Abstract
While Inonotus obliquus produces a diverse range of bioactive metabolites in its natural habitats, it accumulates less in its submerged cultures. We show here that coculture of I. obliquus with Phellinus punctatus resulted in less production of mycelial biomass but an increased accumulation of phenolic compounds, melanins, and lanostane-type triterpenoids. Metabolites increased in production by coculture include phelligridin C, phelligridin H, methyl inoscavin A, inoscavin C, inoscavin B, davallialactone, methyl davallialactone, foscoparianol D, 21,24-cyclopentalanosta-3β,21,25-triol-8-en, lanosta-7,9(11),23-triene-3β,22,25-triol, and inotodisaccharide and melanins. Metabolites from coculture also showed an increased potential for scavenging free radicals and inhibiting the proliferation of HeLa 229 cells. Davallialactone, methyl davallialactone, and minor phenolic components are the major contributors for scavenging DPPH and hydroxyl radical in monoculture, and phelligridin C, phelligridin H, methyl inoscavin A, inoscavin C, methyl davallialactone, foscoparianol D, and inotodisaccharide are those for scavenging the tested radicals in coculture. Lanostane-type triterpenoids indicated limited roles in scavenging free radicals. Nearly all the detected metabolites correlate positively with inhibiting proliferation of HeLa 229 cells. Thus, coculture of I. obliquus with other fungi seems to be a cost-effective strategy for upregulating biosynthesis of bioactive metabolites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The medicinal fungus Inonotus obliquus (Fr.) Pilat (Hymenochaetaceae) has been used as a folk remedy in Russia and Eastern Europe for more than four centuries, where its beneficial influence on treating several human diseases, in the absence of unacceptable toxic side effects, has become established (Zheng et al. 2009c). In nature, this fungus inhabits primarily the trunks of Betula trees and forms sterile conks (sclerotia) termed “Chaga” (Campbell and Davidson 1938). Chemical investigations show that this fungus produces a diverse range of metabolites including lanostane-type triterpenoids (Shin et al. 2002), hispidin analogs (Lee and Yun 2007), and melanins (Babitskaia et al. 2000). Among these are biologically active compounds possessing hypoglycaemic, hepato-protective, antifungal, antitumor, and antiviral activities (Zheng et al. 2009c). Recently, evidence was provided that hispidin analogs were the potent antioxidant agents and the effective remedy for the protection against oxidative stress-induced human diseases including cardiovascular, neurodegenerative, and autoimmune diseases (Zheng et al. 2009b).
Geographically, however, I. obliquus is restricted to very cold habitats (45° N–50° N latitude), and it grows very slowly, suggesting that Chaga cannot be used for industrial production of these compounds (Zheng et al. 2009c). Previous attempts to grow this fungus axenically in a continuously stirring tank reactor resulted in an increased accumulation of melanins and flavonoid aglycones in the presence of H2O2, but hispidin analogs were only the minor components (Zheng et al. 2009b). Moreover, pharmacological activities of ethanol/acetone (1:1) extracts of mycelia or culture broth reached only about 50% immunostimulating effects of ethanol/acetone extracts of Chaga (Zheng et al. 2008b).
In natural habitats where the fungus is grown, I. obliquus is exposed to various environmental stresses including the attempted invasion of pathogenic microbes (Zheng et al. 2009a) and the competition for nutrient and space with other microbial species (Pettit 2009). Thus, strategies for surviving such conditions involve minimizing any stress-induced damage of lipids, protein, DNA, and attack by pathogenic microbes or antagonism from competitors. In this context, production of secondary metabolites by I. obliquus is thought to be one such defense response able to increase its competitiveness. Under laboratory culture conditions where these environmental stresses disappear, I. obliquus produces only small amounts of these biologically active metabolites (Zheng et al. 2009a). Recently, several attempts have been made to enhance the production of bioactive metabolites by growing this fungus in shake flasks under darkness (Zheng et al. 2009b) and in the presence of a fungal elicitor (cell debris of Alternaria alternata) (Zheng et al. 2009a), which resulted in an increased production of hispidin analogs together with enhanced capacities for scavenging free radicals by total phenolic compounds. Yet levels of these compounds by cultured mycelia were still less than those found in Chaga (Zheng et al. 2009c), and production of lanostane-type triterpenoids has not been well documented in submerged culture conditions.
Genomic studies indicate that certain groups of fungi have dozens of secondary metabolite pathways that are not expressed under physiological growth conditions. One approach to more fully access the metabolic potential of cultured microbes is the coculture with other microbes, where the presence of neighboring microbes may induce the synthesis of antagonistic secondary metabolites (Pettit 2009). During the studies on production of bioactive metabolites, we found that simultaneous inoculation of I. obliquus and Phellinus punctatus resulted in a significant increase in the production of melanins and phenolic compounds (unpublished data), which provoked an investigation into any possible enhancement in upregulating and diversifying pharmacologically active metabolites produced in this coculture system.
P. punctatus is one of the species in the genus Phellinus that has been recognized as a medicine in some folklore recipes to treat malicious tumors since the last century (Ying et al. 1987). It produces hispidin analogs without accumulating melanins in its natural habitats (Wang et al. 2007). In this study, we grew I. obliquus in the presence of P. punctatus and quantified the accumulation of lanostane-type triterpenoids, polyphenols, and melanins together with their capacities for scavenging free radicals and inhibiting tumor cell proliferation. Particular attention was directed at any changes in the biosynthesis of these metabolites by the culture simultaneously inoculated with I. obliquus and P. punctatus and the involvements of the metabolites in scavenging free radical and inhibiting tumor cell proliferation.
Materials and methods
Fungal materials, inoculum preparation, and conditions in submerged cultures
I. obliquus (Persoon: Fries) Pila ATCC 28281 was obtained from the fungal collection center of the Institute of Microbiology, Chinese Academy of Sciences, Beijing China. P. punctatus (0646) was purchased from the LE (BIN) culture collection of Basidiomycetes of the Komarov Botanical Institute, St. Petersburg, Russia. The two strains were maintained on malt extract agar slants containing 3% malt extract, 0.3% peptone, and 2% agar at pH 5.6. The slants were cultivated at 25°C for 2 weeks. When the mycelia overgrew the slants, they were stored at 4°C and subcultured every 3 months. The preparation of standardized inoculum was conducted as described (Zheng et al. 2009a). An aliquot of 200 ml medium containing the components identical to those described previously (Zheng et al. 2009c) in 500-ml conical flasks was inoculated with I. obliquus only (monoculture) or simultaneously with I. obliquus and P. punctatus at a ratio of 5:1 (w/w) (coculture) and incubated in the above quoted conditions for 13 days (the culture time usually covering the whole growth phases for submerged cultures of I. obliquus). Samples (20 ml) were taken 24 h (day 1) after inoculation followed by every other day up to the end of incubation and analyzed as described below.
Analytical protocols
Mycelial biomass (MB) was estimated as previously detailed (Zheng et al. 2009c). For measuring the concentrations of total mycelial phenolic compounds (TMP) and total mycelial triterpenoids (TMT), mycelial samples were washed three times with pure water and then extracted with anhydrous ethanol/acetone (1:1) using an ultrasonic cell disrupter (JY92-2D, Scientz, China) as described (Zheng et al. 2009a). Total phenol levels were measured with the Folin-Ciocalteu reagent method (Singleton and Rossi 1965) and expressed as gallic acid equivalents (GAE), using a standard curve generated with 0–80 mg/l gallic acid (Sigma, USA). Total triterpenoid concentrations were determined by the vanillic acid method as described (Lu et al. 2008), using a standard curve created with 20–200 mg/l inotodiol isolated from Chaga (collected from Betula woods located in Jingbo Lake, Mudanjiang, Northeast China) with purities more than 98%. Melanins in culture filtrates were determined as described (Zheng et al. 2009c).
Bioassay for capacities for scavenging free radicals
A total of 100 μg extract of mycelia withdrawn at different culture age was used for the bioassay of free radical scavenging. Capacities for scavenging free radicals were represented by potentials for scavenging 1,1-diphenyl-2-picrylhydrazyl (DPPH) and hydroxyl radicals, which were performed following protocols as described (Shyu and Hwang 2002; Zheng et al. 2009b). Scavenging capacities were expressed as percentage inhibition of the tested free radicals by 1 mg extracts of cultured mycelia.
Bioassay for inhibiting tumor cell proliferation
Inhibition for tumor cell proliferation was evaluated using HeLa 229 cells. HeLa 229 cells (kindly donated by tumor research center, Affiliated Hospital of Xuzhou Medical College, Xuzhou, China) were cultured using a 96-well plate at an initial density of 10 × 105 cells per milliliter in a DMEM with 10% FBS, penicillin (100 U/ml), streptomycin (100 μg/ml), and amphotericin B (0.25 μg/ml) in a humidified atmosphere of 5% CO2 at 37°C for 48 h followed by adding mycelial extracts and being incubated for another 24 h. At the end of incubation, the cells were determined for their viability using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide as described (Gieni et al. 1995). Embryo rat hepatocytes were utilized as the reference of growth inhibition by mycelial extracts against normal cells. Controls consisted of the wells containing cells without adding mycelial extracts. Growth inhibition was expressed as the percentage for inhibiting proliferation of HeLa 229 cells and rat hepatocytes, which was calculated according to the formula: \( {\hbox{inhibition rate}} = \left( {{{{1 - {\hbox{O}}{{\hbox{D}}_{\rm{sample}}}}} \left/ {{{\hbox{O}}{{\hbox{D}}_{\rm{control}}}}} \right.}} \right) \times 100\% \), where OD is the optical density at 570 nm.
NMR-based metabolomic analysis of the metabolites produced during culture process
Mycelial extracts sampled at different culture time were lyophilized, and a total of 50 mg of each was dissolved in a mixed solution of 600 μl deuterated methyl disulfide (DMSO-d6) and 50 μl of 100 mM sodium phosphate (prepared with D2O) (pH 7.4) in a nuclear magnetic resonance (NMR) tube in preparation for 1H NMR (1D and 2D) measurements following the parameters previously described (Zheng et al. 2009b). The standards included hispidin analogs, benzoic acid derivatives, and flavonoids. Hispidin analogs (with purities more than 96%) were isolated from Chaga according to the procedure as described (Zheng et al. 2009c). These consisted of inoscavins A, B, C, and D, phelligridins C, D, E, and J, davallialactone, and methyl davallialactone. The standards of benzoic acid derivatives and flavonoids and their sources were the same as previously described (Zheng et al. 2009c). Those for triterpenoids included lanosterol, inotodiol, trametenolic acid, foscoparianol D, 21,24-cyclopentalanosta-3β,21,25-triol-β-en, lanosta-7,9(11),23-triene-3β,22,25-triol, and lanosta-8,23-diene-3β,22,25-triol.
For detecting changes of metabolic profiles in the culture simultaneously inoculated with I. obliquus and P. punctatus, 1H NMR spectra were determined for data reduction and pattern recognition. In I. obliquus, proton resonances of phenolic compounds are present predominantly down field of 1H NMR spectra with chemical shifts of more than δ 6.0 ppm (Lee and Yun 2006), while those of triterpenoids are present in higher field ranging from 0.50 to 5.80 ppm. Thus, 1H NMR spectra (δ 10–0.5 ppm) were automatically data-reduced to 1,000 integral segments of equal length (δ 0.0095 ppm) using Mestrec 4.86 software (Mestrelab Research, Alicante, Spain), with each segment consisting of the integral of the NMR region to which it was associated. The data were normalized to total spectral area, and centered scaling was applied before pattern recognition analyses. Principal component analysis (PCA) was performed using a mean-centered approach with SIMCA P-11 (Umetrics, Umea, Sweden) software. Spectral filters were applied, where necessary, to remove any unrelated components and partial least squares (PLS) was used for pattern recognition. Data were displayed using the principal component (PC) score and loading plots. The quality of the models was described by R 2 and Q 2 values. R 2 is defined as the proportion of variance in the data explained by the models and indicates goodness of fit, and Q 2 is specified as the proportion of variance in the data predictable by the model and shows predictability (Trygg and Wold 2002).
Statistics
All the experiments were performed by ten independent repeats. Results from representative experiments are expressed as means ± standard deviation. Data of all experiments were analyzed by t test (SPSS 11.0). The assumptions of analysis of variance were considered to be statistically significant at p < 0.05.
Results
Accumulation of mycelial biomass and secondary metabolites
Figure 1 describes the accumulation patterns of MB, TMP, TMT, and extracellular melanins in monoculture and coculture. In monoculture, mycelial accumulation showed an exponential increase beginning at day 3 and culminating at day 11 with levels reaching nearly 7.60 ± 0.81 g/l. Similar time courses of MB accumulation were also seen in coculture, yet maximum biomass levels were reduced to 5.21 ± 0.25 g/l (Fig. 1a). In contrast, metabolites accumulated in coculture were substantially higher than those found in monoculture. In melanin production, for example, maximum level in monoculture reached only 1.23 ± 0.34 g/l but increased to 3.61 ± 0.31 g/l in coculture (Fig. 1b). Maximum TMP level in monoculture attained 29.89 ± 3.5 mg/g and enhanced to 43.91 ± 4.6 mg/g in coculture (Fig. 1c). TMT accumulation in coculture was also drastically raised in contrast to that in monoculture (Fig. 1d).
Patterns for accumulating mycelial biomass (a), melanins (b), total mycelial phenolic compounds (TMP) (c), and total mycelial triterpenoids (TMT) (d) by I. obliquus in monoculture and I. obliquus and P. punctatus in coculture. Results are the mean of ten independent experiments and error bars indicate standard deviations
1H NMR spectroscopy measurements and signal assignments
1H NMR spectroscopy of mycelial extracts differed markedly between monoculture and coculture in samples taken at different culture ages. Very weak resonances for phenolic protons appeared in extracts of I. obliquus in monoculture taken after days 3 and 5, but these were enhanced after day 7, where several broadened singlets overlapped at 7.04–7.10 ppm, representing typical resonances contributed collectively by H-9 in hispidin analogs (Lee and Yun 2006) (Fig. S1). In addition, down field singlets between 8.10 and 8.36 ppm suggested the presence of phelligridins C and H, and typical doublets with a coupling constant at 13.2 Hz at 5.76 and 5.66 ppm are consistent with those from davallialactone and methyl davallialactone (Lee and Yun 2006). Moreover, signals representing the presence of methyl inoscavins A and C and inoscavin B were also observed (Table S1). These proton signals, however, were reduced considerably after day 9, but those of methyl inoscavin A and inoscavin B appeared again at days 11 and 13 (Fig. S1b). In comparison, resonances of phenolic protons observed in monoculture at day 7 were also perceivable in coculture taken at day 3 but enhanced only after day 9 and decreased after day 11 (Fig. S1a).
Proton resonances for non-phenolic compounds were also seen both in monocultures and cocultures. Again, in Fig. S1, double doublets appeared around 3.20 ppm, and multiple singlets overlapped between 0.65 and 1.10 ppm, indicating the presence of lanostane-type triterpenoids (Taji et al. 2005). Further assignments of these resonances resulted in the tentative identification of foscoparianol D (Zheng et al. 2007b), 21, 24-cyclopentalanosta-3β, 21, 25-triol-8-en (Yusoo et al. 2001), and lanosta-7, 9(11), 23-triene-3β, 22,25-triol (Taji et al. 2005) (Table S1). Multiple signals between 3.80 and 2.80 ppm and two doublets at 4.88 and 4.28 ppm are diagnostic sugar resonances (Table S1). Further assignments of these signals in combination with the 13C NMR DEPT (135°), 1H –1H COSY, HSQC, and HMBC spectra led to the identification of inotodisaccharide, a new disaccharide constructed by α-glucose and β-glucose in 1→2 linkage (Figs. S2, S3, S4, S5, and S6). By the end of incubation, however, all the detected proton resonances were reduced. These resonances were all perceivable in coculture (Fig. S1B), but those for 21,24-cyclopentalanosta-3β,21,25-triol-8-en disappeared in monoculture (Fig. S1A).
Differences in metabolic profiles between monocultures and cocultures during culture progression
In order to evaluate the changes in metabolic profiles in coculture, pairwise comparisons were conducted to identify the metabolites responsible for the discrepancies between the monocultures and cocultures in PCA score plots as a reflection of culture age. To do this, PLS model was applied to remove non-correlated variation in X variables (1H NMR spectra) to Y variables (intensities of proton resonances), or for variability in X orthogonal to Y. Figure 2 describes the pairwise comparisons in score plots (Fig. 2a–f) at Hotelling T2 region (an eclipse defines a 95% confidence of interval of the modeled variation) and loading plots (Fig. 2g–l). In PCA loading plots, the upward sections represent metabolites that were relatively higher in production than the downward sections, whereas the downward sections reveal metabolites that were higher in production than the upward.
PCA score (a–f) and loading plots (g–l) derived from the 1H NMR spectra of the extracts of mycelia from monoculture and coculture, demonstrating their differences in metabolite profiles. t[1] and t[2] are the scores for first and second principal component, respectively; p[1] is the loading of the first principal component. 1, phelligridin C; 2, phelligridin H; 3, methyl inoscavin A; 4, inoscavin C; 5, inoscavin B; 6, davallialactone; 7, methyl davallialactone; 8, foscoparianol D; 9, 21,24-cyclopentalanosta-3β,21,25-triol-8-en; 10, lanosta-7,9(11),23-triene-3β,22,25-triol; 11, inotodisaccharide; d, day
Quantitative differentiations of metabolites at day 3 arose from a slightly higher accumulation of phelligridin C and H, inoscavin C, davallialactone, and methyl davallialactone and dramatically higher accumulation of foscoparianol D, lanosta-7,9(11),23-triene-3β,22,25-triol, and inotodisaccharide in extracts from coculture (Fig. 2a, g). These metabolic profiles were changed at day 5 by an enhanced production of phelligridin C, davallialactone, and methyl davallialactone in monoculture and phelligridin H, inoscavin C, foscoparianol D, and inotodisaccharide in coculture (Fig. 2b, h). A substantial increase was seen in the production of methyl inoscavin A and inotodisaccharide, together with unidentified phenolic compounds resonating at 7.47, 7.62, and 8.03 ppm in monoculture, and inoscavin C, davallialactone, 21,24-cyclopentalanosta-3β,21,25-triol-8-en, and lanosta-7,9(11),23-triene-3β,22,25-triol together with unknown compounds resonating at 5.96, 6.22, 6.43, 6.59, 6.61, 7.85, and 8.20 ppm in coculture (Fig. 2c, i). At the later stage of coculture enhanced production of phelligridin H, methyl inoscavin A, davallialactone, foscoparianol D, lanosta-7,9(11),23-triene-3β,22,25-triol, 21,24-cyclopentalanosta-3β,21,25-triol-8-en, and inotodisaccharide was found at days 9 (Fig. 2d, j) and 11 (Fig. 2e, k) and methyl inoscavin A, inoscavins C and B, foscoparianol D, 21,24-cyclopentalanosta-3β,21,25-triol-8en, lanosta-7,9(11),23-triene-3β,22,25-triol, and inotodisaccharide at day 13 (Fig. 2f, l). For day-to-day pairwise comparisons from the PCA model, differences between monoculture and coculture over the culture period showed high goodness of fit and predictability with R 2 values from 0.91 to 0.96 and Q 2 values from 0.71 to 0.93.
Metabolites contributing to free-radical scavenging and tumor cytotoxicity
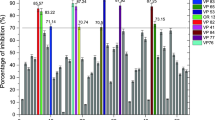
Figure 3 describes the capacities for scavenging free radicals and inhibiting the proliferation of HeLa 229 cells and rat hepatocytes by metabolites produced in monoculture and coculture. Extracts from mycelia taken at different culture time all possessed potentials for scavenging tested free radicals. For scavenging DPPH, potentials by extracts from monoculture increased time-dependently from 11 days onwards with peak levels reaching 765.89%/mg, while those enhanced from 9 days onwards with culminating levels reaching 997.69%/mg. Similar time-coursed patterns for scavenging hydroxyl radical were also observed, where the peak levels all culminated at day 9, reaching 596.45%/mg and 786.97%/mg in monoculture and coculture, respectively.
Capacities for scavenging free radicals and inhibiting proliferation of HeLa 229 cells and rat hepatocytes by ethanol/acetone (1:1, v/v) extracts of mycelia from monoculture and coculture. Results are the mean of ten independent experiments and error bars indicate standard deviations. a Capacities for scavenging DPPH; b capacities for scavenging hydroxyl radical; c inhibition for Hela 229 cells; d inhibition for rat’s hepatocytes. Capacities for scavenging free radical were expressed by 1 mg extract, while inhibition rates were calculated by 100 μg extracts against 10 × 105 Hela 229 cells per milliliter and 10 × 105 hepatocytes per milliliter, respectively
Extracts from mycelia withdrawn at different culture age also possessed time-dependent potential for inhibiting the growth of HeLa 229 cells from 9 days onwards in coculture and 11 days onwards in monoculture with peak levels at 59.8% and 46.7%, respectively (Fig. 3c). In contrast, these extracts did not show evident inhibition to the growth of rat hepatocytes (Fig. 3d), implying that mycelial extracts both from monoculture and coculture, withdrawn at different culture age, possessed higher selective toxicity to tumor cells.
To determine the metabolites involved in scavenging free radicals and inhibiting the growth of HeLa 229 cells, a PLS model was applied, in which correlations between scavenging potentials or inhibiting the growth of HeLa 229 cells and 1H NMR spectra were calculated. As shown in Fig. 4, the resultant score plots (Fig. 4a–d) all showed clear separations between samples taken at different incubation times. The first two PCs explained 89.74% of the total variance of X variables (1H NMR spectra) and 86.19% of the total variance of the Y variables (scavenging potentials) with R 2 and Q 2 values of 0.85 and 0.78, respectively (Fig. 4a), for scavenging DPPH, and 81.40% of the X and 87.11% of the Y with R 2 and Q 2 values of 0.87 and 0.81, respectively, for scavenging hydroxyl radicals by extracts from monoculture (Fig. 4c). Those explained 88.23% of the X and 92.41% of the Y with R 2 and Q 2 values of 0.92 and 0.89, respectively, for scavenging DPPH (Fig. 4b) and 91.34% of the X and 96.73% of the Y with R 2 and Q 2 values of 0.97 and 0.91, respectively, for scavenging hydroxyl radical by extracts from coculture (Fig. 4d). The PLS loading plots (Fig. 4e–h) demonstrated the metabolites contributing to scavenging the two tested free radicals. In monoculture, polyphenols, foscoparianol D, and 21,24-cyclopentalanosta-3β,21,25-triol-8-en together with several unidentified lanostane-type triterpenoids were positively associated with scavenging hydroxyl radical (Fig. 4e) and DPPH (Fig. 4g), respectively; whereas lanosta-7,9(11),23-triene-3β,22,25-triol, inotodisaccharide, and some unidentified triterpenoids were negatively involved in scavenging these free radicals. In coculture, hispidin analogs phelligridins C and H, methyl inoscavin A, inoscavin C, davallialactone, and methyl davallialactone and inotodisaccharide were the major contributors for scavenging hydroxyl radical (Fig. 4f), and phelligridin H, davallialactone, methyl davallialactone, and inotodisaccharide for DPPH (Fig. 4h); foscoparianol D, 21,24-cyclopentalanosta-3β,21,25-triol-8-en, and lanosta-7,9(11),23-triene-3β,22,25-triol were all negatively involved. For inhibiting proliferation of HeLa 229 cells, the resultant score plots also suggested unambiguous separations between the samples withdrawn at different culture time. The first two PCs explained 88.4% of the total variance of X variables (1H NMR spectra) and 91.2% of the total variance of the Y variables (growth inhibition rate) with R 2 and Q 2 values of 0.89 and 0.81, respectively, by extracts from monoculture (Fig. 5a) and 0.94 and 0.91, respectively, by those from coculture (Fig. 5b). The PLS loading plots showed that nearly all the metabolites, both from monoculture and coculture, correlate positively with its growth inhibition against HeLa 229 cells (Fig. 5c, d). The goodness of fit of this model was further evidenced by performing predictability of the scavenging potential for the tested free radicals and inhibition capacity for the growth of tumor cells, and the results coincided well with the observed scavenging (Fig. S7a–d) and inhibiting capacity (Fig. S7e, f).
PLS scores (a–d) and loading plots (e–h) derived from 1H NMR spectra of the extracts of mycelia from monoculture and coculture, demonstrating metabolites involved in scavenging DPPH and hydroxyl radicals. a and e and c and g, scavenging DPPH radical by extracts from monoculture; b and g and d and h scavenging hydroxyl radical by the extracts from coculture. t[1] and t[2] are the scores for first and second principal component, respectively; w*c [1] is the weights of the first principal component, indicating the contribution to the biological activities. 1, phelligridin C; 2, phelligridin H; 3, methyl inoscavin A; 4, inoscavin C; 5, inoscavin B; 6, davallialactone; 7, methyl davallialactone; 8, foscoparianol D; 9, 21,24-cyclopentalanosta-3β,21,25-triol-8-en; 10, lanosta-7,9(11),23-triene-3β,22,25-triol; 11, inotodisaccharide; d, day
PLS scores (a–b) and loading plots (c–d) derived from 1H NMR spectra of extracts of mycelia from monoculture and coculture, demonstrating metabolites involved in inhibiting proliferation of HeLa 229 cells. a and c are the score and loading plots by the extracts from monoculture; b and d are those from coculture. t[1] and t[2] are the scores for first and second principal component, respectively; w*c is the weights of the principal component
Discussion
The data presented here showed that coculture of I. obliquus and P. punctatus resulted in a reduced production of mycelial biomass, but an increased accumulation of phenolic compounds, melanins, and lanostane-type triterpenoids. Mycelial extracts from coculture suggested an increased potential for scavenging free radicals and inhibiting the growth of HeLa 229 cells. Davallialactone, methyl davallialactone, and minor phenolic components were the major contributors for scavenging DPPH and hydroxyl radical in monoculture and phelligridins C and H, methyl inoscavin A, inoscavin C, davallialactone, methyl davallialactone, and inotodisaccharide for scavenging these tested radicals in coculture. Lanostane-type triterpenoids indicated limited roles in scavenging free radicals but evident contribution in inhibiting the growth of HeLa 229 cells.
It is believed that potentially interesting gene clusters can possibly be expressed to produce metabolites that increase competitiveness in natural environments (Pettit 2009). The absence of environmental growth limitations under laboratory conditions might be the reasons that silence these gene clusters, leading to a decreased production of bioactive metabolites in the vast majority of fungi grown in submerged cultures. This might also be responsible for the differences in production of secondary metabolites between cultured mycelia of I. obliquus and Chaga. Simultaneous inoculation of the two microbes will lead to the competition for the nutrients and space, and antagonistic substances are released to inhibit the growth of each other (Pettit 2009). In natural habitats, P. punctatus does not produce melanins (Zjawiony 2004). In this study, the presence of P. punctatus in submerged culture of I. obliquus also enhanced the activities of PAL, TAL, and HMGR (Fig. S8), the key enzymes responsible for the metabolisms of phenolic compounds, melanins, and lanostane-type triterpenoids, respectively (Zheng et al. 2008a, 2009a, c). These seem to articulate that the gene clusters in I. obliquus or P. punctatus expressing metabolites for the defense against microbial invasion in natural habitats can also be activated when these two fungi are cocultured. The most conspicuous feature of this coculture system is the dramatically increased production of melanins. Fungal melanins possess structure domains of ortho-quinones, hydroquinone, and semiquinones, able to act as electron acceptors, donors, or both, and have been demonstrated to be the most powerful antioxidants (Shcherba et al. 2000) that protect microorganisms from oxidative damage and resist the attack of host immune system. This might underpin the successful invasion of many human pathogenic melanin-producing fungi (Langfelder et al. 2003). Thus, overproduction of melanins might be one of the reasons for the reduction of mycelial biomass. It has been well established that phenolic compounds particularly hispidin analogs in I. obliquus and P. punctatus are effective scavengers for free radicals (Zheng et al. 2009a) capable of protecting proteins, lipids, and DNA from oxidative damage (Park et al. 2004). In potato dextrose agar plates, simultaneous inoculation of these two fungi resulted in mutual antagonism and in the production of large quantities of secondary metabolites in the border of mycelia of the two fungi (data not shown). Owing to the mutual antagonism between I. obliquus and P. punctatus, production of antioxidant polyphenols seems to be another line of defense for cell growth. Lanostane-type triterpenoids also inhibited microbial replication (Mothana et al. 2000). Thus, enhanced production of these metabolites is one of the consequences for these incompatible interactions between the two fungi. In this context, enhanced production of secondary metabolites inevitably reduces the involvements of carbon and nitrogen source for mycelial growth, which may also underlie less accumulation of mycelial biomass in coculture.
It has been proposed that low O–H bond dissociation enthalpy is necessary to facilitate the H abstraction from a radical (Zhang et al. 2003) and the presence of a –OH group in the O-phenol moiety tends to lower the bond dissociation enthalpy value by stabilizing the formed radical and thereby enhancing the antioxidant activity (Wright et al. 2001). Antioxidant activity is also affected by intra-molecular hydrogen bonds that can exist in the catechol moiety and inter-molecular hydrogen bonds between these functional groups that contain polar protonic solvents (Jung et al. 2008). Correlation between metabolic profiles and antioxidant activity showed that polyphenols, primarily hispidin analogs possessing catechol moieties, were positively associated with the capacity for scavenging the tested free radicals both in monoculture and coculture, which coincided well with the data reported previously (Jung et al. 2008). Thus, polyphenols in the extracts, particularly those with catechol moieties, are the major contributors for scavenging free radicals.
Evidences have also shown that phenolic compounds and lanostane-type triterpenoids are the active constituents able to inhibit proliferation of tumor cells (Zheng et al. 2007a) or possessing antitumor-promoting activities (Taji et al. 2005). Correlation between metabolic profiles and inhibition for tumor cell growth suggested that polyphenols and lanostane-type triterpenoids were all positively involved in inhibiting proliferation of HeLa 229 cells, reinforcing that these metabolites were the antitumor constituents.
Metabolic analysis designed to evaluate the advantages of coculture over monoculture requires a rapid and accurate determination of key metabolites. Metabolomic approaches, capable of measuring the dynamic multiparametric responses of living systems to the internal and external influences, are claimed to evaluate comprehensively multiparametric metabolic responses to all pathophysiological stimuli and genetic modification (Gao et al. 2008). As one of the major techniques in metabonomics, NMR spectroscopy has the disadvantage of low detection limits but possesses many other advantages over HPLC/MS in that measurements are non-destructive and non-selective. It is also feasible to acquire profiles of a comprehensive range of organic metabolites (Li et al. 2007). In this study, NMR-based metabolomic analysis of mycelial extracts leads to the identification of metabolites that differed monoculture and coculture and the metabolites involved positively or negatively in free-radical scavenging and tumor cell growth inhibition, demonstrating clearly the enhanced biosynthesis of hispidin analogs and lanostane-type triterpenoids by this cocultured system. Yet, in NMR spectroscopy, proton resonances of melanins are not detectable in mycelial extracts or culture filtrates. This might be one of the limitations of NMR in determining some polymers and the reasons that numerous unidentified weak resonances were also the components contributing to scavenging free radical and inhibiting tumor cell proliferation.
This study has examined the production of secondary metabolites by coculture of I. obliquus and P. punctatus and their antioxidant activity and tumor cytotoxicity. Further experiments are needed to determine the metabolites produced by each fungus and the substances responsible for inducing the enhanced production of the stated metabolites and mechanisms of action. Regardless, this study uncovered for the first time the changes of metabolic profiles in the culture simultaneously inoculated with I. obliquus and P. punctatus and the components involved positively in scavenging free radicals and inhibiting proliferation of tumor cells using NMR-based metabolomic analysis. Moreover, increased inhibition to tumor cell growth by the extracts from coculture of these two fungi provided optimistic prospects for producing bioactive metabolites.
References
Babitskaia VG, Shcherba VV, Ikonnikova NV (2000) Melanin complex of the fungus Inonotus obliquus. Prikl Biokhim Mikrobiol 36:439–444
Campbell WA, Davidson RW (1938) A Poria as the fruiting stage of the fungus causing the sterile conks on birch. Mycologia 30:553–560
Gao XX, Ge HM, Zheng WF, Tan RX (2008) NMR-based metabonomics for detection of Helicobacter pylori infection in gerbils: which is more descriptive? Helicobacter 13:103–111
Gieni RS, Yan L, Hayglass KT (1995) Comparison of [3H] thymidine incorporation with MTT- and MTS-based bioassays for human and murine IL-2 and IL-4 analysis tetrazolium assays provide markedly enhanced sensitivity. J Immunol Meth 187:85–93
Jung JY, Lee IK, Seok SJ, Lee HJ, Kim YH, Yun BS (2008) Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J Appl Microbiol 104:1824–1832
Langfelder K, Streibel M, Jah B, Haase G, Brakhage AA (2003) Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet Biol 38:143–158
Lee IK, Yun BS (2006) Hispidin analogs form the mushroom Inonotus xeranticus and their free radical scavenging activity. Bioorg Med Chem Lett 16:2376–2379
Lee IK, Yun BS (2007) Highly oxygenated and unsaturated metabolites providing a diversity of hispidin class antioxidants in the medicinal mushrooms Inonotus and Phellinus. Bioorg Med Chem 15:3309–3314
Li L, Wang JN, Ren J, Xiang JF, Tang YL, Liu JX, Han D (2007) Metabonomics analysis of the urine of rats with Qi deficiency and blood stasis syndrome based on NMR techniques. Chin Sci Bull 52:3068–3073
Lu X, Zhu W, Yang Z, Zhou Y, Huang C, Peng X (2008) Research on the extraction process of the total triterpenes from common self heal. J Math Med 21:202–204
Mothana RAA, Jansen R, Julich WD, Lindequist U (2000) Ganomycins A and B, new antimicrobial farnesyl hydroquinones from the basidiomycete Ganoderma pfeifferi. J Nat Prod 63:416–418
Park YK, Lee HB, Jeon EJ, Jung HS, Kang MH (2004) Chaga mushroom extract inhibits oxidative DNA damage in human lymphocytes as assessed by comet assay. Biofactors 21:109–112
Pettit RK (2009) Mixed fermentation for natural product drug discovery. Appl Microbiol Biotechnol 83:19–25
Shcherba VV, Babitskaia VG, Kurchenko VP, Ikonnikova NV, Kukukianskaia TA (2000) Antioxidant features of fungal melanin pigments. Prikl Biokhim Mikrobiol 36:569–574
Shin Y, Tamai Y, Terazawa M (2002) Triterpenoids, steroids, and a new sesquiterpene from Inonotus obliquus (Pers.: Fr.) Pilat. Intl J Med Mush 4:77–84
Shyu YS, Hwang LS (2002) Antioxidant activity of the crude extract of lignan glycosides from unroasted Burma black sesame meal. Food Res Intl 35:357–365
Singleton VL, Rossi JAJ (1965) Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Taji S, Yamada T, S-i W, Tokuda H, Sakuma K, Tanaka R (2005) Lanostane-type triterpenoids from the sclerotia of Inonotus obliquus possessing anti-tumor promoting activity. Eur J Med Chem 43:2373–2379
Trygg J, Wold S (2002) Orthogonal projections to latent structures, O-PLS. J Chemometr 16:119–128
Wang Y, Shang XY, Wang SJ, MS Y, Li S, Yang YC, Ye F, Shi JG, He L (2007) Structures, biogenesis, and biological activities of pyrano[4, 3-c]isochromen-4-one derivatives from the fungus Phellinus igniarius. J Nat Prod 70:296–299
Wright JS, Johnson ER, DiLabio GA (2001) Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123:1173–1183
Ying J, Mao X, Ma Q (1987) Icons of medicinal fungi from China. Science, Beijing
Yusoo S, Yutaka T, Terazawa M (2001) Chemical constituents of Inonotus obliquus II: a new triterpene, 21, 24-cyclopentalanosta-3b, 21, 25-triol-8-en. J Wood Sci 47:313–316
Zhang HY, Sun YM, Wang XL (2003) Substituent effects on O–H bond dissociation enthalpies and ionization potentials of catechols: a DFT study and its implications in the rational design of phenolic antioxidants and elucidation of structure-activity relationships for flavonoids antioxidants. Chem- A Eur J 9:502–508
Zheng W, Gao X, Gu Q, Chen C, Wei Z, Shi F (2007a) Antitumor activity of daphnodorins from Daphne genkwa roots. Intl Immunopharmacol 7:128–134
Zheng W, Liu T, Xiao X, Qi G (2007b) Sterol composition in field-grown and cultured mycelia of Inonotus obliquus. Acta Pharm Sin 42:750–756
Zheng W, Xiang X, Chen C, Wang Y, Zhao Y, Jiang J, Chu C (2008a) Effects of culture media and three metal ions on the accumulation of lanosterol and ergosterol in cultured mycelia of Inonotus obliquus. Mycosystema 27:126–139
Zheng W, Zhao Y, Zhang M, Yin Z, Chen C, Wei Z (2008b) Phenolic compounds from Inonotus obliquus and their immune stimulating effects. Mycosystema 27:39–47
Zheng W, Miao K, Zhao Y, Zhang M (2009a) Nitric oxide mediates fungal elicitor-enhanced biosynthesis of antioxidant polyphenols in Inonotus obliquus in submerged cultures. Microbiol 155:3340–3348
Zheng W, Zhang M, Zhao Y, Miao K, Jiang H (2009b) NMR-based metabonomic analysis on effect of light on production of antioxidant phenolic compounds in submerged cultures of Inonotus obliquus. Biores Technol 100:4481–4487
Zheng W, Zhang M, Zhao Y, Wang Y (2009c) Accumulation of antioxidant phenolic constituents in submerged cultures of Inonotus obliquus. Biores Technol 100:1327–1335
Zjawiony JK (2004) Biologically active compounds from Aphyllophorales (Polypore) fungi. J Nat Prod 67:300–310
Acknowledgments
Financial support was provided by grants from Natural Science Foundation of China (31070052), the Ministry of Science and Technology of China (2005DFA30280, 2007021506) and the Natural Science Foundations of Jiangsu Province, China (BK2009084 and SBK201040012).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Representative 1H NMR spectra of the extracts from mycelia of I. obliquus growing in a monoculture and b coculture at different culture time. 1, phelligridin C; 2, phelligridin H; 3, methyl inoscavin A; 4, inoscavin C; 5, inoscavin B; 6, davallialactone; 7, methyl davallialactone; 8, foscoparianol D; 9, 21,24-cyclopentalanosta-3β,21,25-triol-8-en; 10, lanosta-7,9(11),23-triene-3β,22,25-triol; 11, inotodisaccharide; d, day (DOC 396 kb)
Fig. S2
1H NMR spectrum of inotodisaccharide. a Full spectrum; b zoom-in of the protons resonating between δ 2.8 and δ 3.8 ppm (DOC 128 kb)
Fig. S3
13C NMR of inotodisaccharide. a Full spectrum; b DEPT 135°; c zoom-in of the carbons resonating between δ 70 and δ 77 ppm (DOC 224 kb)
Fig. S4
1H–1H COSY of inotodisaccharide (DOC 156 kb)
Fig. S5
HSQC spectrum of inotodisaccharide (DOC 158 kb)
Fig. S6
HMBC spectrum of inotodisaccharide (DOC 161 kb)
Fig. S7
PLS observed vs predicted plots derived from the calculation between 1H NMR spectra and capacities for scavenging DPPH by the metabolites from monoculture (a) and coculture (b) and hydroxyl radicals by those from monoculture (c) and coculture (d) and the calculation between 1H NMR spectra and inhibition to the growth of HeLa 229 cells by the metabolites from monoculture (e) and coculture (f) (DOC 81 kb)
Fig. S8
Time-coursed changes in activities of PAL (a), TAL (b), and HMGR (c) of the mycelia in monoculture and coculture. Results are the mean of ten independent experiments and error bars are the standard deviations. PAL, TAL, and HMGR activity was determined, respectively, by established methods (Mori et al. 2001, Plant Sci 160:335–360; Khan et al. 2003, J Plant Physiol 160:859–863; Zheng et al. 2008, Mycosystema 27:126–139). Enzyme activities were expressed as the units, where one unit is defined in capacity to produce 1 nmol cinnamic acid (for PAL), 4-hydroxyl cinnamic acid (for TAL), and mevalonic acid (for HMGR), respectively. Unit per milligram protein is the units of enzymes in 1 mg protein (DOC 52 kb)
Table S1
Metabolites and their 1H chemical shifts identified by 400 MHz 1H NMR spectroscopy (DOC 40 kb)
Rights and permissions
About this article
Cite this article
Zheng, W., Zhao, Y., Zheng, X. et al. Production of antioxidant and antitumor metabolites by submerged cultures of Inonotus obliquus cocultured with Phellinus punctatus . Appl Microbiol Biotechnol 89, 157–167 (2011). https://doi.org/10.1007/s00253-010-2846-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2846-2