Abstract
A mathematically based fed-batch bioprocess demonstrated the suitability of using a relatively cheap and renewable substrate (butyric acid) for Pseudomonas putida CA-3 high cell density cultivation. Butyric acid fine-tuned addition is critical to extend the fermentation run and avoid oxygen consumption while maximising the biomass volumetric productivity. A conservative submaximal growth rate (μ of 0.25 h−1) achieved 71.3 g L−1 of biomass after 42 h of fed-batch growth. When a more ambitious feed rate was supplied in order to match a μ of 0.35 h−1, the volumetric productivity was increased to 2.0 g L−1 h−1, corresponding to a run of 25 h and 50 g L−1 of biomass. Both results represent the highest biomass and the best biomass volumetric productivity with butyrate as a sole carbon source. However, medium chain length polyhydroxyalkanoate (mcl-PHA) accumulation with butyrate grown cells is low (4 %). To achieve a higher mcl-PHA volumetric productivity, decanoate was supplied to butyrate grown cells. This strategy resulted in a PHA volumetric productivity of 4.57 g L−1 h−1 in the PHA production phase and 1.63 g L−1 h−1over the lifetime of the fermentation, with a maximum mcl-PHA accumulation of 65 % of the cell dry weight.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Worldwide concern about uncertainty and volatility of crude oil prices together with demand for greener alternatives has increased the interest in producing biobased products using chemical and biological processes (Philp et al. 2013). Butyric acid is a carboxylic acid composed of four atoms of carbon and is one of the constituents of volatile fatty acids (VFAs). It is currently produced by oxidation of butyraldehyde produced by hydroxyformylation of propylene (a crude oil derivative) (Sudheesh et al. 2012). However, it can also be made from renewable resources using anaerobic digestion of biomass (Zhang et al. 2009; Forster-Carneiro et al. 2008; Neves et al. 2009; Selvam et al. 2010). While the majority of anaerobic digestion processes are dedicated to biogas production, VFAs are a critical intermediate in the process (Lehtomaki et al. 2008). In fact, VFA yield is a main driver for efficient production of biomethane, so the careful monitoring of VFA production is critical to a successful biomethane production process (Aymerich et al. 2013). Butyric acid can be the predominant volatile fatty acid intermediate in methane production during anaerobic digestion of biomass (Zhu et al. 2002). Given that methane can be produced very cheaply using anaerobic digestion (1.50 to 300€/t depending on the waste used) (Patterson et al. 2011) and the process can be easily manipulated to produce butyric acid, it is a very attractive starting material for bioprocesses (Cerrone et al. 2014; Zhu et al. 2002; Browne et al. 2011). Butyric acid has not been investigated as a substrate for biomass production potentially due to the fact that it is viewed as a fossil-based and inhibitory resource with other simple substrates derived from biomass taking priority (Albuquerque et al. 2010; Ng et al. 2011; Muhr et al. 2013). While one study has shown the accumulation of short chain length polyhydroxyalkanoate (PHA; PHB) using a mixture of propionic and butyric acid (Grousseau et al. 2014), no study to date has investigated butyric acid as a substrate for high cell density biomass generation and medium chain length PHA production.
While butyric acid is a readily available carbon and energy source for a variety of bacteria, it is also toxic at relatively low concentrations resulting in osmotic shock (Cheung et al. 2010). Consequently, the feeding strategy in a fed-batch fermentation needs to balance the supply of carbon for high biomass and the negative impact of substrate toxicity on growth rate. To achieve this balance, we used mathematical modelling of the fermentation which uses data from substrate utilisation, bacterial growth rate and oxygen consumption rates (Vrana Špoljaric et al. 2013).
High cell density (g L−1) and high volumetric productivity of biomass (g L−1 h−1) is a must for industrial fermentation strategies aiming for bacterial metabolite production. While some studies have employed oxygen enrichment in the air feed (up to 100 %) to increase bacterial biomass by delaying the oxygen limitation (Lee et al. 2000; Sun et al. 2009), this increases the cost of the fermentation process compared to air feeding. While increased costs can be tolerated for high value products such as pharmaceuticals or fine chemicals, PHA is proposed for use in commodity products, and so, the additional costs of enriching air with oxygen are undesirable. Thus, the current study aimed to establish a high cell density fed-batch fermentation with the air supply not enriched with oxygen.
Pseudomonas are a key target for biocatalysis as they have the capacity to produce a range of value added products such as fine chemicals and polymers (Ren et al. 2010; Wittgens et al. 2011). We investigate the use of butyric acid as a substrate for the generation of high cell density cultures of P. putida and subsequently demonstrate the usefulness of this biocatalyst for medium chain length (mcl)-PHA production.
Materials and methods
Bacterial strains
Two strains of P. putida were chosen based on their ability to grow with the short-chain fatty acid butyric acid. They were P. putida CA-3 (NCIMB 41162) (O’Connor et al. 1995) and KT2440 (Bagdasarian et al. 1981). The strains were maintained as freeze-dried (lyophilised) cultures for long-term storage and on mineral salts medium (MSM) agar plates supplemented with sodium gluconate as the sole carbon and energy source for periods of up to 2 weeks. From the MSM plates, a single colony was inoculated into 5 mL of MSM media with 20 mM sodium butyrate as C source and incubated for 18 h at 30 °C shaking. This pre-inocula (1 mL) was used to inoculate 50 mL of MSM media with 1 g L−1 NH4Cl and 40 mM of sodium butyrate as C source. Four flasks of 50 mL (200 mL in total) were used as inocula (after 20 h of growth = late exponential phase) for the batch fermenter. A range of butyric acid concentrations (10–40 mM) were used in this case. The trials were performed in triplicate for each concentration of sodium butyrate tested.
Bacterial growth medium
The minimal MSM contained (per litre) were 4.70 g (NH4)2SO4, 3.00 g MgSO4 × 7H2O, 15.00 g Na2HPO4 × 7H2O, 3.40 g KH2PO4 and trace elements as defined by Sun et al. (2007). Pure butyric acid was used as a feed (for the fed-batch process) while a salt form of sodium butyrate was used for the batch phase experiments. The inoculum (400 mL) was incubated shaking (220 rpm) for 20 h at 30 °C.
Batch mode fermenter
A Sartorius® 5-L total working volume vessel was chosen for batch fermentation. Dissolved oxygen (DO) and pH were constantly monitored for the duration of the growth period using online probes. In particular, DO was maintained using a constant flow of air (provided with an air compressor) of 2 vvm; a limit of 40 % of oxygen saturation into the media was maintained as a reference point; whenever this limit was reached, an increase in agitation rate (RPM) was activated as a cascade control. The pH was controlled automatically at 6.9 ± −0.1 by the addition of 20 % NH4OH solution (Sigma) or 15 % v/v H2SO4 (Sigma); foaming was controlled by the automatic addition of antifoam (polypropylene glycol P2000, Sigma) when required. A laptop computer was used to remotely record data every 5 s. The accumulated data were recorded into BioPAT® MFCS SCADA fermentation software (Sartorius AG, Germany). An inoculum of 400 mL was used for a 3-L working volume. Peristaltic pumps were used to precisely control feed addition.
Mathematical model development
In order to aid in the development and improvement of the fermentation process, a kinetic model was constructed to account for biomass growth along with substrate and oxygen utilisation, while the physical process of oxygen supply and mass transfer was also modelled.
Model type
An unstructured, non-segregated model was applied to this system. This type of model considers neither internal structure of cells (unstructured) nor any diversity in the cell population (non-segregated) (Nielsen and Villadsen 1992). Another key assumption of this model type is that the time constants for the biological kinetic processes are much larger than the mixing and mass transfer time constants (Roels 1983).
Rate of cell growth
The specific exponential growth rate of the cells was expressed as a function of substrate concentration using the Monod equation with an extra oxygen inhibition term added:
where, S is the concentration of the growth limiting substrate, O is the concentration of dissolved oxygen, K s is the saturation constant for this substrate, K O is the saturation constant for dissolved oxygen and \( {\mu}_{max} \) is the maximum growth rate of the cell under substrate sufficient conditions.
Rate of nutrient utilisation
The specific rate at which the substrate, oxygen and ammonium were consumed was related to the specific growth rate predicted by the Monod equation by the means of a yield coefficient (Enfors and Häggström 2000):
where q s and q o are the specific rates of consumption of substrate (S) and oxygen respectively, and Y x/s and Y x/o are the yield coefficients of biomass on substrate and oxygen respectively.
Mass balances
Mass balances were constructed to track changes in concentration over time (Enfors and Häggström 2000):
The rate of production of biomass and the rates of consumption of substrate and oxygen were related to both the growth rate and concentration of biomass present at any given time.
Oxygen mass transfer
Oxygen was fed in the form of air, which formed a dispersed gas phase in the reactor medium. The rate of mass transfer of oxygen from this dispersed gas phase to the medium was described as follows (Doran 1995):
where C L * is the saturation concentration of oxygen in the medium, C L is the instantaneous oxygen concentration in the medium and k L a is oxygen gas-liquid mass transfer coefficient.
Estimation of oxygen mass transfer coefficient (kLaO2)
The volumetric mass transfer coefficient of oxygen (k L a) from the dispersed gas phase to the continuous aqueous phase was measured experimentally via the dynamic gassing out method (Vantriet 1979).
Model assumptions
A number of assumptions were made during the model construction:
-
1.
Inhibition of growth due was assumed to occur at low dissolved oxygen levels (below ~10 %), and an oxygen limitation term was included in Eq. 1 to account for this.
-
2.
Inhibition of cellular growth by high concentrations of butyric acid was neglected in the model. The butyric acid concentration only exceeded 1 g L−1 during the very final stages of fermentation when oxygen limitation was experienced by the cells. At this point, the fermentations were ended so inhibition of growth due to the butyric acid accumulation could be neglected.
-
3.
Butyric acid is highly soluble in water, so no physical transport issues were accounted for in the model.
Model parameters
Batch data and a bank of fed-batch data were gathered in order to determine the model parameters. μ max was calculated directly from experimental batch data while K s was estimated from batch data and the value refined by fitting it to the bank of fed-batch data. Y X/S and Y X/NH4 were determined by fitting values to the bank of fed-batch data. Y X/O was also fitted to the fed-batch data, as the rate of oxygen supply was determined experimentally as described above. The final model parameters applied are shown in Table 1.
Model implementation
The model was constructed and implemented using the DynoChem (Scale-up Systems Ltd., Dublin, Ireland) computer modelling package. This package was chosen due to its solver power, built-in tools and suitability as a cross disciplinary tool. Once constructed, the ordinary differential equations were solved using the built-in Rosenbrock optimisation routine. Model parameters were fitted to the experimental batch data using the Dynochem built-in fitting routine. This routine uses the Levenberg-Marquardt algorithm to minimise the sum of squares between the model predictions and experimental data (Levenberg 1944).
Feeding strategies
There were a number of feeding strategies applied to the fed-batch fermentations performed using butyric acid as substrate. The aim of the strategies was to maximise the cell dry weight and productivity obtained. Further details about the strategies are given below.
Simple exponential feeding with a set specific growth rate
After an initial batch phase (5 h), additional substrate was fed at the rate shown in Eq. 8.
where S t is a total butyric acid required to produce biomass X t at time t, Y X/S is the yield of biomass from butyric acid, X 0 is the initial biomass and μ is the desired specific growth rate.
Novel feeding profile developed to maximise productivity
The aim of this strategy was to maintain the maximum specific growth rate for as long as possible, and then, the model was used to predict when oxygen supply would become limiting and thus the feed rate adjusted so as to reduce oxygen consumption and prolong the run.
Novel sub-maximal specific growth rate runs
As problems were encountered while trying to maintain the maximum specific growth rate when the novel strategy was imposed (further details in the results section), a number of strategies were employed which imposed lower specific growth rates over the early stages of the run. Specific growth rates of μ = 0.25, 0.30, 0.35 and 0.40 h−1 were applied at the beginning of successive runs. The aim was to identify the highest specific growth rate that could be sustained over a prolonged period. The model was then used to identify a suitable constant feed rate in order to maximise the cell dry weight yields and productivity achieved by extending the run and delaying oxygen limitation. The fatty acid mixture (butyrate/decanoate 80:20 (v/v)) was added for 14 h, after the μ = 0.35 h−1 feeding strategy was maintained to allow co-production of biomass and PHA under phosphorous limitation in the bioreactor.
Freeze-drying process
Fermentation broth was centrifuged using a continuous flow centrifuge (Heraeus Centrifuge Stratos, Thermo Fisher Scientific, Germany) at 4 °C and 25,040×g in a titan rotor (HCT22.300, Thermo Fisher Scientific, Germany). Cells were first frozen at −20 °C for 4–6 h and transferred to a −80 °C freezer (overnight) and subsequently lyophilised using a freeze-dryer system (FreeZone bulk tray dryer, Labconco, USA) until fully dried.
Polyhydroxyalkanoate extraction for gas chromatography analysis
The cell pellets arising from centrifugation of 50-mL culture samples from shaken flasks and fermentor were frozen at −80 °C and freeze dried overnight. A method of methylation/sulphuric acid extraction was used to generate methyl esters of the PHA monomers (Lageveen et al. 1988). The samples were analyzed in a Hewlett-Packard 6890 N gas-chromatograph equipped with a HP-INNOWAX capillary column (25 m × 0.25 mm, 0.32-μm film thickness; SGE Analytical Sciences) and a flame ionisation detector to detect 3-hydroxyalkanoic methyl esters with the temperature program previously described (Lageveen et al. 1988).
MCL-PHA extraction for polymer characterisation
Polymer isolation
Cells were harvested from cultivations in a Thermo Sorvall Contifuge Stratos continuous flow centrifuge (Fisher Scientific, Dublin, Ireland) at 25,040×g. Harvested cells were frozen at −80 °C for 24 h and then lyophilised (Labconco, Fisher Scientific). PHA was isolated from freeze-dried cells using Soxhlet extraction with recirculation of the chloroform solvent at 60 °C for 4 h. The supernatant was filtered using a 0.2-μm PTFE filter. Chloroform containing PHA was then subjected to rotary evaporation under vacuum until approximately 40 mL of chloroform had been recovered. The polymer was precipitated using 2 cm3 of a wash solution consisting of 35 % methanol, 35 % ethanol and 30 % distilled water (Elbahloul and Steinbuchel 2009). The supernatant was then decanted, and the precipitated PHA was allowed to dry before further analysis.
Polymer analysis
Differential scanning calorimetry (DSC) was performed as previously described to determine the glass transition temperature (Tg) and the melting temperature (Tm) of the polymer with a Perkin Elmer Pyris Diamond Calorimeter calibrated to indium standards (Kenny et al. 2008). To determine the thermal stability and decomposition profile of the samples, thermogravimetric analysis (TGA) was carried out on a Perkin Elmer Pyris 1 thermogravimetric analyser calibrated using nickel and iron standards (Kenny et al. 2008). Dynamic mechanical analysis (DMA) was carried out on a Perkin Elmer Mechanical Analyser in order to confirm the Tg. The Tg was identified by the sharp drop in storage modulus and the corresponding peak in the loss modulus (Galego et al. 2000). Molecular weight distribution was obtained by gel permeation chromatography (GPC) using PL gel 5 mm mixed C + PL gel column (Perkin Elmer) with PELV 290 UV–Vis detector set at 254 nm as previously described (Kenny et al. 2008).
Results
Batch experiments
Batch experiments were conducted in a 5-L working volume fermenter in order to examine the growth characteristics of P. putida KT2440 and CA-3 when supplied with varying concentrations of butyric acid as their sole growth substrate. Increasing concentrations (10 to 40 mM) of sodium butyrate were used as a carbon and energy source. The data arising from the batch experiments was used to determine a number of growth parameters (e.g. maximum specific growth rate and yield coefficient of biomass on substrate) of P. putida KT2440 and CA-3. The most suitable bacterium for growth with butyric acid as sole substrate was seen to be P. putida CA-3. The growth parameters determined for P. putida CA-3 from these batch experiments are shown in Table 1. Similar results were already achieved in the past (Cerrone et al. 2014). The well-known P. putida KT2440 exhibited a lower maximum specific growth rate across a range of butyric acid concentrations compared to P. putida CA-3 (Table S1). P. putida CA-3 is a better accumulator of the inorganic polymer polyphosphate (Tobin et al. 2007), and this ability could provide energy contained in the ortophosphate bonds for coping with environmental stresses (Casey et al. 2013).
Fed-batch processes
Establishing a feeding strategy for butyric acid addition
Based on the higher maximum specific growth rate and greater tolerance for butyric acid witnessed in batch experiments, P. putida CA-3 was chosen as the strain for development of a fed-batch process. The data arising from the batch experiments were used to establish the butyric acid feeding profile. This was mathematically modelled in order to provide a consistent feed to sustain the specific growth rate of P. putida CA-3 without limiting or overfeeding butyric acid.
Simple exponential feeding
From the batch data, it was observed that a maximum specific growth rate of 0.45 h−1 was theoretically possible. It was decided to impose a constant specific growth rate of 0.25 h−1 on the system by feeding as per Eq. 8. This conservative value was chosen as there was little risk of overfeeding (only 55 % of maximum specific growth rate), and process information could be gathered for the mathematical model. The fermentation lasted for 23 h, at which point oxygen limitation (followed by butyric acid accumulation) ended the run. A cell dry weight (CDW) of 28.3 g L−1 h−1 was achieved by this time. Good agreement between the model and experimental data was also found (Fig. 1). A maximum biomass generation rate (μX) of 6.5 g L−1 h−1 was also achieved. Maclean et al. (2008) achieved a maximum cell generation rate (μX) of 14 g L−1 h−1 when feeding pure nonanoic acid but supplying pure oxygen to guarantee sufficient dissolved oxygen. The only data in the literature for the growth of bacterial biomass using butyric acid in a stirred tank reactor for high biomass productivity was reported by (Grousseau et al. 2013) where a maximum of 46.7 g L−1 (as CDW) of a Cupriavidus necator population was obtained in 67.4 h (0.69 g L−1 h−1).
Growth of P. putida CA-3 on butyric acid fed exponentially to control μ = 0.25 h−1. Cell dry weight (CDW) (g L−1, triangle), butyric acid concentration (g L−1, x mark) and dissolved oxygen (%, circle) were tracked over time. The experimental data were compared to model predictions (corresponding lines)
Novel feeding profile developed to maximise bacterial biomass productivity
In order to maximise volumetric productivity, it is desirable to maintain a specific growth rate as high as possible. As such, a novel strategy, aiming at maintaining the maximum specific growth rate for as long as possible, before switching to a constant feed rate (based on the model predictions), was implemented. From the start of the fermentation, the substrate was fed so as to maintain the maximum specific growth rate until time 12 h (Fig. 2). At this point, the feed was switched to a constant rate until the end of the fermentation in order to limit the specific growth rate (and thus the oxygen consumption), prolong the run and increase the total biomass yield. The run went according to plan over the first 9 h with the experimental data matching the model predictions very well (Fig. 2). From this point on, the model and data began to diverge considerably. Growth ceased at less than 10 g L−1 and butyric acid accumulated rapidly. Subsequent repeats showed the same behaviour.
Growth of P. putida CA-3 on butyric acid fed according to a novel maximum productivity feeding profile. Cell dry weight (CDW) (g L−1, triangle), butyric acid concentration (g L−1, x mark) and dissolved oxygen (%, circle) were tracked over time. The experimental data were compared to model predictions (corresponding lines)
Novel sub-maximal specific growth rate runs
As it was suspected that prolonged growth at the maximum specific growth rate was the cause of the poor performance witnessed for the hybrid run, it was decided to perform a number of runs with lower specific growth rates applied to the early fermentation hours to see whether these runs could then be extended.
Extended μ = 0.25 h−1 run
In order to extend the run (described in Fig. 1), the exponentially increasing feed rate was switched to a constant feed rate from 20 h. This strategy delayed oxygen limitation dramatically. The fermentation time was increased from 23 to 42 h, allowing for the accumulation of more 71.3 g of bacterial biomass (dry weight) per litre, corresponding to an overall biomass volumetric productivity of 1.7 g L−1 h−1 (Fig. 3). The fermentation ended when oxygen was no longer detectable in the fermenter. The depletion of oxygen was followed by the accumulation of butyric acid and foaming out of the culture.
Growth of P. putida CA-3 on butyric acid according to a novel extended μ = 0.25 h−1 feeding strategy. Cell dry weight (CDW) (g L−1, triangle), butyric acid concentration (g L−1, x mark) and dissolved oxygen (%, circle) were tracked over time. The experimental data were compared to model predictions (corresponding lines)
Novel μ = 0.30 h−1 run
Considerable cell densities were achieved by maintaining low specific growth rates over long periods. The next step was to improve biomass productivity. This involved incorporating higher (but still sub maximal) specific growth rates into the earlier stages of the run followed by a constant feed rate after 20 h of incubation. When a specific growth rate of 0.30 h−1 was applied, it was seen that the system could accumulate 61.1 g L−1 of cell dry weight in 32 h, corresponding to an overall biomass volumetric productivity of 1.9 g L−1 h−1 (Fig. 4). After this point, the oxygen depleted below detectable levels, cells started to foam, and the fermentation was terminated.
Growth of P. putida CA-3 on butyric acid according to a novel extended μ = 0.30 h−1 feeding strategy. Cell dry weight (CDW) (g L−1, triangle), butyric acid concentration (g L−1, x mark) and dissolved oxygen (%, circle) were tracked over time. The experimental data were compared to model predictions (corresponding lines)
Novel μ = 0.35 h−1 run
The final successful step in improving biomass productivity was to impose a specific growth rate of 0.35 h−1 in the early stages of the run. It was seen that the system could accumulate only 49.2 g L−1 of cell dry weight but that it was achieved in 25 h. This resulted in an overall biomass volumetric productivity of 2.0 g L−1 h−1 (Fig. 5). As in previous fermentation runs, the fermentation was terminated when oxygen was not detectable in the bioreactor which coincided with uncontrollable foaming of the culture.
Growth of P. putida CA-3 on butyric acid according to a novel extended μ = 0.35 h−1 feeding strategy. Cell dry weight (CDW) (g L−1, triangle), butyric acid concentration (g L−1, x mark) and dissolved oxygen (%, circle) were tracked over time. The experimental data were compared to model predictions (corresponding lines)
Novel μ = 0.40 h−1 run
The final fed-batch strategy imposed a specific growth rate of 0.40 h−1 at the beginning of the run. However, this showed similar behaviour to that of the hybrid run (Fig. 2), i.e. the run went according to plan over the first ~10 h with the experimental data matching the model predictions very well (data not shown). From this point on, the model and data began to diverge considerably. Growth ceased at less than 10 g L−1, and butyric acid accumulated rapidly. Subsequent repeats showed the same behaviour.
Table 2 summarises the key fermentation results outlined above for each of the different strategies tested. The only data in the literature for the growth of bacterial biomass using butyric acid in a stirred tank reactor for high biomass productivity was reported by Grousseau et al. (2013) where a maximum of 46.7 g L−1 CDW of a C. necator population was achieved in 67 h. As can be seen in Table 2, the maximum CDW reached in the current study of 71.3 g L−1 exceeds the literature value by 52 %, and the maximum biomass productivity achieved of 2.0 g L−1 h−1 is the first high volumetric productivity value reported in the scientific literature using any bacterial species supplied with butyric acid.
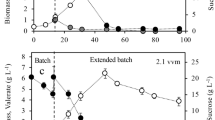
mcl-PHA production with μ = 0.35 h−1 strategy
Despite a phosphate limitation in the growth medium, butyric acid grown cells accumulated low levels of medium chain length PHA (<5 % of CDW) (data not shown). To accumulate mcl-PHA using butyric acid grown cells (μ = 0.35 h−1), we supplied decanoate as part of the butyric acid feed (ratio of butyrate/decanoate feed (v/v) = 20:80) for the mcl-PHA accumulation phase. We introduced phosphate limitation at 28, for 12 h, to induce cells to accumulate mcl-PHA. In a total fed-batch process of 40 h, 90 g L−1 CDW was obtained with 65 % of this as mcl-PHA (Fig. 6). The rate of mcl-PHA accumulation was maximally 4.57 g L−1 h−1 within the mcl-PHA production phase and 1.63 g L−1 h−1 over the full course of the fermentation. When decanoate was not supplied, the cells accumulated a maximum of 4.1 % of the cell dry weight as mcl-PHA (data not shown). Thus, the decanoic acid supplied is responsible for the 94 % of all the mcl-PHA accumulated in the 14 h co-feeding period. Furthermore, 173 g of mcl-PHA was produced from a total of 282 g of carbon supplied in the 40 h feeding period representing a 61 % carbon yield. A total of 61.6 g (69 mL) of decanoic acid was provided during the mcl-PHA production phase.
Polymer characterisation
The extracted mcl-PHA polymer from a decanoic acid/butyric acid (80:20) fed-batch fermentation was mainly composed of (R)-3-hydroxydecanoic acid and (R)-3-hydroxyoctanoic acid monomers in the same proportions (Figure S1). The melting temperature was 15 °C while Tg was −77 °C and Mw and Mn were 104,351 and 63,024 g mol−1 respectively (polydispersity index (PDI) of 1.6). The polymer produced when only butyrate was supplied was also composed predominantly of (R)-3-hydroxydecanoic acid monomers (around 50 %) with (R)-3-hydroxyoctanoic acid (maximum 12 %) (Figure S1) and (R)-3-hydroxydodecanoic acid monomers (maximum 25 %) as the major other monomers. The PHA isolated from butyrate grown cells has a similar Mw and Mn to the PHA from decanoate/butyrate grown cells. It has a melting temperature of 34 °C, and Tg was −69 °C, while its ΔH was 10.8 J g−1.
Discussion
The mathematical modelling of batch and initial fed-batch data allowed us to generate a substrate feeding strategy to greatly improve the biomass productivity. However, mcl-PHA accumulation from butyric acid was very low, and so, an alternative strategy of decanoic acid co-feeding with butyric acid was undertaken to increase mcl-PHA productivity. Given the expense of decanoic acid, a two phase (sequential) approach to biomass and PHA accumulation was undertaken with high cell densities produced first from butyric acid alone, which is three times cheaper, and subsequent use of the generated biomass as a biocatalyst for mcl-PHA accumulation.
After achieving a CDW of 28 g L−1 with a simple exponential feeding, one approach was to match the maximum growth rate with a suitable feed rate and extend the run while maintaining that feed rate. However, subsequent runs revealed that there are substrate-specific issues when butyrate is fed at a maximum feed rate. The termination of the bioprocess after 10 h when P. putida CA-3 was grown at μMAX (Fig. 2) was not due to over feeding of substrate as the butyric acid concentration in the medium was negligible and butyric acid accumulated only after cell growth had slowed down/ceased (Fig. 2). Cessation of the fermentation was also not due to substrate limitation as cells exposed to prolonged carbon limitation in the previous submaximal growth rate (μ = 0.25 h−1) grew for up to 23 h before termination of the run (Fig. 1). Dissolved oxygen was seen to be in excess (Fig. 2) with a submaximal impeller speed, suggesting remaining capacity for oxygen mass transfer. Thus, cells could not maintain the maximum specific growth rate over a prolonged period of time due to biological and not physical limitations. This behaviour was not witnessed in a previous study, with medium chain fatty acids, with P. putida where in the presence of adequate substrate, the maximum specific growth rate could be maintained until nutrient limitation or other stresses were applied to or occurred within the system (Sun et al. 2007).
Achieving a high growth rate and avoiding early termination of the bioprocess was needed to attain high biomass (g L−1) and high volumetric productivity (g L−1 h−1). A hybrid approach to substrate feeding which would result in a high (submaximal) initial growth rate followed by a lower growth rate was implemented (Figs. 3–5, Table 2). The upper threshold for the growth rate of P. putida CA-3 that would not terminate the fermentation was unknown and required empirical investigation. The hybrid run of 0.25 h−1 combined with a constant feed of substrate at 20 h demonstrated that the fermentation could be prolonged beyond 23 h and that high cell density and volumetric productivity could be achieved (Fig. 3). Runs where the initial growth rate was set at 0.30 and 0.35 h−1 resulted in increase in biomass productivity over the 0.25 h−1 runs of 12 and 18 % respectively. However, the 0.40 h−1 run ended at the 9–10-h period indicating the upper limit of the sustainable initial specific growth rate was 0.35 h−1.
C4 compounds have been investigated previously as substrates for the growth of a variety of bacteria. P. putida Gpo1 achieved a maximum biomass of 1.8 g L−1 CDW in 10 days when supplied with butane as a substrate (Johnson and Hyman 2006). Matsuyama and co-workers (2001) achieved 7.9 g L−1 CDW (0.44 g L−1 h−1) with Kluyveromyces lactis grown on 4-hydroxy-2-butanone. Finally, Grousseau et al. (2013) achieved 46.7 g L−1 CDW with butyric acid grown C. necator in 67.4 h, corresponding to 0.69 g L−1 h−1, which is 35 % of the value achieved in this study.
The current study outlines both the first high cell density and high volumetric productivity cultivation of bacterial cultures using butyric acid as a sole source of carbon and energy in a stirred tank reactor supplied with air and without oxygen enrichment. Both high cell density and high volumetric productivity of bacterial biomass are critical for industrial application of biocatalysts. Furthermore, it was also demonstrated that with the aid of a mathematical model a high cell density culture with P. putida CA-3 can be achieved with an inhibitory substrate such as butyric acid. Based on volumetric productivities of well-established anaerobic digestion systems using waste materials (e.g. municipal solid waste, slaughter house waste) butyric acid can be produced cheaply (1 to 220€/t depending on the waste source) using anaerobic digestion of biomass and wastes and thus represents a significant biobased carbon resource that could be used to replace non-biobased carbon (Cerrone et al. 2014; Browne et al. 2011). This biobased substrate can be used to produce bacterial biomass that can act as a biocatalyst for the synthesis of multiple value added products (Poblete-Castro et al. 2012). As a proof of concept for the use of this high cell density biocatalyst, we co-fed decanoate to butyrate grown cells (20:80 v/v ratio butyrate/decanoate) to produce mcl-PHA at high volumetric productivity (4.57 g L−1 h−1). This rate of mcl-PHA productivity is double the highest PHA productivities reported, for example, P. putida KT2440 (Maclean et al. 2008) obtained a volumetric productivity of PHA of 2.3 g L−1 h−1 using nonanoic acid. Based on the data supplied by Maclean et al. (2008), 899 g of nonanoic acid (611 g of carbon) were consumed by P. putida KT2440 to achieve 206 g of mcl-PHA resulting in a yield of 0.34 g of mcl-PHA/g of carbon. In the current study, the yield of PHA by P. putida CA-3 from decanoic acid/butyric acid dual feed over the course of the fermentation was 54 % higher (0.52 g of mcl-PHA/g of carbon) than that achieved by P. putida KT2440 with nonanoic acid.
Butyric acid (commercial grade) is currently at least 3-fold lower cost than decanoic acid (1,500 €/t compared to 4,500 €/t). Since the production of butyric acid from renewable and low-cost resources is now an established process using anaerobic digestion (Zhu et al. 2002), the price differential between medium chain fatty acids such as decanoic acid and butyric acid is likely to dramatically increase. Given that butyrate is cheaper than medium chain fatty acids and can be used in a dual substrate feeding process to generate a higher yield of PHA than feeding the medium chain fatty acid alone, it can be considered as an interesting substrate to generate biomass for production of PHA. Lee et al. (2000) and Maclean et al. (2008) both report the use of oxygen enriched air for high cell density cultivation and PHA production while here, we report the use of compressed air which is cheaper making the current approach economically more attractive.
Decanoic acid, as a mcl-PHA-related substrate, will be converted to 3-hydroxyalkanoyl-CoA (in the β-oxidation pathway as a substrate for the PhaC (mcl-PHA polymerase). Consequently, accumulation of mcl-PHA is immediate after the feeding of decanoic acid (Fig. 6). Just 14 h of co-feeding are enough to increase (16-fold) the mcl-PHA accumulation, compared to using butyric acid as the sole substrate. Based on the pathways through which building blocks for PHA synthesis are made in P. putida, the monomer composition of PHA accumulated by butyric acid grown cells and decanoic acid supplemented cells was as predicted, i.e. butyric acid, metabolised via de novo fatty acid synthesis, produced PHA with (R)-3-hydoxydecanoic acid as the predominant monomer (Rehm et al. 2001) and PHA accumulated in decanoic acid fed cells contained an equal mixture of (R)-3-hydroxydecanoic acid and (R)-3-hydroxyoctanoic acid due to metabolism through β-oxidation. β-oxidation is very efficient at producing intermediates for PHA production and thus the higher PHA productivity from decanoic acid-fed compared to butyric acid grown cells. The low melting temperature and Tg of the PHA produced when decanoic acid was present in the feed reflects the soft appearance of the polymer. The low Tg value is in accordance with that reported by Abe and co-workers (2012) where the presence of (R)-3-hydroxydecanoic acid is responsible for lowering the Tg compared with monomers with a lower number of carbons, which was observed for mcl-PHA isolated from cells supplied with butyrate alone. The higher melting temperature of the decanoate-derived polymer is likely due to a less diverse monomer composition compared to the PHA isolated from butyrate grown cells (Figure S1).
A mathematically developed substrate feeding approach has resulted in a high cell density culture with butyric acid; this is an essential step to producing sufficient biocatalyst. These biocatalysts can then be supplied with specific substrates to achieve high rates of bioproduct accumulation without having to also use these often expensive precursor substrates for biomass generation, e.g. mcl-PHA generated using decanoic acid.
References
Abe H, Ishii N, Sato S, Tsuge T (2012) Thermal properties and crystallization behaviours of medium-chain-length poly(3-hydroxyalkanoate)s. Polymer 53:3026–3034
Albuquerque MGE, Torres MG, Reis MAM (2010) Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: effect of the influent substrate concentration on culture selection. Water Res 44(11):3419–3433
Aymerich E, Esteban-Gutierrez M, Sancho L (2013) Analysis of the stability of high-solids anaerobic digestion of agro-industrial waste and sewage sludge. Bioresour Technol 144:107–114
Bagdasarian M, Lurz R, Rckert B, Franklin FCH, Bagdasarian MM, Frey J, Timmis KN (1981) Specific-purpose plasmid cloning vectors II. Broad host range, high copy number, RSF 1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16(1–3):237–247
Browne J, Nizami AS, Thamsiriroj T, Murphy JD (2011) Assessing the cost of biofuel production with increasing penetration of the transport fuel market: a case study of gaseous biomethane in Ireland. Renew Sust Energ Rev 15:4537–4547
Casey WT, Nikodinovic-Runic J, Fonseca-Garcia P, Guzik MW, McGrath JW, Quinn JP, Cagney G, Prieto MA, O’Connor KE (2013) The effect of polyphosphate kinase (ppk) deletion on polyhydroxyalkanoate accumulation and carbon metabolism in Pseudomonas putida KT2440. Environ Microbiol Rep 5(5):740–746
Cerrone F, Choudhari S, Davis R, Cysneiros D, O’Flaherty V, Duane G, Casey E, Guzik MW, Kenny TS, Babu PR, O’Connor KE (2014) Medium chain length polyhydroxyalkanoate (mcl-PHA) production from volatile fatty acids derived from the anaerobic digestion of grass. Appl Microbiol Biotechnol 98(2):611–620
Cheung HNB, Huang GH, Yu H (2010) Microbial-growth inhibition during composting of food waste: effects of organic acids. Bioresour Technol 101:5925–5934
Doran PM (1995) Bioprocess engineering principles. Academic, London
Elbahloul Y, Steinbuchel A (2009) Large-scale production of poly (3-hydroxyoctanoic acid) by Pseudomonas putida GPO1 and a simplified downstream process. Appl Environ Microbiol 75(3):643–651
Enfors SO, Häggström L (2000) Bioprocess technology—fundamentals and applications (a textbook for introduction of the theory and practice of biotechnical processes). Royal Institute of Technology (KTH), Stockholm
Forster-Carneiro T, Perez M, Romero LI (2008) Thermophilic anaerobic digestion of source-sorted organic fraction of municipal solid waste. Bioresour Technol 99:6763–6770
Galego N, Rozsa C, Sanchez R, Fung F, Vazquez A, Tomas JS (2000) Characterization and application of poly(β-hydroxyalkanoates) family as composite biomaterials. Polym Test 19:485–492
Grousseau E, Blanchet E, Déléris S, Albuquerque MGE, Etienne P, Uribellarea JL (2013) Impact of sustaining a controlled residual growth on polyhydroxybutyrate yield and production kinetics in Cupriavidus necator. Bioresour Technol 148:30–38
Grousseau E, Blanchet E, Déléris S, Albuquerque MGE, Etienne P, Uribellarea JL (2014) Phosphorus limitation strategy to increase propionic acid flux towards 3-hydroxyvaleric acid monomers in Cupriavidus necator. Bioresour Technol 153:206–215
Johnson EL, Hyman MR (2006) Propane and n-butane oxidation by Pseudomonas putida GPo1. Appl Environ Microbiol 72(1):950–952
Kenny ST, Nikodinovic J, Babu RP, Woods T, Blau WJ, O’Connor KE (2008) Up-cycling of PET (polyethylene terephthalate) to the biodegradable plastic PHA (polyhydroxyalkanoate). Environ Sci Technol 42:7696–7701
Lageveen R, Huisman G, Preusting H, Witholt B (1988) Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly-(R)-3-hydroxyalkanoates and poly-(R)-3-hydroxyalkanoates. Appl Environ Microbiol 54(12):2924–2932
Lee SY, Wong HH, Choi J, Lee SH, Lee SC, Han CS (2000) Production of medium-chain-length polyhydroxyalkanoates by high-cell-density cultivation of Pseudomonas putida under phosphorus limitation. Biotechnol Bioeng 68:466–470
Lehtomaki A, Huttunen S, Lehtinen TM, Rintala JA (2008) Anaerobic digestion of grass silage in batch leach bed processes for methane production. Bioresour Technol 99:3267–3278
Levenberg K (1944) A method for the solution of certain non-linear problems in least squares. Q Appl Math II 2(1):164–168
Maclean H, Sun ZY, Ramsay J, Ramsay B (2008) Decaying exponential feeding of nonanoic acid for the production of medium-chain-length poly(3-hydroxyalkanoates) by Pseudomonas putida KT2440. Can J Chem-Rev Can Chim 86(6):564–569
Matsuyama A, Yamamoto H, Kawada N, Kobayashi Y (2001) Industrial production of _R/-1,3-butanediol by new biocatalysts. J Mol Catal B Enzym 11:513–521
Muhr A, Rechberger EM, Salerno A, Reiterer A, Malli K, Strohmeier K, Schober S, Mittlebach M, Koller M (2013) Novel description of mcl-PHA biosynthesis by Pseudomonas chlororaphis from animal-derived waste. J Biotechnol 165(1):45–61
Neves L, Oliveira R, Alves MM (2009) Co-digestion of cow manure, food waste and intermittent input of fat. Bioresour Technol 100:1957–1962
Ng KS, Wong YM, Tsuge T, Sudesh K (2011) Biosynthesis and characterization of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) copolymers using jatropha oil as the main carbon source. Process Biochem 46:1572–1578
Nielsen J, Villadsen J (1992) Modelling of microbial kinetics. Chem Eng Sci 47(17–18):4225–4270
O’Connor K, Buckley CM, Hartmans S, Dobson ADW (1995) Possible regulatory role for nonaromatic carbon sources in styrene degradation by Pseudomonas putida CA-3. Appl Environ Microbiol 61:544–548
Patterson T, Esteves S, Dinsdale R, Guwy A (2011) An evaluation of the policy and techno-economic factors affecting the potential for biogas upgrading for transport fuel use in the UK. Energ Policy 39(3):1806–1816
Philp JC, Ritchie RJ, Guy K (2013) Biobased plastics in a bioeconomy. Trends Biotechnol 31(2):65–67
Poblete-Castro I, Becker J, Dohn K, Martins dos Santos V, Wittmann C (2012) Industrial biotechnology of Pseudomonas putida and related species. Mini review. Appl Microbiol Biotechnol 93:2279–2290
Rehm BHA, Mitsky TA, Steinbuchel A (2001) Role of fatty acid de novo biosynthesis in polyhydroxyalkanoic acid (PHA) and rhamnolipid synthesis by pseudomonads: establishment of the transacylase (PhaG)-mediated pathway for PHA biosynthesis in Escherichia coli. Appl Environ Microbiol 67:3102–3109
Ren Q, Ruth K, Thny-Meyer L, Zinn M (2010) Enantiomerically pure hydroxycarboxylic acids: current approaches and future perspectives. Mini-Review. Appl Microbiol Biotechnol 87(1):41–52
Roels JA (1983) Energetics and kinetics in biotechnology. Elsevier Biomedical, Amsterdam
Selvam A, Xu SY, Gu XY, Wong JWC (2010) Food waste decomposition in leachbed reactor: role of neutralizing solutions on the leachate quality. Bioresour Technol 101:1707–1714
Sudheesh N, Parmar JN, Shukla RS (2012) Hydroformylation of propene heterogeneously catalyzed by HRh(CO)(PPh3)3 encapsulated into hexagonal mesoporous silica—parametric variation and mass transfer study. Appl Catal A Gen 415–416:124–131
Sun Z, Ramsay JA, Guay M, Ramsay BA (2007) Carbon-limited fed-batch production of medium-chain-length polyhydroxyalkanoates from nonanoic acid by Pseudomonas putida KT2440. Appl Microbiol Biotechnol 74:69–77
Sun Z, Ramsay JA, Guay M, Ramsay BA (2009) Enhanced yield of medium-chain-length polyhydroxyalkanoates from nonanoic acid by co-feeding glucose in carbon-limited, fed-batch culture. J Biotechnol 143(4):262–267
Tobin KM, McGrath JW, Mullan A, O’Connor KE (2007) Polyphosphate accumulation by Pseudomonas putida CA-3 and other medium chain length polyhydroxyalkanoate accumulating bacteria under aerobic growth conditions. Appl Environ Microbiol 73:1383–1387
Vantriet K (1979) Review of measuring methods and results in nonviscous gas-liquid mass-transfer in stirred vessels. Ind Eng Chem Process Des Dev 18(3):357–364
Vrana Špoljaric I, Lopar M, Koller M, Muhr A, Salerno A, Reiterer A, Malli K, Angerer H, Strohmeier K, Schober S, Mittelbach M, Horvat P (2013) Mathematical modeling of poly[(R)-3-hydroxyalkanoate] synthesis by Cupriavidus necator DSM 545 on substrates stemming from biodiesel production. Bioresour Technol 133:482–494
Wittgens A, Tiso T, Arndt TT, Wenk P, Hemmerich J, Müller C, Wichmann R, Küpper B, Zwick M, Wilhelm S, Hausmann R, Syldatk C, Rosenau F, Blank LM (2011) Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440. Microb Cell Factories 10:80
Zhang C, Yang H, Yang F, Ma Y (2009) Current progress on butyric acid production by fermentation. Curr Microbiol 59:656–663
Zhu Y, Wu Z, Yang ST (2002) Butyric acid production from acid hydrolysate of corn fibre by Clostridium tyrobutyricum in a fibrous-bed bioreactor. Process Biochem 38:657–666
Acknowledgments
Federico Cerrone was funded by Enterprise Ireland and the Irish Industrial Development Agency (IDA) through the Technology Center for Biorefining and Bioenergy (project no. CC20090004). The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 143 kb)
Rights and permissions
About this article
Cite this article
Cerrone, F., Duane, G., Casey, E. et al. Fed-batch strategies using butyrate for high cell density cultivation of Pseudomonas putida and its use as a biocatalyst. Appl Microbiol Biotechnol 98, 9217–9228 (2014). https://doi.org/10.1007/s00253-014-5989-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5989-8