 vvm, and the agitation speed was 140 rev/min. The initial glycerol concentration was 40 g/L, and it was maintained between 15 and 25 g/L by continuously feeding with 800 g/L of glycerol into the reactor throughout the fermentation process. The pH was maintained at 6.8 by automatically adding 50 % (w/v) KOH. The fermentation medium was the same as that reported by Cui et al. (2014).
Construction of poxB and poxB-pta-ackA mutant strains using lambda Red recombination
The poxB and pta-ackA genes were deleted by lambda Red recombination (Yamamoto et al. 2009). Gene disruption cassettes with a kanamycin-resistance marker and a flippase recognition site were amplified by two-step polymerase chain reactions from the chromosomal DNA of K. pneumoniae strain KG2 and the pKD4 vector, respectively, using the primers listed in Table 1. To construct the poxB deletion mutant, pKD46-Tc was first transformed into the KG2 strain, followed by transformation with the poxB disruption cassette. The recombinant strain was screened by growth on LB plates supplemented with kanamycin at 37 °C for 24 h. Finally, pCP20-Tc was transformed into the recombinant strain to remove the kanamycin-resistance gene. Cells were cultured overnight at 42 °C, diluted, and plated onto solid LB to obtain single colonies, which were further screened for loss of kanamycin and tetracycline hydrochloride resistance; the resulting poxB mutant strain was named KG4 (Table 1). Using the same procedure, the pta-ackA genes were deleted from strain KG4; the poxB-pta-ackA mutant strain was named KG5 (Table 1).
Analytical methods
The optical density (OD) of bacterial cultures was related to the dry cell weight by an experimentally determined calibration curve. The OD at 620 nm of the bacterial cultures was measured after appropriate dilution. 1,3-PD and 2,3-BD concentrations in the fermentation samples were quantified using gas chromatography. The analysis conditions included N2 as the carrier gas, a detector temperature of 270 °C, and a column temperature of 120 °C. Other metabolites present in the fermentation broths were quantified using a high-performance liquid chromatography system equipped with a 2487 Dual-Wavelength Absorbance Detector (Waters Corporation, Milford, MA, USA) and a Plastisil ODS column (AQ-C18, 5 μm, 250 × 4.6 mm; Welch Material, Inc., Ellicott City, MD, USA) at a flow rate of 0.8 mL min−1 and a column temperature of 65 °C. The mobile phase was 0.005 M H2SO4. Samples were filtered through 0.45-μm filters before analysis.
Results
Inhibitory effect of the poxB deletion on cell growth
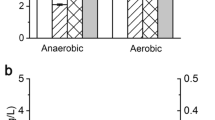
Because lactate-deficient K. pneumoniae strains are commonly used for industrial applications, the lactate-deficient strain KG2 was used as the parent strain in our study. The growth and acetate formation of the poxB mutant strain KG4 and the parent strain KG2 after 24 h of flask-scale cultivation under different conditions are shown in Fig. 2. An inhibitory effect of the poxB deletion on bacterial growth was found. Compared with the KG2 strain, the growth of the KG4 strain significantly decreased, regardless of the carbon source used and whether the cultures were grown under anaerobic or aerobic conditions. The KG4 strain exhibited larger growth defects under anaerobic conditions (64 and 55 % of the cell concentration of the KG2 strain in glycerol- and glucose-based media, respectively), while its cell concentrations were 70 and 76 % of those of the KG2 strain in glycerol and glucose-based media, respectively, under aerobic culture conditions (Fig. 2a).
Cell growth (a) and acetate formation (b) of KG2 and KG4 after 24 h of flask cultivation under anaerobic and aerobic conditions. Black bars KG2 in glycerol-based medium, cross-hatched bars KG4 in glycerol-based medium, white bars KG2 in glucose-based medium, hatched bars KG4 in glucose-based medium. Error bars represent the standard deviations from three independent experiments
Under anaerobic culture conditions, although the total acetate formation in strain KG4 decreased, the acetate formation per cell (g/g) was 0.17 and 0.42 in glycerol- and glucose-based media, respectively, which were almost the same as those in the KG2 strain, indicating that deleting poxB did not affect acetate formation. In contrast, compared with strain KG2, acetate formation in the KG4 strain was dramatically increased under aerobic conditions; meanwhile, more acetate (9.84 g/L) accumulated in a glycerol-based medium at the end of the cultivation (Fig. 2b).
In addition to acetate formation, another role of poxB is to generate CO2. To assess the effect of CO2 on the growth defect of the KG4 strain, 1 g/L of NaHCO3, an efficient source of CO2 for bacterial growth (Kozliak et al. 1995; Repaske and Clayton 1978), was added to the medium. As shown in Fig. 3, the addition of NaHCO3 had no effect on the growth of the KG2 strain under all culture conditions, and it did not affect the growth of the KG4 strain under aerobic culture conditions. However, the addition of NaHCO3 stimulated the anaerobic growth of strain KG4 in both glycerol- and glucose-based media, indicating that the growth defect of the KG4 strain was probable due to the shortage of CO2 under anaerobic conditions.
The effect of NaHCO3 on cell growth of KG2 and KG4 at the end of flask cultivation under anaerobic and aerobic conditions in glycerol-based medium (a) or glucose-based medium (b). Black bars KG2 without NaHCO3, white bars KG4 without NaHCO3, cross-hatched bars KG2 with NaHCO3, hatched bars KG4 with NaHCO3. Error bars represent the standard deviations from three independent experiments
Recovery of cell growth in the poxB mutant by deleting pta and ackA
In contrast to the adverse effects of the poxB deletion, deleting the other main acetate pathway, Pta-AckA, in strain KG2 had almost no effect on cell growth and the formation of metabolites, such as acetate and 1,3-PD, in all of the test conditions (data not shown). However, according to the aforementioned experiments, deleting poxB resulted in an extreme accumulation of acetate under aerobic culture conditions, which could be the reason for the aerobic growth defect of strain KG4. Thus, the pta and ackA genes were deleted from strain KG4. The growth and acetate formation of the resulting mutant, KG5, in a glycerol-based medium after 24 h of flask cultivation are shown in Table 2. Compared with strain KG4, acetate formation in strain KG5 dramatically decreased to 1.85 g/L (19 % of that of the KG4 strain) under aerobic conditions (Table 2). As a result of the decreased accumulation of acetate, the concentration of KG5 cells significantly increased at the end of aerobic cultivation, almost reaching the level of the parent strain KG2 (Table 2). More interestingly, it was shown that deleting the pta-ackA genes alleviated the growth defect of the poxB mutant, even under aerobic condition. At the end of anaerobic cultivation, the cell concentration of KG5 was the same as that of KG2 (Table 2). Such recovery of cell growth in the KG5 strain under both aerobic and anaerobic conditions was confirmed in a glucose-based medium (data not shown).
Although the growth of strain KG5 was almost the same as that of the KG2 strain in aerobic and anaerobic cultures, the varieties of metabolites formed, especially those involved in the TCA cycle, differed (Table 2). The levels of α-ketoglutarate and succinate in strain KG5 were only 62 and 41 %, respectively, of those in strain KG2 at the end of aerobic cultivation. In contrast, the levels of α-ketoglutarate and succinate substantially increased in strain KG5 at the end of anaerobic cultivation, as they were approximately 211 and 53 %, respectively, greater than those in strain KG2. Interestingly, the highest level of 1,3-PD production (20.8 g/L) at the end of anaerobic cultivation was found in strain KG5, which was 15 % greater than that in strain KG2 (Table 2).
Pyruvate accumulation is usually reported to be the reason for the growth defects in Pta-AckA-deficient E. coli strains (Chang et al. 1999; Liao et al. 1996). Based on our experiments, deleting poxB resulted in a high accumulation of pyruvate in strain KG4 under anaerobic culture condition, while strain KG5 did not accumulate high levels of pyruvate at the end of anaerobic cultivation (Table 2). Because of the importance of the pyruvate node in metabolism, variations in the level of pyruvate in strain KG5 during flask cultures were further analyzed. As shown in Fig. 4a, pyruvate accumulation in strain KG5 was found during the early stage of flask cultivation. The peak values of pyruvate occurred after approximately 6 h and reached 0.72 g/L (aerobic conditions) and 0.31 g/L (anaerobic conditions). The growth of strain KG5 during flask cultivation is also shown in Fig. 5. Compared with the KG2 strain, the growth of strain KG5 was retarded during the early stage of flask cultivation. The growth retardation was greater under aerobic conditions. At 10 h of cultivation, the cell concentration of strain KG5 was 66 % of that of strain KG2, while under anaerobic conditions, it reached 77 % of that of the KG2 strain (Fig. 4b).
Cell growth and metabolites formation in KG2 (open symbol) and KG5 (solid symbol) during the fed-batch fermentation process under micro-aerobic condition. a 1,3-PD (triangle), glycerol (circle), and cell growth (square); b acetate (square) and pyruvate (triangle); c α-ketoglutarate (square), 2,3-BD (circle), and succinate (triangle). Date points are averages of three identical experiments
1,3-PD production by the poxB-pta-ackA mutant KG5 during fed-batch fermentation
Compared with anaerobic fermentation, micro-aerobic fermentation for 1,3-PD production by K. pneumoniae is commonly used recently due to its high process productivity and convenient industrial application (Chen et al. 2003; Durgapal et al. 2014). As expected, the strain of KG5 also showed the high ability for 1,3-PD production under micro-aerobic condition in a flask batch process (Table 3). To confirm the ability of strain KG5 to produce large amounts of 1,3-PD, fed-batch fermentations under micro-aerobic conditions were performed in a 5-L bioreactor. The detailed fermentation results are shown in Fig. 5 and Table 3. During the fed-batch fermentation, the cell concentration increased in the early stage (0–16 h) and decreased in the later stage (16–30 h). Compared with the KG2 strain, the growth of strain KG5 was lower in the early stage, although a small decrease in the cell concentration was found in the later stage. In contrast to growth, a high level of 1,3-PD production was observed in strain KG5, especially in the later stage of fermentation (Fig. 5a). At the end of the fed-batch fermentation, 1,3-PD production by strain KG5 reached 76.8 g/L, which was 15 % greater than that of strain KG2.
Deleting the poxB, pta, and ackA genes resulted in a significant decrease in acetate formation during fed-batch fermentation. Acetate production by strain KG5 was 1.58 g/L, only 23 % of that of strain KG2 at the end of the fed-batch fermentation. In contrast with strain KG2, greater accumulations of α-ketoglutarate and succinate were observed in strain KG5 during fed-batch fermentation, and a high accumulation of pyruvate during the early stage of fermentation was also found. As shown in Table 3, strain KG5 completely consumed 140.1 g/L of glycerol within 30 h of fed-batch fermentation, which was less than that consumed by strain KG2 (141.3 g/L). Meanwhile, the formation of the dominant by-product, 2,3-BD, was almost unchanged; thus, the total yield of 1,3-PD from glycerol by strain KG5 in fed-batch fermentation reached 0.66 (mol/mol), a 16 % increase compared with strain KG2 (Table 3).
Discussion
Acetate, which is highly toxic, is a major by-product that is excreted during the formation of 1,3-PD from glycerol in K. pneumoniae (Celinska 2010; Zeng et al. 1994). Although a low level of acetate seems to have a positive effect on 1,3-PD fermentation (Grahame et al. 2013; Lu et al. 2013), acetate overflow could lead to inhibition of cell growth and a decrease in 1,3-PD production (Cui et al. 2014; He et al. 2013; Szymanowska-Powalowska and Kubiak 2015). In this study, the possibility of improving 1,3-PD production by directly blocking acetate synthesis pathways was investigated. Our results indicated that, in addition to decreasing acetate formation, deleting the poxB, pta, and ackA genes significantly improved 1,3-PD production in K. pneumoniae.
PoxB catalyzes the oxidative decarboxylation of pyruvate to acetate. In E. coli, pyruvate is mainly converted into acetyl-CoA by the dehydrogenase complex (PDHC) and/or pyruvate formate-lyase (PFL), so PoxB generally has been regarded as non-essential (Chang et al. 1994; Grabau and Cronan 1984). Although previous studies have elucidated some functions of PoxB, deleting poxB had almost no effect on aerobic growth and acetate formation in E. coli (Chang and Cronan 1983; Phue et al. 2010). In our experiments, an extreme growth defect occurred in the poxB mutant, indicating that, in contrast to E. coli, PoxB plays a more important role in K. pneumoniae. As the recycling of PoxB-generated acetate to acetyl-CoA via ACS requires additional energy, the involvement of PoxB in pyruvate oxidation seems wasteful. However, PoxB was actually confirmed to contribute to aerobic growth in E. coli (Abdel-Hamid et al. 2001). Thus, the sizeable acetate flux generated by PoxB could be an adaptation to ensure good growth in K. pneumoniae.
Although the deletion of poxB inhibited growth in both aerobic and anaerobic culture conditions, the reasons for the growth defects differ. Under aerobic conditions, the extra carbon flux obtained by blocking PoxB should directly flow to acetyl-CoA via PDHC and/or PFL. Meanwhile, the TCA cycle and acetate assimilation were reported to be suppressed in a poxB mutant (Kumari et al. 2000; Li et al. 2006; Martinez-Gomez et al. 2012; Phue and Shiloach 2004); thus, a high concentration of acetate accumulated in the poxB mutant, probably through the Pta-AckA pathway. Compared with the glucose-based medium, greater acetate accumulation in the poxB mutant correlated with a more severe aerobic growth defect in the glycerol-based medium. Consequently, the accumulation of acetate in aerobic cultures could explain the growth defect of the poxB mutant. Carbon-dependent accumulation of acetate in the poxB mutant may be caused by regulatory differences in acetate assimilation (Wolfe 2005). The suppression of the TCA cycle in an aerobic culture of the poxB mutant was confirmed, as evidenced by the decrease in metabolites involved in the TCA cycle, such as α-ketoglutarate and succinate (Table 2). Additionally, under anaerobic conditions, CO2 is important for bacterial growth (Dharmadi et al. 2006; Merlin et al. 2003; Tran et al. 2014), and metabolic pathways, such as the PoxB pathway, should be involved in producing an endogenous supply of CO2 because of the lack of TCA. Thus, the serious growth defect of the poxB mutant implies that an appreciable amount of PoxB-generated CO2 is required for bacterial growth. The alleviation of the anaerobic growth defect of the poxB mutant by the addition of NaHCO3 (an external CO2 donor) to the medium strongly supports the above assumption (Fig. 3). It was reported that the requirement for CO2 for bacterial growth mainly depended on PDHC in anaerobically grown E. coli (Murarka et al. 2010). Here, it was shown that PoxB affected the generation of CO2 during the anaerobic growth of K. pneumoniae. Based on our data, compared with aerobic growth, deleting poxB resulted in a great accumulation of pyruvate and a more severe inhibition of anaerobic growth of K. pneumoniae (Table 2 and Fig. 2).
The Pta-AckA pathway is considered to be the primary acetate synthesis pathway in E. coli (De Mey et al. 2007; Merlin et al. 2003). In our study, the deletion of pta-ackA, unlike the deletion of poxB, had no effect on bacterial growth and metabolite formation in K. pneumoniae. However, the growth and metabolic characteristics of the poxB-pta-ackA mutant significantly differed from those of the poxB mutant. Under aerobic conditions, a large decrease in acetate formation occurred in the poxB-pta-ackA mutant, which strongly suggests that Pta-AckA also plays an important role in acetate formation, although the Pta-AckA pathway is dispensable in a K. pneumoniae strain that expresses PoxB. Accompanying the decrease in acetate formation, the aerobic growth of the poxB-pta-ackA mutant was better than that of the poxB mutant. The cell concentration of the poxB-pta-ackA mutant at the end of flask cultivation almost recovered to the same level of the parent strain. Thus, this further confirmed that the high accumulation of acetate was responsible for the aerobic growth defect of the poxB mutant. More interestingly, the alleviation of the growth defect of the poxB mutant by deleting the pta-ackA even occurred under aerobic conditions. This could mean that, in addition to the decrease in acetate formation in aerobic conditions, deleting pta-ackA could also overcome the shortage of CO2 in the poxB mutant under anaerobic conditions. According to our data, the increase in metabolites involved in the TCA cycle (α-ketoglutarate and succinate) clearly suggests that carbon flux could be forced to the TCA cycle in the poxB-pta-ackA mutant under anaerobic conditions, and an increased flux of the TCA cycle can supply sufficient CO2 for the anaerobic growth of K. pneumoniae.
Increasing the carbon flux to the TCA cycle could generate extra dihydronicotinamide adenine dinucleotide (NADH), which is helpful for 1,3-PD production. Further studies indicated that the cell growth rate of poxB-pta-ackA mutant was still little lower than that of parent strain, and accumulation of pyruvate was also found in the early stage of cultivation, which was observed in Pta-AckA deficient E. coli strains (Chang et al. 1999; Liao et al. 1996). Nevertheless, the poxB-pta-ackA mutant showed an excellent ability to produce 1,3-PD under both anaerobic and micro-aerobic conditions. Based on our data, deleting poxB-pta-ackA severely decreased acetate formation and increased the carbon flux to the TCA cycle, while there was no effect on 2,3-BD formation. As a result, the 1,3-PD concentration and yield reached 76.8 g/L and 0.66 (mol/mol), respectively, after 30 h of fed-batch fermentation, which were 15 and 16 %, respectively, greater than those of the parent strain.
In summary, a K. pneumoniae poxB mutant exhibited an extreme growth defect. The growth defect of the poxB mutant was alleviated by deleting pta-ackA, probably due to the decrease in acetate formation under aerobic conditions and the improvement in the CO2 supply under anaerobic conditions. According to our data, deleting poxB, pta, and ackA in K. pneumoniae severely decreased acetate formation and increased carbon flux to the TCA cycle, which was improved 1,3-PD production.
References
Abdel-Hamid AM, Attwood MM, Guest JR (2001) Pyruvate oxidase contributes to the aerobic growth efficiency of Escherichia coli. Microbiology 147:1483–1498
Ashok S, Raj SM, Rathnasingh C, Park S (2011) Development of recombinant Klebsiella pneumoniae delta dhaT strain for the co-production of 3-hydroxypropionic acid and 1,3-propanediol from glycerol. Appl Microbiol Biotechnol 90:1253–1265
Celinska E (2010) Debottlenecking the 1,3-propanediol pathway by metabolic engineering. Biotechnol Adv 28:519–530
Celinska E (2012) Klebsiella spp as a 1, 3-propanediol producer: the metabolic engineering approach. Crit Rev Biotechnol 32:274–288
Chang DE, Shin S, Rhee JS, Pan JG (1999) Acetate metabolism in a pta mutant of Escherichia coli W3110: importance of maintaining acetyl coenzyme A flux for growth and survival. J Bacteriol 181:6656–6663
Chang YY, Cronan Jr JE (1983) Genetic and biochemical analyses of Escherichia coli strains having a mutation in the structural gene (poxB) for pyruvate oxidase. J Bacteriol 154:756–762
Chang YY, Wang AY, Cronan Jr JE (1994) Expression of Escherichia coli pyruvate oxidase (PoxB) depends on the sigma factor encoded by the rpoS(katF) gene. Mol Microbiol 11:1019–1028
Chen X, Zhang DJ, Qi WT, Gao SJ, Xiu ZL, Xu P (2003) Microbial fed-batch production of 1,3-propanediol by Klebsiella pneumoniae under micro-aerobic conditions. Appl Microbiol Biotechnol 63:143–146
Cui YL, Zhou JJ, Gao LR, Zhu CQ, Jiang X, Fu SL, Gong H (2014) Utilization of excess NADH in 2,3-butanediol-deficient Klebsiella pneumoniae for 1,3-propanediol production. J Appl Microbiol 117:690–698
De Mey M, De Maeseneire S, Soetaert W, Vandamme E (2007) Minimizing acetate formation in E. coli fermentations. J Ind Microbiol Biotechnol 34:689–700
Dharmadi Y, Murarka A, Gonzalez R (2006) Anaerobic fermentation of glycerol by Escherichia coli: a new platform for metabolic engineering. Biotechnol Bioeng 94:821–829
Dittrich CR, Vadali RV, Bennett GN, San KY (2005) Redistribution of metabolic fluxes in the central aerobic metabolic pathway of E. coli mutant strains with deletion of the ackA-pta and poxB pathways for the synthesis of isoamyl acetate. Biotechnol Prog 21:627–631
Durgapal M, Kumar V, Yang TH, Lee HJ, Seung D, Park S (2014) Production of 1,3-propanediol from glycerol using the newly isolated Klebsiella pneumoniae J2B. Bioresour Technol 159:223–231
Gao L, Jiang X, Fu S, Gong H (2014) In silico identification of potential virulence genes in 1,3-propanediol producer Klebsiella pneumoniae. J Biotechnol 189:9–14
Grabau C, Cronan Jr JE (1984) Molecular cloning of the gene (poxB) encoding the pyruvate oxidase of Escherichia coli, a lipid-activated enzyme. J Bacteriol 160:1088–1092
Grahame DA, Kang TS, Khan NH, Tanaka T (2013) Alkaline conditions stimulate the production of 1,3-propanediol in Lactobacillus panis PM1 through shifting metabolic pathways. World J Microbiol Biotechnol 29:1207–1215
He L, Zhao X, Cheng K, Sun Y, Liu D (2013) Kinetic modeling of fermentative production of 1, 3-propanediol by Klebsiella pneumoniae HR526 with consideration of multiple product inhibitions. Appl Biochem Biotechnol 169:312–326
Huang Y, Li Z, Shimizu K, Ye Q (2012) Simultaneous production of 3-hydroxypropionic acid and 1,3-propanediol from glycerol by a recombinant strain of Klebsiella pneumoniae. Bioresour Technol 103:351–359
Khan NH, Kang TS, Grahame DA, Haakensen MC, Ratanapariyanuch K, Reaney MJ, Korber DR, Tanaka T (2013) Isolation and characterization of novel 1,3-propanediol-producing Lactobacillus panis PM1 from bioethanol thin stillage. Appl Microbiol Biotechnol 97:417–428
Kozliak EI, Fuchs JA, Guilloton MB, Anderson PM (1995) Role of bicarbonate/CO2 in the inhibition of Escherichia coli growth by cyanate. J Bacteriol 177:3213–3219
Kumar V, Sankaranarayanan M, Durgapal M, Zhou S, Ko Y, Ashok S, Sarkar R, Park S (2013) Simultaneous production of 3-hydroxypropionic acid and 1,3-propanediol from glycerol using resting cells of the lactate dehydrogenase-deficient recombinant Klebsiella pneumoniae overexpressing an aldehyde dehydrogenase. Bioresour Technol 135:555–563
Kumar V, Sankaranarayanan M, Jae KE, Durgapal M, Ashok S, Ko Y, Sarkar R, Park S (2012) Co-production of 3-hydroxypropionic acid and 1,3-propanediol from glycerol using resting cells of recombinant Klebsiella pneumoniae J2B strain overexpressing aldehyde dehydrogenase. Appl Microbiol Biotechnol 96:373–383
Kumari S, Simel EJ, Wolfe AJ (2000) sigma(70) is the principal sigma factor responsible for transcription of acs, which encodes acetyl coenzyme A synthetase in Escherichia coli. J Bacteriol 182:551–554
Lee SM, Hong WK, Heo SY, Park JM, Jung YR, Oh BR, Joe MH, Seo JW, Kim CH (2014) Enhancement of 1,3-propanediol production by expression of pyruvate decarboxylase and aldehyde dehydrogenase from Zymomonas mobilis in the acetolactate-synthase-deficient mutant of Klebsiella pneumoniae. J Ind Microbiol Biotechnol 41:1259–1266
Li M, Yao S, Shimizu K (2006) Effect of poxB gene knockout on metabolism in Escherichia coli based on growth characteristics and enzyme activities. World J Microbiol Biotechnol 23:573–580
Li M, Zhang X, Agrawal A, San KY (2012) Effect of acetate formation pathway and long chain fatty acid CoA-ligase on the free fatty acid production in E. coli expressing acy-ACP thioesterase from Ricinus communis. Metab Eng 14:380–387
Liao JC, Hou SY, Chao YP (1996) Pathway analysis, engineering, and physiological considerations for redirecting central metabolism. Biotechnol Bioeng 52:129–140
Lu S, Han Y, Duan X, Luo F, Zhu L, Li S, Huang H (2013) Cell morphology variations of Klebsiella pneumoniae induced by acetate stress using biomimetic vesicle assay. Appl Biochem Biotechnol 171:731–743
Martinez-Gomez K, Flores N, Castaneda HM, Martinez-Batallar G, Hernandez-Chavez G, Ramirez OT, Gosset G, Encarnacion S, Bolivar F (2012) New insights into Escherichia coli metabolism: carbon scavenging, acetate metabolism and carbon recycling responses during growth on glycerol. Microb Cell Factories 11:46
Merlin C, Masters M, McAteer S, Coulson A (2003) Why is carbonic anhydrase essential to Escherichia coli? J Bacteriol 185:6415–6424
Murarka A, Clomburg JM, Moran S, Shanks JV, Gonzalez R (2010) Metabolic analysis of wild-type Escherichia coli and a pyruvate dehydrogenase complex (PDHC)-deficient derivative reveals the role of PDHC in the fermentative metabolism of glucose. J Biol Chem 285:31548–31558
Peebo K, Valgepea K, Nahku R, Riis G, Oun M, Adamberg K, Vilu R (2014) Coordinated activation of PTA-ACS and TCA cycles strongly reduces overflow metabolism of acetate in Escherichia coli. Appl Microbiol Biotechnol 98:5131–5143
Petrov K, Petrova P (2009) High production of 2,3-butanediol from glycerol by Klebsiella pneumoniae G31. Appl Microbiol Biotechnol 84:659–665
Phue JN, Lee SJ, Kaufman JB, Negrete A, Shiloach J (2010) Acetate accumulation through alternative metabolic pathways in ackA − pta − poxB − triple mutant in E. coli B (BL21). Biotechnol Lett 32:1897–1903
Phue JN, Shiloach J (2004) Transcription levels of key metabolic genes are the cause for different glucose utilization pathways in E. coli B (BL21) and E. coli K (JM109). J Biotechnol 109:21–30
Repaske R, Clayton MA (1978) Control of Escherichia coli growth by CO2. J Bacteriol 135:1162–1164
Seo MY, Seo JW, Heo SY, Baek JO, Rairakhwada D, Oh BR, Seo PS, Choi MH, Kim CH (2009) Elimination of by-product formation during production of 1,3-propanediol in Klebsiella pneumoniae by inactivation of glycerol oxidative pathway. Appl Microbiol Biotechnol 84:527–534
Skraly FA, Lytle BL, Cameron DC (1998) Construction and characterization of a 1,3-propanediol operon. Appl Environ Microbiol 64:98–105
Szymanowska-Powalowska D, Bialas W (2014) Scale-up of anaerobic 1,3-propanediol production by Clostridium butyricum DSP1 from crude glycerol. BMC Microbiol 14:45
Szymanowska-Powalowska D, Kubiak P (2015) Effect of 1,3-propanediol, organic acids, and ethanol on growth and metabolism of Clostridium butyricum DSP1. Appl Microbiol Biotechnol 99:3179–3189
Tang X, Tan Y, Zhu H, Zhao K, Shen W (2009) Microbial conversion of glycerol to 1,3-propanediol by an engineered strain of Escherichia coli. Appl Environ Microbiol 75:1628–1634
Tran KT, Maeda T, Wood TK (2014) Metabolic engineering of Escherichia coli to enhance hydrogen production from glycerol. Appl Microbiol Biotechnol 98:4757–4770
Wolfe AJ (2005) The acetate switch. Microbiol Mol Biol Rev 69:12–50
Xu YZ, Guo NN, Zheng ZM, Ou XJ, Liu HJ, Liu DH (2009) Metabolism in 1,3-propanediol fed-batch fermentation by a d-lactate deficient mutant of Klebsiella pneumoniae. Biotechnol Bioeng 104:965–972
Yamamoto S, Izumiya H, Morita M, Arakawa E, Watanabe H (2009) Application of lambda Red recombination system to Vibrio cholerae genetics: simple methods for inactivation and modification of chromosomal genes. Gene 438:57–64
Yen HW, Li FT, Chang JS (2014) The effects of dissolved oxygen level on the distribution of 1,3-propanediol and 2,3-butanediol produced from glycerol by an isolated indigenous Klebsiella sp. Ana-WS5. Bioresour Technol 153:374–378
Zeng AP, Ross A, Biebl H, Tag C, Gunzel B, Deckwer WD (1994) Multiple product inhibition and growth modeling of Clostridium butyricum and Klebsiella pneumoniae in glycerol fermentation. Biotechnol Bioeng 44:902–911
Zheng ZM, Xu YZ, Liu HJ, Guo NN, Cai ZZ, Liu DH (2008) Physiologic mechanisms of sequential products synthesis in 1,3-propanediol fed-batch fermentation by Klebsiella pneumoniae. Biotechnol Bioeng 100:923–932
Zhu C, Jiang X, Zhang Y, Lin J, Fu S, Gong H (2015) Improvement of 1,3-propanediol production in Klebsiella pneumoniae by moderate expression of puuC (encoding an aldehyde dehydrogenase). Biotechnol Lett 37:1783–1790
Zhuge B, Zhang C, Fang HY, Zhuge JA, Permaul K (2010) Expression of 1,3-propanediol oxidoreductase and its isoenzyme in Klebsiella pneumoniae for bioconversion of glycerol into 1,3-propanediol. Appl Microbiol Biotechnol 87:2177–2184
Acknowledgments
This work was supported by the National Natural Science Foundation of China under Grant No. 31271862.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Human and animal studies
This article does not contain any studies with human participants or animals performed by any of the author.
Rights and permissions
About this article
Cite this article
Lin, J., Zhang, Y., Xu, D. et al. Deletion of poxB, pta, and ackA improves 1,3-propanediol production by Klebsiella pneumoniae . Appl Microbiol Biotechnol 100, 2775–2784 (2016). https://doi.org/10.1007/s00253-015-7237-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7237-2