Abstract
Glycerol is an attractive carbon source for biofuel production since it is cheap and abundant due to the increasing demand for renewable and clean energy sources, which includes production of biodiesel. This research aims to enhance hydrogen production by Escherichia coli from glycerol by manipulating its metabolic pathways via targeted deletions. Since our past strain, which had been engineered for producing hydrogen from glucose, was not suitable for producing hydrogen from glycerol, we rescreened 14 genes related to hydrogen production and glycerol metabolism. We found that 10 single knockouts are beneficial for enhanced hydrogen production from glycerol, namely, frdC (encoding for furmarate reductase), ldhA (lactate dehydrogenase), fdnG (formate dehydrogenase), ppc (phosphoenolpyruvate carboxylase), narG (nitrate reductase), focA (formate transporter), hyaB (the large subunit of hydrogenase 1), aceE (pyruvate dehydrogenase), mgsA (methylglyoxal synthase), and hycA (a regulator of the transcriptional regulator FhlA). On that basis, we created multiple knockout strains via successive P1 transductions. Simultaneous knockouts of frdC, ldhA, fdnG, ppc, narG, mgsA, and hycA created the best strain that produced 5-fold higher hydrogen and had a 5-fold higher hydrogen yield than the parent strain. The engineered strain also reached the theoretical maximum yield of 1 mol H2/mol glycerol after 48 h. Under low partial pressure fermentation, the strain grew over 2-fold faster, indicating faster utilization of glycerol and production of hydrogen. By combining metabolic engineering and low partial pressure fermentation, hydrogen production from glycerol was enhanced significantly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycerol is the main by-product from biodiesel production, accounting for roughly 10 % of the products (w/w). The production of biodiesel (and therefore glycerol) is increasing, and it is estimated that the world production of biodiesel and crude glycerol will reach 37 and 4 billion gallon by 2016 (Anand and Saxena 2012). As a result, the surplus of crude glycerol has been negatively affecting the refined glycerol market in recent years. To assure the sustainable development and commercial competitiveness with other fuel sources for both the glycerol and biodiesel industries, by-product glycerol should be converted into other value added chemicals and fuels such as ethanol and hydrogen (Deckwer 1995). Given its low carbon structure, glycerol has been used to produce bio-hydrogen and bio-ethanol via the fermentative process with a significant and promising production (Hu and Wood 2010; Murarka et al.2008).
Hydrogen is a compelling future energy source because it does not produce greenhouse gases or other pollutants since the products of hydrogen oxidation are only water and energy. Furthermore, hydrogen has higher energy content than fossil fuels by 2.75 times. Bio-hydrogen can be produced via four main ways: (a) bio-photolysis of water using algae and cyanobacteria, (b) photodecomposition of organic compounds by photosynthetic bacteria, (c) fermentative hydrogen production from organic compounds, and (d) hybrid systems using photosynthetic and fermentative bacteria (Das and Veziroğlu 2001). The first two processes require photosynthesis where hydrogen production is dependent on light while the third is a dark fermentation or anaerobic process, which is strictly light independent. Note that fermentative hydrogen production is more efficient for industrial production compared to the other methods because it can utilize organic pollutants as input sources, requires less energy consumption, and requires simple technology (Das and Veziroğlu 2001; Maeda et al. 2008; Navarro et al. 2009; Ust’ak et al. 2007; Vardar-Schara et al. 2008). Recently, hydrogen production by both dark fermentative and photosynthesis organisms such as Enterobacter, Bacillus, Clostridium, and green alga has been well studied and steadily enhanced (Fabiano and Perego 2002; Hawkes et al. 2002; Kalia et al. 1994; Srirangan et al. 2011; Yokoi et al. 1998, 2002). For example, the highest hydrogen yield of 2.81 mol H2/mol glucose from Clostridium beijerinckii L9 has been reported (Lin et al. 2007). However, it is far lower than a theoretical stoichiometric yield of 4 mol H2/mol glucose. Among various fermentative hydrogen producing microorganisms, Escherichia coli bears a high potential for hydrogen production at a large scale because it is easily manipulated genetically, can achieve a high hydrogen yield, and sustains a rapid growth (Maeda et al. 2008; Vardar-Schara et al. 2008).
Recently, the fermentation flux in E. coli has been improved for hydrogen production, typically from glucose metabolism (Bagramyan et al. 2002; Blattner 1997; Maeda et al. 2007a, 2008), with the goal of producing formate, which is converted to CO2 and H2. Inactivation of hycA, a negative regulator of the FHL define complex, together with the overexpression of the FHL complex (encoded by fhlA), results in a significant increase in hydrogen production (Kim et al. 2009; Wang et al. 2012; Yoshida et al. 2005). So far, the role of hydrogenase 3 (Hyd-3) is clear since a defect in hycE, which encodes the large subunit of Hyd-3, significantly impairs the hydrogen yield (Maeda et al. 2007b). Furthermore, the FHL complex is essential because its inactivation is detrimental to hydrogen production (Dharmadi et al. 2006). E. coli converts pyruvate into CO2, lactate, and acetate by pyruvate dehydrogenase (encoded by aceE), D-lactate dehydrogenase (encoded by ldhA), and pyruvate oxidase (encoded by poxB), respectively (Bunch et al. 1997; Chang and Cronan 1983; Jiang et al. 2001). Regarding the respiratory pathway, two formate dehydrogenases, formate dehydrogenase N (encoded by fdnG) and formate dehydrogenase-O (encoded by fdoG), play a pivotal role in formate oxidation to CO2 (Abaibou et al. 1995; Jormakka et al. 2002; Rossmann et al. 1991; Sawers et al. 1991). The cell exports formate by the activation of the formate transporter (encoded by focA) to prevent acidification when the pH in the cytoplasm drops (Suppmann and Sawers 1994). Consequently, the concentration of formate, which is a precursor of hydrogen production, is reduced. Hence, in addition to the FHL complex, redirection of both metabolic and respiratory pathways should enhance hydrogen production from glycerol.
To date, the production of hydrogen in E. coli from glucose has been achieved by applying metabolic engineering (Maeda et al. 2007a, 2008). From glycerol, inactivation of fumarate reductase (∆frdA) increases the production of hydrogen and ethanol (Shams Yazdani and Gonzalez 2008). Hydrogen production could be also increased by improving the conversion of glycerol. In principle, glycerol is converted to dihydroxylacetone (DHA), which is the first intermediate product in the glycolytic pathway by glycerol dehydrogenase (encoded by gldA; Truniger and Boos 1994). Apparently, glycerol dehydrogenase is one of the most important enzymes in the glycerol metabolism since either its overexpression or inactivation highly affects hydrogen production in E. coli (Hu and Wood 2010; Sanchez-Torres et al. 2013; Shams Yazdani and Gonzalez 2008). Hence, blocking key pathways and overexpression of the glycerol dehydrogenase should improve the hydrogen yield from glycerol. In addition, CO2 has a positive role in glycerol conversion, while H2 partial pressure negatively affects hydrogen production and cell growth (Dharmadi et al. 2006; Gonzalez et al. 2008). Therefore, a low partial pressure fermentation is often applied to enhance hydrogen production (Dharmadi et al. 2006; Kim et al. 2006; Maeda et al. 2008; Mizuno et al. 2000).
Although many of the metabolic pathways in E. coli are well studied (Blattner 1997), there remain some gaps in our knowledge base when glycerol is used as a substrate. Recently, some studies have shown that those genes, which are either beneficial or related to hydrogen production from glucose, might not necessarily have a similar effect or even may have the opposite effect when glycerol is used as the substrate. For example, in E. coli, Hyd-1 and Hyd-2 are hydrogen uptake enzymes during glucose metabolism (Menon et al. 1991, 1994), whereas they are reversible enzymes in the presence of glycerol (Sanchez-Torres et al. 2013; Trchounian and Trchounian 2009). In addition, anaerobic growth in the presence of glycerol is quite lower than that of glucose (Hu and Wood 2010).
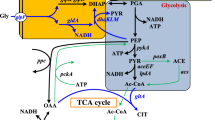
Our previous engineered strain, BW25113 hyaB hybC hycA fdoG frdC ldhA aceE increased hydrogen production by 4.6-fold from glucose and increased hydrogen yield by 2-fold from 0.65 to 1.3 mol H2/mol glucose (Maeda et al. 2007a), while the maximum yield in glucose metabolism is 2 mol H2/mol glucose. However, it could not produce a relatively high amount of hydrogen from glycerol due to the difference in metabolism of glucose and glycerol. Here, we aim to create better metabolic flux for enhancing hydrogen production from glycerol (Fig. 1).
The metabolic and respiratory pathway in Escherichia coli (Altaras and Cameron 1999; Chao et al. 1993; Cooper 1984; Dharmadi et al. 2006; Gonzalez et al. 2008; Murarka et al. 2008; Rossmann et al. 1991; Saikusa et al. 1987; Truniger and Boos 1994). Genetic manipulations are illustrated by cross symbols. The engineered strain is inactivated in fumarate reductase (encoded by frdC), D-lactate dehydrogenase (encoded by ldhA), formate dehydrogenase N, α subunit (encoded by fdnG), phosphoenolpyruvate carboxylase (encoded by ppc), nitrate reductase A, α subunit (encoded by narG), methylglyoxal synthase (encoded by mgsA) and regulator of the transcriptional regulator FhlA (encoded by hycA). Dashed lines and bold words illustrate multiple metabolic steps and the main products. Abbreviations: DHA (dihydroxylacetone) PEP (phopshoenolpyruvate); FHL (formate hydrogen-lyase); gldA (glycerol dehydrogenase)
Materials and methods
Strains, P1 transduction, plasmids, and primers
The parent strain, BW25113, was obtained from the Yale Coli Genetic Stock Center (the USA), while single KEIO and ASKA strains were provided from the KEIO library (National Institute of Genetics, Japan). P1 transduction (Cherepanov and Wackernagel 1995) was used to create the multiple deletion strains (Table 1). Prior to the P1 transduction, the kanamycin resistance gene of the recipient strain was removed by helper plasmid, pCP20, which is able to facilitate homologous DNA recombination with the flanking repeated site (FRT) located at either end of the resistance gene. The helper plasmid containing the chloramphenicol (CmR) antibiotic marker was then cured by growing at 43 °C due to its sensitivity to this temperature (Cherepanov and Wackernagel 1995; Datsenko and Wanner 2000). After each round of P1 transduction, the knockout strain with its newly disrupted gene was verified by PCR using a set of specific primers (Table 1).
Four plasmids were used in this study including pCA24N as the empty vector; pCA24N-FdhF, expressing the subunit of formate dehydrogenase; pCA24N-FhlA, expressing the regulator of the FHL complex; and pCA24N-GldA, expressing E. coli glycerol dehydrogenase. These plasmids were originated from Kitagawa et al. (2005). A set of primers was designed for the confirmation purpose.
Culture medium, growth condition, and chemicals
Luria-Bertani (LB) medium and LB agar plates containing appropriate antibiotics were used to grow the strains when conducting P1 transduction. Minimal glycerol medium supplemented with 10 g/ L of glycerol, 0.02 mg/ L of NiCl2, and 0.172 mg/ L of Na2SeO3 (Murarka et al. 2008; Sanchez-Torres et al. 2013) and appropriate antibiotics was used as the medium for preparing the overnight culture and fermentation. Note that selenium is toxic to the cell, however, a small amount of selenium is essential for the synthesis of selenocysteine, which is one of the constituents of the FDH H (Sawers 1991, 1994; Takahata et al. 2008). Furthermore, a low concentration of selenite (below 5 mM) does not affect the growth of E. coli (Bebien et al. 2002). The pH of the medium was adjusted by using 5 M NaOH to 7.5. All the experiments were conducted at 37 °C at 120 rpm in a bio-shaker (BR-180LF, Taitec). Chemicals were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan), Dojindo Molecular Technologies, Inc. (Kumamoto, Japan) and Sigma Al-rich Co. Llc (Tokyo, Japan) and Nacalai Tesque, Inc (Kyoto, Japan).
Growth rate and hydrogen assay
For the growth rates, the strains were streaked from the −80 °C glycerol stock on LB agar plates, and then incubated overnight at 37 °C. After 12–18 h, a single colony was inoculated into a 250-mL flask (Iwaki, Japan) containing 25 mL of minimal glycerol medium, and the strains were grown aerobically. Cell turbidity was measured at 600 nm wavelength by using a UV/VIS spectrophotometer (JASCO V-530, Tokyo, Japan).
For the hydrogen assay, sealed crimp top vials (34 or 68 mL) were used for screening hydrogen production of the single mutant strains and for examining the kinetic growth, glycerol utilization, and hydrogen production of the highest hydrogen producing strains and the wild type strains. The pre-culture, closed vials, and fresh minimal glycerol medium were sparged with nitrogen gas for 5 and 10 min to remove oxygen. Inside the anaerobic chamber, an appropriate volume of overnight culture was added to vials containing sparged minimal glycerol medium with 0.1 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG). The initial optical density at 600 nm (OD600) of the fermentative culture was adjusted to 0.05–0.06. Samples were then grown anaerobically at 37 °C, 120 rpm. After each 24 h, 50 μL of gas from the headspace of the fermentation vial was consecutively measured by a gas chromatograph (Agilent 6890 Series GC System) equipped with a thermal conductive detector (Maeda et al. 2007c). The cell mass was calculated based on the value of OD600 with the amount of 0.22 mg protein/OD/mL (Fishman et al. 2005). Hydrogen production and productivity was calculated based on the method reported elsewhere (Maeda et al. 2007c).
Hydrogen low partial pressure assay
The pre-culture and closed vial assays were performed as above using a 68-mL-sealed crimp top vial. To reach a low partial pressure condition, the fermentative vials were connected to an inverted cylinder and the generated gases such as H2 and CO2 were released into the headspace of the vial prior to the inverted cylinder (Maeda et al. 2008). The fermentation vials were slightly stirred and kept at 37 °C. Hydrogen was measured after 24 h when the fermentation likely reached the log phase. The generated hydrogen volume was calculated as the sum of headspace volume of the fermentative vial and the inverted cylinder, and the volume of the connecting tube.
Quantification of organic acids, cell, and glycerol consumed
The fermentation culture was filtered by a 0.2-μm membrane (Sartorious, Germany) for organic acids and glycerol quantification. Organic acids were measured after 24, 48, 72, 96, and 120 h of fermentation by high performance liquid chromatography (8 mm × 300 mm, Shimazu Co., Tokyo, Japan) using a Shim-Pack SCR-102H column and a CDD-6A electric conductivity detector (Maeda et al. 2009). The mobile phase was set to be 5 mM p-toluenesulfonic acid monohydrate with a flow rate of 0.8 mL/ min, and the column was set at 40 °C. The organic acids were quantified after 24 h of fermentation when the hydrogen low partial pressure was applied. The Free glycerol determination Kit (Sigma, San Louis, MO) was used to determine glycerol concentration. Ethanol and 1, 2 propanediol (1, 2-PDO) were quantified by a GC 2025 gas chromatograph equipped with a CBP20-M25-025 capillary column and a flame ionization detector (Shimadzu, Japan). The temperature of the column, oven, and detector were set at 50 °C, 250 °C, and 250 °C, respectively (Sanchez-Torres et al. 2013). Product yields (hydrogen, cell, organic acids, 1, 2-PDO, and ethanol) were calculated in mmole of product per mmole glycerol consumed. Cell yield was calculated based on the estimated molecular weight of 26.535 g provided by the formula of CH1.94O0.52N0.25P0.025 of E. coli dry mass (Villadsen et al. 2011).
Results
Screening of hydrogen production of the single mutant strain
In our previous study (Maeda et al. 2007a), we created the metabolically engineered strain, BW25113 hyaB hybC hycA frdC ldhA fdoG aceE, which produces 4.6-fold more hydrogen than wild-type BW25113 during glucose metabolism. However, its hydrogen productivity increased only 1.8-fold over the parent strain during glycerol metabolism (Table 2). Furthermore, the growth rate of this strain decreased by 2.7-fold with glycerol. Therefore, we hypothesized that the enzymes of the related pathways may play different roles in glycerol metabolism. Hence, we investigated the pathways most suitable to delete to increase hydrogen production under glycerol metabolism. Next, beneficial genes were introduced to enhance hydrogen production. Based on the knowledge on the metabolic pathway of E. coli under both glucose and glycerol metabolism (Fig. 1), we investigated 14 single KEIO mutant strains individually (Table 2).
Table 2 shows that except for BW25113 hybC, the growth rate of the other 13 single knockout strains deceased from 1.1 to 3.3-fold. Noticeably, introduction of multiple gene disruptions does not necessarily lower the growth rate of the mutant strains. In fact, the highest hydrogen producing mutant-septuple strain grew negligibly lower than strain BW25113 with the growth rate of 0.31/h with glycerol.
Similar to previous work, inactivation of fumarate reductase (∆frdC) led to a 1.6-fold increase in hydrogen production with glycerol (Shams Yazdani and Gonzalez 2008). Disruption of ldhA, fdnG, ppc, narG, focA, hyaB, and hycA have been reported to have a positive effect on the production of either intermediates or end products such as succinate or hydrogen in glucose metabolism (Kabir et al. 2005; Maeda et al. 2007a; Redwood et al. 2008; Wang and Gunsalus 2003; Yoshida et al. 2005). Single disruption of these genes boosted hydrogen production from 1.1 to 1.4-fold during glycerol metabolism. Consistent with the results that have been previously reported in glucose metabolism (Maeda et al. 2007a), single inactivation of pyruvate oxidase (encoded by poxB) was detrimental to the hydrogen production and reduced viability with glycerol. In contrast to that with glucose (Maeda et al. 2007a), inactivation of formate dehydrogenase O (encoded by fdoG) did not improve hydrogen production with glycerol. Additionally, blocking the synthesis pathway to alcohol by inactivating dehydrogenase (encoded by adhE) severely impaired hydrogen production and cell growth and this result is consistent with those reported previously with glycerol (Blankschien et al. 2010; Murarka et al. 2008). In agreement with previous studies with glycerol, inactivation of the large subunit of Hyd-2 (encoded by hybC) resulted in almost no hydrogen produced indicating that Hyd-2 plays the main role in hydrogen production from glycerol under alkaline pH (Sanchez-Torres et al. 2013; Trchounian et al. 2011; Trchounian and Trchounian 2009). In E. coli, 1, 2-PDO is the end product of glycerol metabolism, and it is synthesized by the conversion of dihydroxylacetone phosphate into methylglyoxal prior to other intermediates (Fig. 1). Therefore, in order to direct the metabolic flux toward hydrogen production, we blocked this sub-pathway by inactivating methylglyoxal synthase (encoded by mgsA). This disruption resulted in 1.2-fold increase of hydrogen productivity.
From these screening results with single mutants, we conducted successive P1 transductions by introducing multiple disruptions into the host strain. Surprisingly, there was little increase in hydrogen production for the double and the triple knockout strains namely, BW25113 frdC ldhA and BW25113 frdC ldhA fdnG. However, hydrogen productivity remarkably increased by 3.2-fold in the quadruple knockout strain BW25113 frdC ldhA fdnG ppc. Single inactivation of formate transporter (∆focA) and the large subunit of Hyd-1 (∆hyaB) somewhat increased hydrogen production, though, when disrupted in the quadruple mutant strain, they did not contribute to any improvement of hydrogen production (Table 2). Rather, disruption of focA in the septuple mutant strain also decreased by 1.9-fold of hydrogen productivity, indicating that the formate transporter is necessary for increasing hydrogen production in the multiple mutant strain, and this finding agrees with those reported previously for the conversion of glucose into hydrogen (Maeda et al. 2007a). Successive transductions of narG and mgsA increased hydrogen production with glycerol by 3.8 and 4.3-fold, respectively. Since we were not able to remove the kanamycin maker gene for BW25113 aceE, disruption of aceE was made the final step. Hydrogen productivity significantly increased by 5.5-fold when the negative regulator of the hyc operon-hycA was inactivated. As mentioned above, hydrogen productivity of the strain BW25113 aceE increased by 2.1-fold. However, when this gene was disrupted in the septuple mutant strain, hydrogen productivity dramatically dropped due to its extremely slow growth rate. The possible reason for this decrease of both hydrogen production and cell growth is a deficiency in electron acceptors when the metabolic pathway of E. coli was highly engineered. The best strain was the septuple mutant, BW25113 frdC ldhA fdnG ppc narG mgsA hycA, which had a reasonable growth rate and 5.5-fold higher hydrogen productivity than that of its parent strain.
Since the conversion of glycerol is highly mediated by glycerol dehydrogenase (encoded by gldA) and the conversion of formate into H2 and CO2 by the FHL complex (Fig. 1), there is a high possibility that overexpression of these enzymes would enhance hydrogen production. Nevertheless, like under glucose metabolism (Maeda et al. 2007a), hydrogen productivity for both the parent and the septuple mutant strain significantly decreased when fhlA was overexpressed (Table 1). Likewise, overexpression of the subunit of formate dehydrogenase (pCA24N-FhdF) also decreased hydrogen productivity by 1.6-fold compared to the septuple mutant strain. This decrease in hydrogen production might result from the selective pressure of the antibiotic added (30 μM chloramphenicol). As previously reported, overexpressing gldA, which converts glycerol into DHA, did not increase pyruvate production, and did not enhance formate and hydrogen production (Blankschien et al. 2010; Gonzalez et al. 2008; Hu and Wood 2010; Truniger and Boos 1994). In fact, overexpression of gldA in the parent strain and in the septuple mutant strain decreased by 3.9 and 1.8-fold of hydrogen productivity, respectively.
Glycerol consumption, cell growth, product yield, and productivity
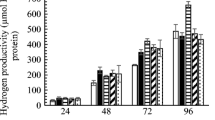
We also examined the kinetic cell growth, glycerol utilization as well as product yields of the septuple mutant strain, BW25113 frdC ldhA fdnG ppc narG mgsA hycA after 120 h. As reported previously, E. coli consumes glycerol at a very slow rate (Dharmadi et al. 2006; Shams Yazdani and Gonzalez 2008). Starting from a glycerol concentration of 10 g/L, the parent strain and the septuple mutant strain consumed glycerol in a similar rate after the first 24 h of fermentation (Fig. 2). However, strain BW25113 utilized glycerol in a faster manner compared to the engineered strain after 48 h and later due to its faster cell growth. It steadily reached the late stage of the log phase after 96 h, while the septuple mutant strain slowly grew and reached the highest cell growth after 96 h before declining (Fig. 2).
As expected, by enhancing the production of formate by blocking the metabolic pathways for the synthesis of succinate (∆frdC) and oxaloacetate (∆ppc; Fig. 1), production of these organic acids, especially succinate of the septuple mutant strain, significantly decreased by 7.2-fold compared to the wild-type strain, BW25113, after 120 h of fermentation (Fig. 3a). A slight decrease in acetate production of the mutant strain also indicated that inactivation of phosphoenolpyruvate carboxylase via disrupting ppc gene negligibly affected the synthesis of acetate and oxalate (data not shown). This was no surprise since acetate may be synthesized from other precursors such as formate and acetyl phosphate. Lactate was not detected in the fermentation broths of both BW25113 and the septuple mutant strain, indicating that D-lactate dehydrogenase (encoded by ldhA), played a minor role in the production of lactate from glycerol at an alkaline pH condition. This finding agrees with those previously reported (Sanchez-Torres et al. 2013).
Our metabolic engineering should lead to a high level of accumulated formate. However, Fig. 3b shows that formate production in the septuple mutant strain was much lower than that of strain BW25113. We speculate that accumulated formate in the septuple mutant was converted into CO2 and H2 at a higher rate compared to strain BW25113. Thus, there were lower concentrations of formate in the fermentation culture for the septuple strain.
As indicated in Fig. 1, ethanol is one of the main products from glycerol metabolism in E. coli. In agreement with a previous study (Shams Yazdani and Gonzalez 2008), the ethanol yield of the wild-type strain was close to the theoretical maximum after 48 h (0.92 mol ethanol/mol glycerol). Meanwhile, the septuple mutant strain achieved 0.88 mol ethanol/mol glycerol after 120 h of fermentation (Table 3). This was no surprise since the wild-type strain anaerobically grew faster than the mutant strain did (Fig. 2), resulting a higher rate of glycerol conversion into other products. In other aspects, a disruption in methylglyoxal synthase (encoded by mgsA), which is required for the synthesis of methylglyoxal, significantly lowered 1, 2-PDO production in the septuple mutant strain (Table 3). The existence of 1, 2-PDO in the septuple mutant is possibly derived from the conversion of dihydroxylacetone into methylglyoxal (Gonzalez et al. 2008).
Critically, after 24 h, the hydrogen yield of the septuple reached 0.67 mol/mol glycerol utilized which is 4.5-fold higher than that of the wild-type BW25113 (Table 3). The mutant strain then reached the maximum theoretical yield of 1 mol H2 formed per 1 mol glycerol consumed after 48 h. To date, this is the highest H2 yield in E. coli from glycerol at pH 7.5 and equal to those conducted at a more favorable acidic condition to co-produce H2 and ethanol (Shams Yazdani and Gonzalez 2008). In principle, the FHL complex is highly active at the acidic condition under both glucose and glycerol metabolism (Bagramyan et al. 2002; Dharmadi et al. 2006; Gonzalez et al. 2008). Meanwhile the conversion efficiency of glycerol depends on glycerol dehydrogenase and dihydroxylacetone kinase, which are most active at neutral to slightly alkaline pH (Dharmadi et al. 2006; Gonzalez et al. 2008; Truniger and Boos 1994). In addition, the intermediate products prior to hydrogen are mainly organic acids that lower intracellular pH during the fermentation. Consequently, acidic pH conditions in the later step during the fermentation would help increase hydrogen production. Therefore, we used glycerol medium starting at pH 7.5 to achieve both objectives: improvement of glycerol conversion in an early period of fermentation and increase in hydrogen production in the later stage under non-pH-controlled fermentation.
Due to the inactivation of those genes required for the synthesis of acetate (encoded by ppc) and succinate (encoded by frdC), disruption of these genes resulted in a lower product yield of acetate and succinate in the mutant strain. Nevertheless, these negligible reductions suggest that the synthesis of acetate and succinate in E. coli under glycerol metabolism did not heavily rely on the activation of phosphophenol pyruvate and fumarate reductase. The productivity rate of either product formed or glycerol utilized of both parent and mutant strain achieved maximum level after 24 h.
Hydrogen low partial pressure
To further improve, hydrogen production, we conducted a low partial pressure fermentation. Unlike under the normal condition, E. coli grew and utilized glycerol in a remarkably faster manner. After 24 h, the BW25113 and the septuple mutant strain reached the middle log phase (OD600 ∼ 0.6), which is about over 2-fold faster that that compared to those under the normal condition.
Under a low partial pressure condition, hydrogen yield and productivity of the mutant strain slightly increased from 0.67 to 0.7 mol H2/mol glycerol and from 6.6 to 6.9 mmol H2/g cell/h, respectively (Table 4). Meanwhile, the formate yield and productivity rate significantly increased over 2-fold. On the contrary, the formate yield and productivity of strain BW25113 tended to decrease which is understandable due to a higher growth rate achieved under low partial pressure conditions.
Results from organic acids quantification show that production of all main organic acids significantly improved under low partial pressure conditions (Fig. 4). Of these, formate production of strain BW25113 and the septuple mutant strain increased by 1.9 and 5.4-fold. A small amount of lactate was detected in the fermentation culture of strain BW25113 (0.03 mmol lactate/mmol glycerol), while it was not detected in those of the septuple mutant strain. These results agree with that under anaerobic and at low pH condition, E. coli converts pyruvate into D-lactate by D-lactate dehydrogenase (encoded by ldhA) (Bunch et al. 1997; Jiang et al. 2001). At the end of the fermentation, the pH became acidic (data not shown) that is a favorite environment for the expression of D-lactate dehydrogenase. Consequently, lactate was formed in the fermentation culture of the parent strain. This result indicates that disruption of ldhA would be more effectively for the hydrogen production at a low pH condition.
Discussion
We previously created a novel strain (BW24113 hyaB hybC hycA frdC ldhA fdoG aceE), which so far produced highest hydrogen production and yield from glucose (Maeda et al. 2007a). Nevertheless, this engineered strain is not suitable for producing a relatively high amount of hydrogen with fast growth rate from glycerol (Table 2). Hence, different pathways (compared to glucose) must be optimized to produce hydrogen from glycerol. Therefore, based on current knowledge on the metabolic and respiratory pathway of E. coli, we investigated various genes related to hydrogen production from glycerol to figure out what genes are beneficial.
In total, we screened the hydrogen producing capacity of 14 single mutant strains under glycerol metabolism (Fig. 1; Tables 1 and 2). We found that 10 genes, namely frdC, ldhA, fdnG, ppc, narG, focA, hyaB, mgsA, aceE, and hycA were critical for hydrogen production in E. coli (Table 2). In principle, the anaerobic respiratory pathways of formate oxidation and nitrate reduction are regulated by two enzymes formate dehydrogenase (FDH) and terminal nitrate reductase (NAR-A; Abaibou et al. 1995; Sawers et al. 1991; Sawers 2005). Inactivation of formate dehydrogenase N (encoded by fdnG) and terminal nitrate reductase (encoded by narG), consistent with those under glucose metabolism, hydrogen production of multiple mutant strains also increased (Maeda et al. 2008). It is thought that formate dehydrogenase O (encoded by fdoG) shares a similar subunit structure and amino acid sequence to formate dedydrogenase N (FDH-N). Thus, it would have a similar role in converting formate into CO2. However, inactivation of this enzyme did not improve hydrogen production when using glycerol as a substrate. Since the biochemical characterization of this enzyme is still limited, the possible reason for this may come from its location on the periplasmic side of the cytoplasmic membrane and its activation favors aerobic conditions (Abaibou et al. 1995; Jormakka et al. 2002). In fact, microbial production is inhibited by the accumulation of methyglyoxal—a toxic element synthesized by methylglyoxal synstase under anaerobic fermentation (Saikusa et al. 1987; Subedi et al. 2008; Zhu et al. 2001). Hence, we hypothesized that blocking this metabolic route might help increasing cell growth and producing more precursors for hydrogen production. Interestingly, the result showed that inactivation of methylglyoxal synthase (encoded by mgsA) enhanced about 1.2-fold hydrogen productivity. To date, this is the first report on this matter where blocking of the metabolic pathway to the synthesis of methylglycoxal could enhance hydrogen production in E. coli.
From this foundation, we conducted successive transductions aiming to create a novel strain, which is able to produce high hydrogen production as well as to sustain the cell growth. In the final stage, except for focA, hyaB, and aceE, which encode for a formate transporter, the large subunit of hydrogenase 1 and pyruvate dehydrogenase, respectively, multiple disruptions of the other genes contributed to a significant improvement in hydrogen productivity. The novel strain with defects in frdC, ldhA, fdnG, ppc, narG, mgsA, and hycA had a 5.5-fold higher hydrogen productivity rate and 4.5-fold higher hydrogen yield than that of strain BW25113 (Table 2). Although disruption of focA, hyaB, and aceE alone could enhance hydrogen production, disruption of these genes together with the other beneficial genes in the multiple mutant strains detracted from hydrogen production and cell growth. Hence, blocking some pathways may positively affect hydrogen production as single mutations, but not necessarily have the same effect in the multiple mutant strains. We speculate that in the presence of a high level of accumulated formate resulting from the disruption of the formate transporter in the septuple mutant strain led to uncoupling of the membrane potential, and thus, it was toxic to the cell growth (Beyer et al. 2013; Kirkpatrick et al. 2001; Russell and Diez-Gonzalez 1997). Meanwhile, inactivation of aceE caused less cell viability due to insufficient electron acceptors resulted from redox imbalance. In turn, both of these disruptions lowered hydrogen production in the septuple mutant strain. Under normal pressure conditions, the strain BW25113 frdC ldhA fdnG ppc narG mgsA hycA could achieve the theoretical maximum yield after 48 h (1 mol hydrogen/1 mol glycerol), while the specific maximum yield of the parent strain was just 0.88 mol hydrogen/mol glycerol after 120 h (Table 3). To our knowledge, the hydrogen yield of our engineered strain is superior to those reported previously utilizing the co-overexpression of glycerol dehydrogenase and dihydroxyacetone kinase together with the disruption of fumarate reductase at alkaline pH and is equivalent to those achieved under acidic conditions (Shams Yazdani and Gonzalez 2008).
Given that glycerol dehydrogenase (encoded by gldA) is responsible for the conversion of glycerol into glycolytic intermediates and is activated highly at slightly alkaline pH, in agreement with previous studies, our results show that individual overexpression of this enzyme does not improve hydrogen production (Fig. 1; Table 2) (Gonzalez et al. 2008; Truniger and Boos 1994). However, by co-overexpressing glycerol dehydrogenase and dihydroxyacetone kinase (encoded by dhaKLM), Shams Yazdani and Gonzalez (2008) have created a strain that has remarkably improved glycerol utilization, thus enabling it to produce higher hydrogen production. Therefore, together with such genetic disruptions, hydrogen yield and productivity of E. coli can be enhanced via co-overexpression of these two primary enzymes. We also overexpressed the FHL complex to enhance the conversion efficiency of formate into H2 and CO2 by transforming two different plasmids that constitute the FHL complex: pCA24N-FhlA and pCA24N-FdhF. Nevertheless, overexpression of these genes in both the wild type and the septupe strain lowered hydrogen production and this result is consistent with the previous report on glucose metabolism (Table 2; Maeda et al. 2007a). Possibly, IPTG, an inducer of the FHL complex, and the antibiotic (chloramphenicol) inhibited the cell growth because of their toxicity and selective pressure, respectively.
Aiming to enhance hydrogen production and yield in a shorter time course, we applied a low partial pressure assay for the glycerol metabolism. Despite the minor improvements in hydrogen yield and productivity, it is a step forward since both strains grew much faster than under the closed hydrogen assay (Table 4). Therefore, faster growth of the strain has a potential application for production at a large scale. Additionally, production of formate, a precursor of hydrogen, sharply increased up to 5.4-fold (Table 4). To some extent, hydrogen productivity and yield were not totally formate production dependent. They also depend on various factors such as intracellular pH, extracellular pH, and the diffusion of CO2 into and out of the cell (Kozliak et al. 1995; Merlin et al. 2003; Murarka et al. 2008).
In addition to the metabolic engineering approach, pH is also a critical factor for improving hydrogen production in E. coli. In general, the activation of four hydrogenases is complicated and depends on external pH, F0F1-ATPase and carbon source (Bagramyan et al. 2002; Dharmadi et al. 2006). Hyd-3 is highly active under acidic conditions, enabling it to convert more efficiently H + into H2 (Bagramyan et al. 2002; Dharmadi et al. 2006; Murarka et al. 2008; Trchounian et al. 2012). In contrast, Hyd-2 is most responsible for hydrogen production at neutral and slight alkaline pH (Trchounian and Trchounian 2009). Besides, glycerol dehydrogenase and dihydroxyacetone kinase that determine the efficiency of glycerol conversion are highly active at alkaline pH (Gonzalez et al. 2008). Therefore, to improve hydrogen yield and productivity in E. coli from glycerol, together with metabolic engineering and pressure control, the external pH should be considered. Clearly, metabolic engineering in E. coli has advantages over other hydrogen-producing microorganisms because it can reach the theoretical maximum yield, while sustaining reasonable growth. Hopefully, further improvements in terms of glycerol utilization will lead to large scale application for the producution of hydrogen.
References
Abaibou H, Pommier J, Benoit S, Giordano G, Mandrand-Berthelot MA (1995) Expression and characterization of the Escherichia coli fdo locus and a possible physiological role for aerobic formate dehydrogenase. J Bacteriol 177(24):7141–7149
Altaras NE, Cameron DC (1999) Metabolic Engineering of a 1,2-Propanediol Pathway in Escherichia coli. Appl Environ Microbiol 65(3):1180–1185
Anand P, Saxena RK (2012) A comparative study of solvent-assisted pretreatment of biodiesel derived crude glycerol on growth and 1,3-propanediol production from Citrobacter freundii. New Biotechnol 29(2):199–205
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2(2006):0008
Bagramyan K, Mnatsakanyan N, Poladian A, Vassilian A, Trchounian A (2002) The roles of hydrogenases 3 and 4, and the F0F1-ATPase, in H2 production by Escherichia coli at alkaline and acidic pH. FEBS Lett 516(1–3):172–178
Bebien M, Lagniel G, Garin J, Touati D, Vermeglio A, Labarre J (2002) Involvement of Superoxide Dismutases in the Response of Escherichia coli to Selenium Oxides. J Bacteriol 184(6):1556–1564
Beyer L, Doberenz C, Falke D, Hunger D, Suppmann B, Sawers RG (2013) Coordination of FocA and pyruvate formate-lyase synthesis in Escherichia coli demonstrates preferential translocation of formate over other mixed-acid fermentation products. J Bacteriol 195(7):1428–1435
Blankschien MD, Clomburg JM, Gonzalez R (2010) Metabolic engineering of Escherichia coli for the production of succinate from glycerol. Metab Eng 12(5):409–419
Blattner FR (1997) The Complete Genome Sequence of Escherichia coli K-12. Science 277(5331):1453–1462
Bunch PK, Mat-Jan F, Lee N, Clark DP (1997) The ldhA gene encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 143(Pt 1):187–195
Chang YY, Cronan JE Jr (1983) Genetic and biochemical analyses of Escherichia coli strains having a mutation in the structural gene (poxB) for pyruvate oxidase. J Bacteriol 154(2):756–762
Chao Y, Patnaik R, Roof WD, Young RF, Liao JC (1993) Control of gluconeogenic growth by pps and pck in Escherichia coli. J Bacteriol 175(21):6939–6944
Cherepanov P, Wackernagel W (1995) Gene disruption in Escherichia coli: Tc R and Km R cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Genes 9(0378–1119)
Cooper RA (1984) Metabolism of methylglyoxal in microorganisms. Annu Rev Microbiol 38(0066–4227):49–68
Das D, Veziroǧlu TN (2001) Hydrogen production by biological processes: a survey of literature. Int J Hydrogen Energy 26(1):13–28
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97(12):6640–6645
Deckwer W-D (1995) Microbial conversion of glycerol to 1,3-propanediol. FEMS Microbiol Rev 16(2–3):143–149
Dharmadi Y, Murarka A, Gonzalez R (2006) Anaerobic fermentation of glycerol by Escherichia coli: a new platform for metabolic engineering. Biotechnol Bioeng 94(5):821–829
Fabiano B, Perego P (2002) Thermodynamic study and optimization of hydrogen production by Enterobacter aerogenes. Int J Hydrogen Energy 27(2):149–156
Fishman A, Tao Y, Rui L, Wood TK (2005) Controlling the regiospecific oxidation of aromatics via active site engineering of toluene para-monooxygenase of Ralstonia pickettii PKO1. J Biol Chem 280(1):506–514
Gonzalez R, Murarka A, Dharmadi Y, Yazdani SS (2008) A new model for the anaerobic fermentation of glycerol in enteric bacteria: trunk and auxiliary pathways in Escherichia coli. Metab Eng 10(5):234–245
Hawkes FR, Dinsdale R, Hawkes DL, Hussy I (2002) Sustainable fermentative hydrogen production: challenges for process optimisation. Int J Hydrogen Energy 27(11–12):1339–1347
Hu H, Wood TK (2010) An evolved Escherichia coli strain for producing hydrogen and ethanol from glycerol. Biochem Biophys Res Commun 391(1):1033–1038
Jiang GR, Nikolova S, Clark DP (2001) Regulation of the ldhA gene, encoding the fermentative lactate dehydrogenase of Escherichia coli. Microbiology 147(Pt 9):2437–2446
Jormakka M, Tornroth S, Byrne B, Iwata S (2002) Molecular basis of proton motive force generation: structure of formate dehydrogenase-N. Science 295(5561):1863–1868
Kabir MM, Ho PY, Shimizu K (2005) Effect of ldhA gene deletion on the metabolism of Escherichia coli based on gene expression, enzyme activities, intracellular metabolite concentrations, and metabolic flux distribution. Biochem Eng J 26(1):1–11
Kalia VC, Jain SR, Kumar A, Joshi AP (1994) Frementation of biowaste to H2 by Bacillus licheniformis. World J Microbiol Biotechnol 10(2):224–227
Kim D, Han S, Kim S, Shin H (2006) Effect of gas sparging on continuous fermentative hydrogen production. Int J Hydrogen Energy 31(15):2158–2169
Kim S, Seol E, Oh Y-K, Wang GY, Park S (2009) Hydrogen production and metabolic flux analysis of metabolically engineered Escherichia coli strains. Int J Hydrogen Energy 34(17):7417–7427
Kirkpatrick C, Maurer LM, Oyelakin NE, Yoncheva YN, Maurer R, Slonczewski JL (2001) Acetate and formate stress: opposite responses in the proteome of Escherichia coli. J Bacteriol 183(21):6466–6477
Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E, Toyonaga H, Mori H (2005) Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12(5):291–299
Kozliak EI, Fuchs JA, Guilloton MB, Anderson PM (1995) Role of bicarbonate/CO2 in the inhibition of Escherichia coli growth by cyanate. J Bacteriol 177(11):3213–3219
Lin P-Y, Whang L-M, Wu Y-R, Ren W-J, Hsiao C-J, Li S-L, Chang J-S (2007) Biological hydrogen production of the genus Clostridium: Metabolic study and mathematical model simulation. Int J Hydrogen Energy 32(12):1728–1735
Maeda T, Sanchez-Torres V, Wood TK (2007a) Enhanced hydrogen production from glucose by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 77(4):879–890
Maeda T, Sanchez-Torres V, Wood TK (2007b) Escherichia coli hydrogenase 3 is a reversible enzyme possessing hydrogen uptake and synthesis activities. Appl Microbiol Biotechnol 76(5):1035–1042
Maeda T, Vardar G, Self WT, Wood TK (2007c) Inhibition of hydrogen uptake in Escherichia coli by expressing the hydrogenase from the cyanobacterium Synechocystis sp. PCC 6803. BMC Biotechnol 7:25
Maeda T, Sanchez-Torres V, Wood TK (2008) Metabolic engineering to enhance bacterial hydrogen production. Microb Biotechnol 1(1):30–39
Maeda T, Yoshimura T, Shimazu T, Shirai Y, Ogawa HI (2009) Enhanced production of lactic acid with reducing excess sludge by lactate fermentation. J Hazard Mater 168(2–3):656–663
Menon NK, Robbins J, Wendt JC, Shanmugam KT, Przybyla AE (1991) Mutational analysis and characterization of the Escherichia coli hya operon, which encodes [NiFe] hydrogenase 1. J Bacteriol 173(15):4851–4861
Menon NK, Chatelus CY, Dervartanian M, Wendt JC, Shanmugam KT, Peck HD, Przybyla AE (1994) Cloning, sequencing, and mutational analysis of the hyb operon encoding Escherichia coli hydrogenase 2. J Bacteriol 176(4):4416–4423
Merlin C, Masters M, McAteer S, Coulson A (2003) Why is carbonic anhydrase essential to Escherichia coli? J Bacteriol 185(21):6415–6424
Mizuno O, Dinsdale R, Hawkes FR, Hawkes DL, Noike T (2000) Enhancement of hydrogen production from glucose by nitrogen gas sparging. Bioresour Technol 73(1):59–65
Murarka A, Dharmadi Y, Yazdani SS, Gonzalez R (2008) Fermentative utilization of glycerol by Escherichia coli and its implications for the production of fuels and chemicals. Appl Environ Microbiol 74(4):1124–1135
Navarro E, Montagud A, Fernández de Córdoba P, Urchueguía JF (2009) Metabolic flux analysis of the hydrogen production potential in Synechocystis sp. PCC6803. Int J Hydrogen Energy 34(21):8828–8838
Redwood MD, Mikheenko IP, Sargent F, Macaskie LE (2008) Dissecting the roles of Escherichia coli hydrogenases in biohydrogen production. FEMS Microbiol Lett 278(1):48–55
Rossmann R, Sawers G, Bock A (1991) Mechanism of regulation of the formate-hydrogenlyase pathway by oxygen, nitrate, and pH: definition of the formate regulon. Mol Microbiol 5(11):2807–2814
Russell JB, Diez-Gonzalez F (1997) The Effects of Fermentation Acids on Bacterial Growth. In: Poole RK (ed) Adv Microb Physiol. vol Volume 39. Academic Press, pp 205–234
Saikusa T, H-i R, Watanabe K, Murata K, Kimura A (1987) Metabolism of 2-Oxoaldehydes in Bacteria: Purification and Characterization of Methylglyoxal Reductase from Escherichia coli. Agric Biol Chem 51(7):1893–1899
Sanchez-Torres V, Mohd Yusoff MZ, Nakano C, Maeda T, Ogawa HI, Wood TK (2013) Influence of Escherichia coli hydrogenases on hydrogen fermentation from glycerol. Int J Hydrogen Energy 38(10):3905–3912
Sawers G (1994) The hydrogenases and formate dehydrogenases of Escherichia coli. Antonie Van Leeuwenhoek 66(1–3):57–88
Sawers RG (2005) Formate and its role in hydrogen production in Escherichia coli. Biochem Soc Trans 33(Pt 1):42–46
Sawers G, Heider J, Zehelein E, Bock A (1991) Expression and operon structure of the sel genes of Escherichia coli and identification of a third selenium-containing formate dehydrogenase isoenzyme. J Bacteriol 173(16):4983–4993
Shams Yazdani S, Gonzalez R (2008) Engineering Escherichia coli for the efficient conversion of glycerol to ethanol and co-products. Metab Eng 10(6):340–351
Srirangan K, Pyne ME, Perry Chou C (2011) Biochemical and genetic engineering strategies to enhance hydrogen production in photosynthetic algae and cyanobacteria. Bioresour Technol 102(18):8589–8604
Subedi KP, Kim I, Kim J, Min B, Park C (2008) Role of GldA in dihydroxyacetone and methylglyoxal metabolism of Escherichia coli K12. FEMS Microbiol Lett 279(2):180–187
Suppmann B, Sawers G (1994) Isolation and characterization of hypophosphite–resistant mutants of Escherichia coli: identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol Microbiol 11(5):965–982
Takahata M, Tamura T, Abe K, Mihara H, Kurokawa S, Yamamoto Y, Nakano R, Esaki N, Inagaki K (2008) Selenite Assimilation into Formate Dehydrogenase H Depends on Thioredoxin Reductase in Escherichia coli. J Biochem (Tokyo) 143(4):467–473
Trchounian K, Trchounian A (2009) Hydrogenase 2 is most and hydrogenase 1 is less responsible for H2 production by Escherichia coli under glycerol fermentation at neutral and slightly alkaline pH. Int J Hydrogen Energy 34(21):8839–8845
Trchounian K, Sanchez-Torres V, Wood TK, Trchounian A (2011) Escherichia coli hydrogenase activity and H2 production under glycerol fermentation at a low pH. Int J Hydrogen Energy 36(7):4323–4331
Trchounian K, Pinske C, Sawers RG, Trchounian A (2012) Characterization of Escherichia coli [NiFe]-hydrogenase distribution during fermentative growth at different pHs. Cell Biochem Biophys 62(3):433–440
Truniger V, Boos W (1994) Mapping and cloning of gldA, the structural gene of the Escherichia coli glycerol dehydrogenase. J Bacteriol 176:1796–1800
Ust’ak S, Havrland B, Muñoz JOJ, Fernández EC, Lachman J (2007) Experimental verification of various methods for biological hydrogen production. Int J Hydrogen Energy 32(12):1736–1741
Vardar-Schara G, Maeda T, Wood TK (2008) Metabolically engineered bacteria for producing hydrogen via fermentation. Microb Biotechnol 1(2):107–125
Villadsen J, Nielsen J, Lidén G (2011) Bioreaction Engineering Principles, 3rd edn. Springer US, USA
Wang H, Gunsalus RP (2003) Coordinate Regulation of the Escherichia coli Formate Dehydrogenase fdnGHI and fdhF Genes in Response to Nitrate, Nitrite, and Formate: Roles for NarL and NarP. J Bacteriol 185(17):5076–5085
Wang RY, Shi ZY, Chen JC, Wu Q, Chen GQ (2012) Enhanced co-production of hydrogen and poly-(R)-3-hydroxybutyrate by recombinant PHB producing E. coli over-expressing hydrogenase 3 and acetyl-CoA synthetase. Metab Eng 14(5):496–503
Yokoi H, Mori S, Hirose J, Hayashi S, Takasaki Y (1998) H2 production from starch by a mixed culture of Clostridium butyricum and Rhodobacter sp. M-19. Biotechnol Lett 20(9):895–899
Yokoi H, Maki R, Hirose J, Hayashi S (2002) Microbial production of hydrogen from starch-manufacturing wastes. Biomass Bioenergy 22(5):389–395
Yoshida A, Nishimura T, Kawaguchi H, Inui M, Yukawa H (2005) Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Appl Environ Microbiol 71(11):6762–6768
Zhu MM, Skraly FA, Cameron DC (2001) Accumulation of methylglyoxal in anaerobically grown Escherichia coli and its detoxification by expression of the Pseudomonas putida glyoxalase I gene. Metab Eng 3(3):218–225
Acknowledgements
Special acknowledgment goes to the NBRP-E. coli at the National Institute of Genetics (Japan) for providing KEIO mutants and ASKA clones. The authors would like to thank the Japan Student Services Organization for the scholarship of K. T. Tran during this study. This research was supported by the JGC-S scholarship foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tran, K.T., Maeda, T. & Wood, T.K. Metabolic engineering of Escherichia coli to enhance hydrogen production from glycerol. Appl Microbiol Biotechnol 98, 4757–4770 (2014). https://doi.org/10.1007/s00253-014-5600-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5600-3