Abstract
l-lysine is made in an exceptional large quantity of currently 2,200,000 tons/year and belongs therefore to one of the leading biotechnological products. Production is done almost exclusively with mutants of Corynebacterium glutamicum. The increasing l-lysine market forces companies to improve the production process fostering also a deeper understanding of the microbial physiology of C. glutamicum. Current major challenges are the identification of ancillary mutations not intuitively related with product increase. This review gives insights on how cellular characteristics enable to push the carbon flux in metabolism towards its theoretical maximum, and this example may also serve as a guide to achieve and increase the formation of other products of interest in microbial biotechnology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amino acids are produced by bacteria on an exceedingly large scale. Within 10 years, the market has doubled to currently almost 5,000,000 tons. l-glutamate represents the largest product segment within the amino acid market, and this is exclusively used as monosodium glutamate in food supplements. Instead, l-lysine represents the fastest growing segment. The reason is that l-lysine together with l-threonine, l-tryptophan, and methionine serve primarily as feed additive. This assists to adjust the ratio of the amino acids in feed to ensure that all nutrients can be utilized, which otherwise would end up in the manure.
The leading position among the bacterial amino acid producers is occupied by Corynebacterium glutamicum. C. glutamicum belongs to the order Corynebacteriales, which is a clade within the Actinobacteria including Mycobacterium and Nocardia species (Gao and Gupta 2012). These Gram-positive bacteria are characterized by a periplasmic space and a cell envelope which contains arabinogalactan and mycolic acids (Mishra et al. 2011). C. glutamicum has a long tradition in biotechnology (Eggeling and Bott 2005). It is easy to grow, easy to handle genetically, generally recognized as safe (a GRAS organism), and is remarkably robust against oxygen and substrate supply oscillation as occurs in large-scale fermentations (Buchholz et al. 2014; Käß et al. 2014). All this serves that C. glutamicum is not only a producer and a platform organism in biotechnology but also a model organism to study new prominent biochemical features within the Corynebacteriales.
Whereas previous reviews and monographs treat a variety of aspects on C. glutamicum (Burkovski 2008; Eggeling and Bott 2005; Yukawa and Inui 2013), this review will focus on recent progress in l-lysine production by C. glutamicum together with new aspects on physiology and mutant screening.
The market
l-lysine is made in production plants where typically a series of bioreactors of about 500 m3 in size are operated. The main factors governing the location of the plants are the price of the carbon source and the local market. Since more than half of the l-lysine market is in China and North America and there is convenient access to maize as a feedstock material for the fermentation process, more than half of the l-lysine production capacity is located in these countries (Fig. 1).
Worldwide distribution of major l-lysine plants together with their capacity of annual l-lysine production. The capacity range of major plants is given in tons per year (http://renewablechemicals.agra-net.com). The inset shows the production increase over time (blue line, left y-axis) and the fluctuation of the l- lysine price (red line, right y-axis)
The increase in l-lysine demand over the years is significant (Fig. 1). Estimates assume that the market is currently increasing by 7 % per year. Only recently, new plants were founded, or existing capacities were expanded in the respective corn belts in China and North America, as well as in Brazil, Russia, and Indonesia. The global l-lysine industry is an oligopoly market given the high entry barriers due to high capital intensity and the technology requirements. Just a handful of global players are currently contesting the market. An overcapacity of l-lysine synthesis will result in falling prices as occurred in 2013 which may result in temporary closure of plants as occurred recently for that located in Changchun, China. Low prices and overcapacities result in the consolidation of the lysine industry accompanied by the elimination of weaker competitors. Thus, there is considerable pressure to reduce the manufacturing costs, including technological developments as well as strain developments (Kelle et al. 2005).
Efficient conversion of sugar to l-lysine requires a high yield (l-lysine per sugar), a high productivity (l-lysine per process time), as well as a high titer (l-lysine per volume). Also, the reproducibility decides on success which is in the large-scale bioreactors usually better than 97 % (Takors et al. 2007). l-lysine is provided by the different companies either as a crystalline preparation containing 98.5 % l-lysine hydrochloride, as an alkaline solution of concentrated l-lysine containing 50.7 % l-lysine, or as a lysine sulfate preparation containing 54.6 % l-lysine. The latter product consists of the entire fermentation broth conditioned by spray-drying and granulation. As easily recognizable, these processes differ significantly in investment costs, losses during downstreaming, amount of waste volume, and user friendliness. All this decides the success of the various competitors producing l-lysine. Besides the classical market for l-lysine, which are chicken and poultry feed, l-lysine is now also used as fish feed supplement in aquaculture.
Sugar uptake
The feedstocks used for industrial fermentation by C. glutamicum include glucose, fructose, and sucrose derived from starch hydrolysates of maize, cassava, or wheat. Efficient cellular availability of these sugars for metabolism is of primary importance for rapid high l-lysine formation (Fig. 2). The sugars are imported and phosphorylated by the phosphoenolpyruvate-dependent phosphotransferase carbohydrate uptake system (PTS). Recently, however, it has been discovered that glucose can be utilized by C. glutamicum in a PTS-independent manner (Ikeda et al. 2011; Lindner et al. 2011). Glucose uptake can be mediated by one of the two inositol permeases IolT1 or IolT2 (Krings et al. 2006), followed by phosphorylation of intracellular glucose by glucokinase activity (glk or ppgk). C. glutamicum strains utilizing glucose via an inositol permease show increased l-lysine production and reduced formation of by-products. It is reasonable that increased availability of the C-3 precursor phosphoenolpyruvate in the non-PTS strains contributes to a balanced supply of precursors for the l-lysine assembly line. There are indications that in addition, use of the PTS-independent glucose uptake offers an advantage in terms of cellular robustness in large-scale industrial fermentations with respect to osmotic and temperature stresses (Ikeda et al. 2011). The relevance of high sugar uptake to increase the productivity of useful substances is also known for Escherichia coli (Nishio et al. 2008).
On the left is shown the central metabolism of Corynebacterium glutamicum with specific genes, the location of their enzymes encoded, and different types of mutations used (color coded) to increase l-lysine accumulation. Metabolic pathways are simplified. Starting form oxaloacetate and pyruvate 12 reactions are necessary to result in extracellular l-lysine accumulation. Selected foci relevant for excess l-lysine formation are marked as red spheres. On the right are shown steps to obtain producers with increased performance. The basis are classical strain collections obtained by undirected mutagenesis and screenings. Engineering of strains depends largely on four lines of actions: analysis of genes and genomes, stoichiometric considerations and cytosolic pool determinations, transfer of point mutations, and finally performance of strains in large-scale fermentations
The largest contributor to variable cost in the large-scale amino acid processes is the carbon source. Since the glucose syrup of maize or wheat hydrolysate still contains 3 % or more of unhydrolyzed maltodextrins, cost efficiency is increased when these maltodextrins are also utilized for l-lysine production. Utilization of maltodextrins and even soluble starch with C. glutamicum has been achieved by expression of α-amylase (Seibold et al. 2006; Tateno et al. 2007). As novel cheap substrates, the utilization of xylose by C. glutamicum (Kawaguchi et al. 2006), as well as arabinose (Meiswinkel et al. 2013), methanol (Witthoff et al. 2013), and further carbon sources (Buschke et al. 2013; Neuner et al. 2013) is under investigation.
Provision of reducing power
A crucial point in l-lysine overproduction is an efficient NADPH supply (Fig. 2). Four moles of NADPH are required per mole of l-lysine. Early studies with phosphoglucose isomerase mutants forcing the glucose flux into the pentose phosphate pathway (PPP) indicated a strong positive effect on l-lysine accumulation. This was accompanied by a favorable reduced formation of by-products like glucose-1-phosphate-derived trehalose (Marx et al. 2003). As a direct mutation within genes of the PPP, a gnd-S361F mutation in the 6-phosphogluconate dehydrogenase was identified by whole genome sequencing of a classically obtained producer strain (Ohnishi et al. 2005). The mutant enzyme is less sensitive than the wild-type enzyme to allosteric inhibition by intracellular metabolites, such as fructose 1,6-bisphosphate or NADPH. Introduction of the gnd mutation in producers resulted in increased l-lysine accumulation (Ikeda et al. 2006). Similarly, overexpression of the zwf gene encoding glucose 6-phosphate dehydrogenase (Becker et al. 2007) and that of the entire native operon encoding glucose 6-phosphate dehydrogenase together with transaldolase, transketolase, and 6-phosphogluconolactonase confirmed to rise l-lysine formation (Becker et al. 2011). Fructose-1,6-bisphosphatase activity (fbp) was also identified as a limiting factor to obtain increased l-lysine formation (Becker et al. 2005, 2007; Georgi et al. 2005) (Fig. 2).
Whereas C. glutamicum’s native metabolism enables a maximal theoretical yield of 0.81 mol l-lysine per mol glucose, a transhydrogenase activity would push the theoretical yield to 0.86. The membrane-integral nicotinamide nucleotide transhydrogenase PntAB of E. coli drives the reduction of NADP+ via the oxidation of NADH by using the electrochemical proton gradient across the cytoplasmic membrane. The corresponding genes of E. coli were expressed in C. glutamicum, and significantly increased l-lysine concentrations were obtained (Kabus et al. 2007). In another approach to overcome the recognized NADPH availability, the native NAD-dependent glyceraldehyde 3-phosphate dehydrogenase in C. glutamicum was replaced by the nonphosphorylating NADP-dependent glyceraldehyde 3-phosphate dehydrogenase of Streptococcus mutans (Takeno et al. 2010). Use of this NADPH-generating enzyme enabled increased l-lysine formation in specific settings demonstrating effective l-lysine production upon an additional supply of NADPH. Also, the native but engineered glyceraldehyde 3-phosphate dehydrogenase of C. glutamicum was used to result in a protein accepting NADP in addition to NAD and enabling increased l-lysine formation (Bommareddy et al. 2014).
Increase of precursor supply
Since both oxaloacetate and pyruvate are precursors of l-lysine, cells synthesizing l-lysine require the replenishment of oxaloacetate by carboxylation (Fig. 2). In contrast to many other organisms, C. glutamicum possesses a PEP carboxylase (Pck) together with a pyruvate carboxylase (Pyc) as anaplerotic enzymes. In the genome of a classical producer, the Pyc-P458S protein was identified as an advantageous mutein increasing l-lysine production (Ohnishi et al. 2002), and deregulation of feedback inhibition of PEP carboxylase by aspartate and malate proved to increase l-lysine synthesis, too (Chen et al. 2013). Aside from the two C3-carboxylating enzymes, C. glutamicum possesses three C4-decarboxylating enzymes converting oxaloacetate or malate to PEP or pyruvate, i.e., PEP carboxykinase, malic enzyme, and oxaloacetate decarboxylase. The PEP carboxykinase synthesis is under control of at least four transcriptional regulators (Klaffl et al. 2013), and deletion of the enzyme leads to an increase in l-lysine formation (Petersen et al. 2001). Influencing pyruvate kinase or malic enzyme formation at the anaplerotic node had variable effects on product synthesis. It appears that a well-balanced availability of precursors for high l-lysine synthesis is necessary.
In the wild type of C. glutamicum, the molar flux of pyruvate to acetyl-CoA via pyruvate dehydrogenase activity is about 80 % relative to the molar glucose uptake flux (Bartek et al. 2011). Pyruvate dehydrogenase is part of a multienzyme complex identified in Corynebacteriales consisting of at least five proteins which has also oxo-glutarate dehydrogenase activity (Hoffelder et al. 2010; Niebisch et al. 2006). Reduction of aceE expression, encoding the E1p subunit of the pyruvate dehydrogenase, resulted in increased l-lysine synthesis and other pyruvate-derived products (Buchholz et al. 2013). It is not surprising that reduction of acetohydroxy acid synthase consuming two pyruvate molecules for l-valine synthesis also results in increased l-lysine formation (Blombach et al. 2009). With the same objective of making more precursors for l-lysine synthesis available, isocitrate dehydrogenase activity was reduced to 20 % by changing the start codon ATG to GTG (Becker et al. 2009). As a result, the l-lysine yield was increased from 0. 14 to 0.20 mol (mol glucose)−1. In another approach, in a set of strains, the citrate synthase activity was reduced gradually (van Ooyen et al. 2012). This resulted in a gradually increased l-lysine formation. The systemic analysis at the transcriptome, metabolome, and fluxome level enabled to recognize and overcome further new limitations in the l-lysine biosynthesis pathway to result in a strain accumulating l-lysine with a yield of 0.34 mol (mol glucose)−1.
Precursor assembly line
The conversion of oxaloacetate and pyruvate to l-lysine is catalyzed in a total of 11 reactions, 5 of which are known to be critical to obtain increased flux, and 2 of them are located at branch points (Fig. 2). As typical for long biosynthesis pathways, the aspartate kinase at the beginning of the pathway is feedback regulated, and a number of deregulated kinase enzymes can now be easily generated to result in increased l-lysine formation (Chen et al. 2011; Schendzielorz et al. 2013). The first branch point is the distribution of aspartate semialdehyde towards either l-homoserine or l-lysine. Reducing homoserine dehydrogenase activity by hom mutant alleles results in increased l-lysine formation (Binder et al. 2012; Ohnishi et al. 2002). The same result is obtained by increased dihydrodipicolinate synthase activity (Eggeling et al. 1998). The four-step succinylase reaction to convert piperidine-2,6-dicarboxylate to d,l-diaminopimelate can be bypassed by a one-step dehydrogenase reaction where ammonium is incorporated directly. Increase of this activity is favorable for l-lysine formation (Becker et al. 2011). In an interesting approach, the succinyl-CoA synthetase within the tricarboxylic acid cycle was deleted to have more succinyl-CoA available for the four-step succinylase reaction (Kind et al. 2013). This resulted in a yield increase from 0.12 to 0.17 mol (mol glucose)−1.
The second branch point is the distribution of d,l-diaminopimelate to either peptidoglycan or its decarboxylation to l-lysine. A second copy of lysA catalyzing the decarboxylation reaction increased l-lysine formation (Becker et al. 2011). Probably not unexpected—though identified only recently—also mutations in the d,l-diaminopimelate utilizing UDP-N-acetylmuramoyl-l-alanyl-d-glutamate:meso-diaminopimelate ligase, MurE, resulted in increased l-lysine formation (Binder et al. 2012, 2013).
It is clear that the effective limitation in a producer depends on its genetic background. An interesting tool was developed to assay for several possible limitations at once. The tool consists of an artificial l-lysine operon, OP7, consisting of dapA, dapB, asd, ddh, and lysA, encoding dihydrodipicolinate synthase, dihydrodipicolinate reductase, aspartate semialdehyde dehydrogenase, diaminopimelate dehydrogenase, and diaminopimelate decarboxylase (Blombach et al. 2009). Insertion of this operon into the chromosome of an l-lysine producer—where already increased production was obtained by reduced citrate synthase activity—localized the next target for further flux increase within the l-lysine assembly line (van Ooyen et al. 2012).
l-lysine export
Active l-lysine export is essential for production. Export is catalyzed by LysE, and no l-lysine is excreted upon its deletion. lysE expression is tightly controlled by the l-lysine recognizing transcriptional activator LysG. The natural l-lysine export activity has not yet been recognized as limiting, but strains with 2 lysE copies have been used (Blombach et al. 2009). In contrast to l-lysine, the l-threonine export by C. glutamicum is limited but can be overcome by use of E. coli exporters (Diesveld et al. 2009). This illustrates the extreme importance of export for microbial metabolite formation (Eggeling 2009).
New screening techniques
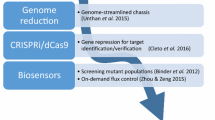
Strain development for products such as l-lysine has not yet proven possible without using classically generated strains or their mutations (Fig. 2). Random and combinatorial approaches are also indispensable for other products such as artemisinin (Tsuruta et al. 2009). Even one of the best l-lysine strains derived from the wild type by metabolic engineering and carrying 12 mutations accumulates 120 g l−1 (Becker et al. 2011), whereas with a classical strain, more than 170 g l−1 are obtained (Eggeling and Sahm 1999). A recent new screening technique expedites strain development enormously (Binder et al. 2012; Mustafi et al. 2012). It is based on i) the naturally l-lysine-sensing transcriptional activator LysG, ii) the fusion of its target gene lysE with eYFP, and iii) the fact that producers have increased l-lysine concentrations (Fig. 3). This enables the analysis of myriads of single cells via fluorescence-activated cell sorting (FACS). The system has been used to isolate a bunch of new l-lysine producers (Binder et al. 2012). Whole genome sequencing of them revealed a number of new mutations. Outstanding was the mutation murE-G81E in the d,l-diaminopimelate utilizing ligase, identifying genes of cell wall synthesis as new targets to improve l-lysine producers.
Metabolite sensors as a high-throughput tool. a The principle of the l-lysine sensor is based on the transcription factor LysG recognizing its effector l-lysine and the fusion of the LysG-target gene with eYFP as reporter. b Cells accumulating higher intracellular l-lysine concentrations and carrying the sensor exhibit increased fluorescence. c Increased l-lysine accumulation in the medium correlates with increased intracellular l-lysine concentrations. d The sensor enables to screen any library for cells with increased production via fluorescence-activated cell sorting (FACS)
The in vivo detection of the desired end-product in single cells enables also to screen plasmid libraries for mutant alleles causing increased product formation. In this manner, the rapid delivery of feedback resistant variants of aspartate kinase as well as N-acetyl-l-glutamate kinase and ATP phosphoribosyltransferase, key enzymes of l-arginine and l-histidine synthesis, respectively, was achieved (Schendzielorz et al. 2013). The realization of introducing chromosomal mutations in C. glutamicum by just using oligonucleotides (recombineering) offers even more exiting possibilities (Fig. 3). Using a mixture of 19 different oligonucleotides targeting codon 81 in murE of the wild-type productive mutants were screened and isolated (Binder et al. 2013). Sequencing revealed 12 different amino acid exchanges in the murE codon, which produced different l-lysine production titers. Thus, this combined mutation and screening technology is suitable to simply create producers as well as genetic diversity in one single step, establishing a new general concept in synthetic biology.
l-lysine beyond feed additives
In addition to its well-established role as a feed additive, l-lysine has a potential to be converted into attractive biopolymers. There are biopolymers which naturally contain l-lysine, and artificial biopolymers can be made derived from the l-lysine decarboxylation product diaminopentane.
Poly-ε-lysine is a naturally occurring polyamide of l-lysine with amide linkages between the ε-amino and α-carboxyl groups. It is biodegradable, edible, and non-toxic, and, therefore, of interest to the food and pharmaceutical industry and to medicine (Shih et al. 2006). Currently, it is mainly used as a natural food additive. It is made extracellularly by Streptomyces albulus or Streptomyces noursei and accumulating in concentrations of up to 98 g l−1 (Bankar and Singhal 2010). Another l-lysine containing biopolymer belongs to the “family of cyanophycins.” Native cyanophycins are polyamides made up of l-arginine and l-aspartate and accumulate intracellularly in selected phototrophic and heterotrophic bacteria where they serve as nitrogen, energy, and carbon storage compound. Heterologous expression of cyanophycin synthetases enabled to synthesize cyanophycin derivatives also containing l-lysine or l-citrulline (Frommeyer et al. 2014). Such polyamide derivatives could be a source of specific dipeptides with applications in medical and cosmetic purposes (Sallam and Steinbüchel 2010).
Probably most interesting is diaminopentane. Since l-lysine production is well established, and l-lysine can be easily decarboxylated by expression of l-lysine decarboxylase, correspondingly engineered C. glutamicum strains accumulate up to 88 g l−1 of diaminopentane (Kind et al. 2014). Diaminopentane is of interest as a component required for the synthesis of polyamides made from a diamine plus a dicarboxylic acid. Best known for this class of polyamides is Nylon which is made from hexamethylene diamine plus adipic acid. Currently, about 6,000,000 tonnes Nylon and its related polyamides are made annually. All this is derived from oil-based material, and the ease to produce diaminopentane with microbes opens up the opportunity to produce the specific polyamide-containing diaminopentane also by a bio-based process (Mimitsuka et al. 2007).
The road ahead
It is well understood how to direct the carbon flux in central metabolism of C. glutamicum to obtain good l-lysine producers. The increasing l-lysine demand favors the dominance of a few companies and also increases the pressure to increase efficiency of production. This relates both to efficiency in terms of yield (l-lysine per glucose), as well as in terms of productivity (l-lysine per process time). It can be easily calculated that at a price of 2 USD kg−1, a company producing 300,000 tons per year, only a 1 % increase is equal to 6 million USD extra money.
However, the better the strains become, the more difficult it is to achieve improvements in product formation. One reason is that the impact of mutations which have a positive effect on l-lysine formation, but which are not present in the central metabolism or the l-lysine assembly line, has been little investigated as yet. For instance, it is not known why in an l-lysine producer obtained by classical mutagenesis, a number of genes for the synthesis of different amino acids are overexpressed (Hayashi et al. 2006). It is also unknown why in an l-arginine producer, the respective biosynthesis genes are strongly upregulated, in a manner not achievable by plasmid overexpression (Ikeda et al. 2009). Patent literature discloses various mutations favorably influencing amino acid production without a broader understanding of their positive effect on production. Examples are oxyR, which is involved in oxidative stress response, sigA encoding the sigma factor A, or genes involved in RNA metabolism, like ssrA, sraD, or rngG. Also, DNA gyrase mutations increase amino acid production (Hayashi and Tabata 2013).
These gaps define the road ahead. One challenge is to identify and characterize new functions operative under conditions of metabolite overproduction. This might relate to RNA processing, transcription control, cell wall synthesis, or protein modification, for instance. Here, the FACS-based techniques are expected to be technology drivers, enabling supply of large collections of producers together with their genome sequences (Binder et al. 2012), and also enabling the rapid introduction of new mutations (Binder et al. 2013). An additional challenge is that industrial amino acid production is a fed-batch process (Kelle et al. 2005). Under such conditions, growth is strongly restricted, but high metabolic activity and production are requested. Molecular studies under such conditions are less frequently targeted in current research. Possibly, an even more concerted interaction of chemical engineers, molecular biologists, chemists, and mathematicians is necessary to contribute to the continuing success of the l-lysine business.
References
Bankar SB, Singhal RS (2010) Optimization of poly-epsilon-lysine production by Streptomyces noursei NRRL 5126. Bioresour Technol 101:8370–8375
Bartek T, Blombach B, Lang S, Eikmanns BJ, Wiechert W, Oldiges M, Noh K, Noack S (2011) Comparative C-13 metabolic flux analysis of pyruvate dehydrogenase complex-deficient, L-valine-producing Corynebacterium glutamicum. Appl Environ Microbiol 77:6644–6652
Becker J, Klopprogge C, Zelder O, Heinzle E, Wittmann C (2005) Amplified expression of fructose 1,6-bisphosphatase in Corynebacterium glutamicum increases in vivo flux through the pentose phosphate pathway and lysine production on different carbon sources. Appl Environ Microbiol 71:8587–8596
Becker J, Klopprogge C, Herold A, Zelder O, Bolten CJ, Wittmann C (2007) Metabolic flux engineering of L-lysine production in Corynebacterium glutamicum—over expression and modification of G6P dehydrogenase. J Biotechnol 132:99–109
Becker J, Klopprogge C, Schroder H, Wittmann C (2009) Metabolic engineering of the tricarboxylic acid cycle for improved lysine production by Corynebacterium glutamicum. Appl Environ Microbiol 75:7866–7869
Becker J, Zelder O, Hafner S, Schröder H, Wittmann C (2011) From zero to hero–design-based systems metabolic engineering of Corynebacterium glutamicumfor L-lysine production. Metab Eng 13:159–168
Binder S, Schendzielorz G, Stäbler N, Krumbach K, Hoffmann K, Bott M, Eggeling L (2012) A high-throughput approach to identify genomic variants of bacterial metabolite producers at the single-cell level. Genome Biol 13:R40
Binder S, Siedler S, Marienhagen J, Bott M, Eggeling L (2013) Recombineering in Corynebacterium glutamicum combined with optical nanosensors: a general strategy for fast producer strain generation. Nucleic Acids Res 41:6360–6369
Blombach B, Hans S, Bathe B, Eikmanns BJ (2009) Acetohydroxyacid synthase, a novel target for improvement of L-lysine production by Corynebacterium glutamicum. Appl Environ Microbiol 75:419–427
Bommareddy RR, Chen Z, Rappert S, Zeng AP (2014) A de novo NADPH generation pathway for improving lysine production of Corynebacterium glutamicum by rational design of the coenzyme specificity of glyceraldehyde 3-phosphate dehydrogenase. Metab Eng 25:30–37
Buchholz J, Schwentner A, Brunnenkan B, Gabris C, Grimm S, Gerstmeir R, Takors R, Eikmanns BJ, Blombach B (2013) Platform engineering of Corynebacterium glutamicum with reduced pyruvate dehydrogenase complex activity for improved production of L-lysine, L-valine, and 2-ketoisovalerate. Appl Environ Microbiol 79:5566–5575
Buchholz J, Graf M, Freund A, Busche T, Kalinowski J, Blombach B, Takors R (2014) CO(2)/HCO(3)(−) perturbations of simulated large scale gradients in a scale-down device cause fast transcriptional responses in Corynebacterium glutamicum. Appl Microbiol Biotechnol 98:8563–8572
Burkovski A (2008) Corynebacteria, Genomics and molecular biology. Caister Academic Press, Norfolk, p 340
Buschke N, Schäfer R, Becker J, Wittmann C (2013) Metabolic engineering of industrial platform microorganisms for biorefinery applications—optimization of substrate spectrum and process robustness by rational and evolutive strategies. Bioresour Technol 135:544–554
Chen Z, Meyer W, Rappert S, Sun J, Zeng A-P (2011) Coevolutionary analysis enabled rational deregulation of allosteric enzyme inhibition in Corynebacterium glutamicum for lysine production. Appl Environ Microbiol 77:4352–4360
Chen Z, Bommareddy RR, Frank D, Rappert S, Zeng AP (2013) Deregulation of feedback inhibition of phosphoenolpyruvate carboxylase for improved lysine production in Corynebacterium glutamicum. Appl Environ Microbiol 80:1388–1393
Diesveld R, Tietze N, Fürst O, Reth A, Bathe B, Sahm H, Eggeling L (2009) Activity of exporters of Escherichia coli in Corynebacterium glutamicum, and their use to increase L-threonine production. J Mol Microbiol Biotechnol 16:198–207
Eggeling L (2009) Microbial metabolite export in biotechnology. Wiley, Hoboken
Eggeling L, Bott M (2005) Handbook of Corynebacterium glutamicum. Taylor & Francis, Boca Raton
Eggeling L, Sahm H (1999) L-Glutamate and L-lysine: traditional products with impetuous developments. Appl Microbiol Biotechnol 52:146–153
Eggeling L, Oberle S, Sahm H (1998) Improved L-lysine yield with Corynebacterium glutamicum: use of dapA resulting in increased flux combined with growth limitation. Appl Microbiol Biotechnol 49:24–30
Frommeyer M, Wiefel L, Steinbüchel A (2014) Features of the biotechnologically relevant polyamide family “cyanophycins” and their biosynthesis in prokaryotes and eukaryotes. Crit Rev Biotechnol 30:1–12
Gao BL, Gupta RS (2012) Phylogenetic framework and molecular signatures for the main clades of the phylum actinobacteria. Microbiol Mol Biol Rev 76:66–112
Georgi T, Rittmann D, Wendisch VF (2005) Lysine and glutamate production by Corynebacterium glutamicum on glucose, fructose and sucrose: roles of malic enzyme and fructose-1,6-bisphosphatase. Metab Eng 7:291–301
Hayashi M, Tabata K (2013) Metabolic engineering for L-glutamine overproduction by using DNA gyrase mutations in Escherichia coli. Appl Environ Microbiol 79:3033–3039
Hayashi M, Ohnishi J, Mitsuhashi S, Yonetani Y, Hashimoto S-I, Ikeda M (2006) Transcriptome analysis reveals global expression changes in an industrial L-lysine producer of Corynebacterium glutamicum. Biosci Biotechnol Biochem 70:546–550
Hoffelder M, Raasch K, van Ooyen J, Eggeling L (2010) The E2 domain of OdhA of Corynebacterium glutamicum has succinyltransferase activity dependent on lipoyl residues of the acetyltransferase AceF. J Bacteriol 192:5203–5211
Ikeda M, Ohnishi J, Hayashi M, Mitsuhashi S (2006) A genome-based approach to create a minimally mutated Corynebacterium glutamicum strain for efficient L-lysine production. J Ind Microbiol Biotechnol 33:610–615
Ikeda M, Mitsuhashi S, Tanaka K, Hayashi M (2009) Reengineering of a Corynebacterium glutamicum L-arginine and L-citrulline producer. Appl Environ Microbiol 75:1635–1641
Ikeda M, Mizuno Y, Awane S, Hayashi M, Mitsuhashi S, Takeno S (2011) Identification and application of a different glucose uptake system that functions as an alternative to the phosphotransferase system in Corynebacterium glutamicum. Appl Microbiol Biotechnol 90:1443–1451
Kabus A, Georgi T, Wendisch VF, Bott M (2007) Expression of the Escherichia coli pntAB genes encoding a membrane-bound transhydrogenase in Corynebacterium glutamicum improves L-lysine formation. Appl Microbiol Biotechnol 75:47–53
Käß F, Hariskos I, Michel A, Brandt HJ, Spann R, Junne S, Wiechert W, Neubauer P, Oldiges M (2014) Assessment of robustness against dissolved oxygen/substrate oscillations for C. glutamicum DM1933 in two-compartment bioreactor. Bioprocess Biosyst Eng 37:1151–1162
Kawaguchi H, Vertes AA, Okino S, Inui M, Yukawa H (2006) Engineering of a xylose metabolic pathway in Corynebacterium glutamicum. Appl Environ Microbiol 72:3418–3428
Kelle R, Hermann T, Bathe B (2005) L-lysine production. CRC Press, Taylor & Francis Group, Boca Raton
Kind S, Becker J, Wittmann C (2013) Increased lysine production by flux coupling of the tricarboxylic acid cycle and the lysine biosynthetic pathway—metabolic engineering of the availability of succinyl-CoA in Corynebacterium glutamicum. Metab Eng 15:184–195
Kind S, Neubauer S, Becker J, Yamamoto M, Völkert M, Abendroth G, Zelder O, Wittmann C (2014) From zero to hero—production of bio-based nylon from renewable resources using engineered Corynebacterium glutamicum. Metab Eng 25:113–123
Klaffl S, Brocker M, Kalinowski J, Eikmanns BJ, Bott M (2013) Complex regulation of the phosphoenolpyruvate carboxykinase gene pck and characterization of its GntR-type regulator IolR as a repressor of myo-inositol utilization genes in Corynebacterium glutamicum. J Bacteriol 195:4283–4296
Krings E, Krumbach K, Bathe B, Kelle R, Wendisch VF, Sahm H, Eggeling L (2006) Characterization of myo-inositol utilization by Corynebacterium glutamicum: the stimulon, identification of transporters, and influence on L-lysine formation. J Bacteriol 188:8054–8061
Lindner SN, Seibold GM, Henrich A, Kramer R, Wendisch VF (2011) Phosphotransferase system-independent glucose utilization in Corynebacterium glutamicum by inositol permeases and glucokinases. Appl Environ Microbiol 77:3571–3581
Marx A, Hans S, Möckel B, Bathe B, de Graaf AA, McCormack AC, Stapleton C, Burke K, O’Donohue M, Dunican LK (2003) Metabolic phenotype of phosphoglucose isomerase mutants of Corynebacterium glutamicum. J Biotechnol 104:185–197
Meiswinkel TM, Gopinath V, Lindner SN, Nampoothiri KM, Wendisch VF (2013) Accelerated pentose utilization by Corynebacterium glutamicum for accelerated production of lysine, glutamate, ornithine and putrescine. Microb Biotechnol 6:131–140
Mimitsuka T, Sawai H, Hatsu M, Yamada K (2007) Metabolic engineering of Corynebacterium glutamicum for cadaverine fermentation. Biosci Biotechnol Biochem 71:2130–2135
Mishra AK, Driessen NN, Appelmelk BJ, Besra GS (2011) Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol Rev 35:1126–1157
Mustafi N, Grünberger A, Kohlheyer D, Bott M, Frunzke J (2012) The development and application of a single-cell biosensor for the detection of L-methionine and branched-chain amino acids. Metab Eng 14:449–457
Neuner A, Wagner I, Sieker T, Ulber R, Schneider K, Peifer S, Heinzle E (2013) Production of L-lysine on different silage juices using genetically engineered Corynebacterium glutamicum. J Biotechnol 163:217–224
Niebisch A, Kabus A, Schultz C, Weil B, Bott M (2006) Corynebacterial protein kinase G controls 2-oxoglutarate dehydrogenase activity via the phosphorylation status of the OdhI protein. J Biol Chem 281:12300–12307
Nishio Y, Usuda Y, Matsui K, Kurata H (2008) Computer-aided rational design of the phosphotransferase system for enhanced glucose uptake in Escherichia coli. Mol Syst Biol 4:160
Ohnishi J, Mitsuhashi S, Hayashi M, Ando S, Yokoi H, Ochiai K, Ikeda M (2002) A novel methodology employing Corynebacterium glutamicum genome information to generate a new L-lysine-producing mutant. Appl Microbiol Biotechnol 58:217–223
Ohnishi J, Katahira R, Mitsuhashi S, Kakita S, Ikeda M (2005) A novel gnd mutation leading to increased L-lysine production in Corynebacterium glutamicum. FEMS Microbiol Lett 242:265–274
Petersen S, Mack C, de Graaf AA, Riedel C, Eikmanns BJ, Sahm H (2001) Metabolic consequences of altered phosphoenolpyruvate carboxykinase activity in Corynebacterium glutamicum reveal anaplerotic regulation mechanisms in vivo. Metab Eng 3:344–361
Sallam A, Steinbüchel A (2010) Dipeptides in nutrition and therapy: cyanophycin-derived dipeptides as natural alternatives and their biotechnological production. Appl Microbiol Biotechnol 87:815–828
Schendzielorz G, Dippong M, Grunberger A, Kohlheyer D, Yoshida A, Binder S, Nishiyama C, Nishiyama M, Bott M, Eggeling L (2013) Taking control over control: use of product sensing in single cells to remove flux control at key enzymes in biosynthesis pathways. ACS Synth Biol 3:21–29
Seibold G, Auchter M, Berens S, Kalinowski J, Eikmanns BJ (2006) Utilization of soluble starch by a recombinant Corynebacterium glutamicum strain: growth and lysine production. J Biotechnol 124:381–391
Shih IL, Shen MH, Van YT (2006) Microbial synthesis of poly(epsilon-lysine) and its various applications. Bioresour Technol 97:1148–1159
Takeno S, Murata R, Kobayashi R, Mitsuhashi S, Ikeda M (2010) Engineering of Corynebacterium glutamicum with an NADPH-generating glycolytic pathway for L-lysine production. Appl Environ Microbiol 76:7154–7160
Takors R, Bathe B, Rieping M, Hans S, Kelle R, Huthmacher K (2007) Systems biology for industrial strains and fermentation processes—example: amino acids. J Biotechnol 129:181–190
Tateno T, Fukuda H, Kondo A (2007) Direct production of L-lysine from raw corn starch by Corynebacterium glutamicum secreting Streptococcus bovis alpha-amylase using cspB promoter and signal sequence. Appl Microbiol Biotechnol 77:533–541
Tsuruta H, Paddon CJ, Eng D, Lenihan JR, Horning T, Anthony LC, Regentin R, Keasling JD, Renninger NS, Newman JD (2009) High-level production of amorpha-4,11-diene, a precursor of the antimalarial agent artemisinin, in Escherichia coli. Plos One 4:e4489
van Ooyen J, Noack S, Bott M, Reth A, Eggeling L (2012) Improved L-lysine production with Corynebacterium glutamicum and systemic insight into citrate synthase flux and activity. Biotechnol Bioeng 109:2070–2081
Witthoff S, Mühlroth A, Marienhagen J, Bott M (2013) C1 metabolism in Corynebacterium glutamicum: an endogenous pathway for oxidation of methanol to carbon dioxide. Appl Environ Microbiol 79:6974–6983
Yukawa H, Inui M (2013) Corynebacterium glutamicum: biology and biotechnology. Springer, Heidelberg, New York
Acknowledgments
This work was funded by the German ministry of education and research (Grant no. 0315589A).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eggeling, L., Bott, M. A giant market and a powerful metabolism: l-lysine provided by Corynebacterium glutamicum . Appl Microbiol Biotechnol 99, 3387–3394 (2015). https://doi.org/10.1007/s00253-015-6508-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6508-2