Abstract
Endophytic fungi isolated from the insectivorous plant Drosera burmannii were tested for their antioxidant potential. The isolate Dro2 was found to have superlative antioxidant activity of the culture broth, scavenging 57.6 ± 0.2% of 2,2-diphenyl-1-picrylhydrazyl free radical. 5.8S rDNA homology led to identification of the isolate as a new strain and it has been named Mucor irregularis isolate Dro2. Optimization studies revealed ethyl acetate to be optimum for extraction of the antioxidant compounds. The crude solvent extract showed 89.6% 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) free radical scavenging activity with respect to 86.1% ABTS radical reducing antioxidant power of ascorbic acid. The sample exhibited 824.0 ± 25.5 µM reducing ability in assay, compared to 1204.0 ± 17.8 µM for ascorbic acid. The sample extract displayed IC50DPPH of 53.6 µg/mL. The crude extract was further purified through various chromatography techniques and GC-MS was performed on the purified active fractions to ascertain the nature and identify compounds conferring antioxidant potential to the endophytic fungal isolate. The active fractions were found to comprise multitudinous compounds with varied biological activity. Antioxidant compounds, such as 2,4-di-tert-butylphenol, myristic acid, pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)-, palmitic acid, were found in significant abundance. This study substantiates the candidature of the endophytic fungus Mucor irregularis isolate Dro2 as an industrially capable source for obtaining bioactive compounds, particularly antioxidant compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Oxidative stress in a biological system arises when the rate of production of oxidants exceeds the rate at which they are removed by endogenous defence systems. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are free radicals constantly formed in the cell as by-products of ATP synthesis in the process of apoptosis and phagocytosis [1]. Their production is also induced by certain environmental factors, such as exposure to ultra-violet radiation [2]. Uncontrolled production of these free radicals has deleterious effects on the structure and function of living systems. These compounds have the ability of initiating chain oxidation reactions with oxidizable substrates present in the cell, which include proteins, carbohydrates, lipids, RNA and DNA [3]. In humans, oxidative stress has been linked to a variety of diseases such as cancer, male factor infertility, autoimmune disorders, cardiovascular and neurodegenerative disorders, cataract, and is pivotal to the process of aging [1]. In plant system, excess of free radicals causes extensive damage of all physiological processes and accumulation of oxidants causes toxicity [4].
The endogenous radical defence system consists of antioxidants—molecules which have the capability of reducing the free radicals, thereby preventing oxidation of oxidizable substrates. Antioxidants are classified into two major groups—enzymatic and non-enzymatic antioxidants. Superoxide dismutase, catalase, glutathione peroxidase, ceruloplasmin are examples of antioxidant enzymes. Some well known non-enzymatic antioxidants are uric acid, vitamin E, ascorbic acid, β-carotene and other carotenoids, metallothionein, xanthophylls, phytic acid, taurine, bilirubin, creatinine, ubiquinol, flavonoids, polyamines, plant phenolic compounds, melatonin, etc. [3]. A supplement of such antioxidative molecules helps neutralize the excess free radicals in case of oxidative stress.
Nutrient antioxidants have been traditionally obtained from plants. In recent years, microorganisms from diverse niches have proved to be potent resources for obtaining multifarious biologically active compounds. Endophytes are one such group of microorganisms with promising biotechnological prospects. Microorganisms living symbiotically in the healthy tissues of plants have been a topic of intense research in the past decade. Their potency as synthesizers of novel antibiotics, antimycotics, anticancer compounds, immunomodulatory substances has been substantiated through several studies [5]. Many aspects of their synthesizing abilities are yet unexplored. The antioxidative property is one such facet. Therefore, it is necessary to evaluate the ability of endophytic microorganisms to produce antioxidant compounds and how these might aid the host plant to thrive under adverse conditions.
The plant selected for this study is Drosera burmannii Vahl. (Droseraceae), commonly known as Burmann’s sundew. It is a small, compact insectivorous plant. The plant grows in waterlogged or wet acidic soils, typically deficient in nutrients. D. burmannii Vahl. has been traditionally used in many medicinal preparations and well known to have rubefacient property.
To our knowledge this is the first study which deals with exploration of endophytic fungal components of D. burmannii Vahl. and evaluation of the antioxidative potential of the isolates. The most promising endophytic fungal isolate in terms of antioxidant activity was studied in detail and the active compounds were identified from its secreted metabolome.
MATERIALS AND METHODS
Analytic grade chemicals and reagents were used in all experiments and HPLC grade reagents (HiMedia, India) were used for the HPLC and GC-MS studies. Media components, solvents and other compounds were acquired from Sisco Research Laboratories Pvt. Ltd., India. Silica gel was purchased from Merck, Germany. Cetyl trimethylammonium bromide (CTAB) was procured from GeNei, India. Poly-L-lysine and primers for PCR were purchased from Sigma-Aldrich, USA.
Isolation of endophytic fungi. Healthy specimens of D. burmannii were collected from the forest of Gurguripal, West Midnapore, West Bengal, India during the month of December. Plants were carefully picked and placed in clean ziplock bags and isolation work was done within 24 h of collection. Plant parts were separated, washed under gentle tap water and then subjected to surface sterilization in accordance with the method described by Woropong et al. [6]. To test the efficacy of surface sterilization, the treated samples were imprinted on potato dextrose agar (PDA) plate (Sisco Research Laboratories Pvt. Ltd., India). The samples were then cut into smaller pieces in an aseptic manner and placed onto water-agar (WA) plates [6]. The plates were incubated at 24°C and checked regularly for emergence of fungal hyphae from the plated fragments. Upon emergence of fungal hyphae, the portion of WA with a single fungal hypha was cut as a small block and transferred onto fresh sterilized Petri plates containing PDA. The plates were then incubated at 24°C.
Cultivation. 5 mm2 blocks of the endophytic isolates maintained on PDA were cut and transferred to 500 mL Erlenmeyer flasks containing 200 mL potato dextrose broth (PDB). The flasks were kept in a rotary shaker incubator at 80 rpm and 24°C for 5 days. Then the culture broths were filtered to separate mycelia from the broth using Whatman filter paper 1 (UK). This filtered culture broth was used as the source of secreted antioxidants of the endophytic fungal isolates.
Determination of antioxidant activity of the culture broths of endophytic fungal isolates. Antioxidant activity of the filtered culture broth was determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay [7]. One mL of the filtered culture broth was added to 1 mL of the freshly prepared 0.04 mM DPPH, vortexed and incubated in dark for 30 min. After incubation, OD517 of the solutions was measured at UV-VIS spectrophotometer (Shimadzu, Japan). Mixture of 1 mL PDB and 1 mL 0.04 mM DPPH incubated for the same period was used as control. Percentage free radical scavenging activity was calculated as follows:
where ODc—absorption of control, ODs—absorption of sample.
The isolate showing maximum antioxidant activity was then recognized among the lot and further studies were carried out to determine the antioxidant compound(s) synthesized by the isolate.
Identification and characterization of the isolate. The morphological and microscopic properties of the isolate were observed and noted. Fungal mycelia were scraped off the surface of PDA plate culture and ground into fine powder in the presence of liquid N2 using a mortar and pestle, following CTAB method [8] employed for extraction of genomic DNA. Fungal universal primers ITS1 and ITS4 were used to amplify the ITS1-5.8S rDNA-ITS2 regions. PCR was carried out in the manner described by Pan et al. [9]. The purified PCR products were outsourced for sequencing. Sequence was analyzed by NCBI BLAST and submitted to NCBI GenBank. Phylogenetic analysis was done using MEGA5.1 by neighbour-joining method [10].
Optimization of fungal extraction. The endophytic fungal isolate was grown in 3 batches of 500 mL PDB. Each batch of filtered culture broth was separately extracted using chilled 2× culture’s volume of ethyl acetate (EA), n-butanol (BA) and cyclohexane (CH). The organic phases were discarded and the solvent phases were dried in a vacuum rotary evaporator (BUCHI Labortechnik AG, Switzerland) under reduced pressure at 35°C and dissolved in methanol. The antioxidant activity of these solvent extracts was then tested via the DPPH assay as previously described. One hundred µL of the solvent extract was added to 900 µL alcoholic DPPH solution. PDB extracted using the respective solvents, rotary dried and dissolved in methanol, was used as a control. The solvent showing maximum free radical scavenging extraction was selected for further tests.
Four L of PDB equally divided into 20 Erlenmeyer flasks of 500 mL capacity were inoculated with the fungal hyphae blocks (5 mm2) and incubated in a rotary shaker under culture conditions previously mentioned. Filtered culture broth was twice extracted with twice the volume of optimal solvent of the culture broth, the dried crude extract was weighed and stored at 4°C.
Antioxidant activity of the crude solvent extract. 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay. For measuring total antioxidant capacity, ABTS solution was prepared, as described by Rajurkar and Hande [11], by mixing equal volumes of 7 mM aqueous ABTS and 2.4 mM aqueous potassium persulfate. The reaction was allowed to continue overnight and then the mixture was diluted with methanol to achieve OD734 of 0.700. This working solution of ABTS (900 µL) was added to 100 µL of methanolic sample solution (1 mg/mL) and incubated for 30 min at room temperature. OD734 was then read. Methanol was used as blank. The antioxidant capacity was calculated according to the equation given below:
Where OD0—absorbance of blank solution, ODsample—absorbance of sample at the end of incubation period.
Ferric ion reducing antioxidant power (FRAP) assay. FRAP assay protocol given by Pan et al. [12] was adopted. One hundred µL of the methanolic sample solution (1 mg/mL) was added to 900 µL of FRAP reagent. The reaction was allowed to continue for 4 min at room temperature after and OD593 was read. Methanol served as a blank. A standard curve was prepared using FeSO4 at concentrations ranging from 20 to 1000 µM with FRAP reagent. Results were expressed in µmol Fe(II).
Determination of DPPH IC50. Dilutions of the crude extract from 40 to 200 µg/mL were prepared in methanol. Radical scavenging activity was determined by the method described by Mensor et al. [13]. Five hundred µL of the sample solutions were mixed with 1500 µL 0.04 mM DPPH and incubated in the dark for 30 min. The DPPH IC50 of ascorbic acid, a standard antioxidant, was determined at concentrations from 50 to 500 µg/mL. The values were plotted on graph and IC50 was calculated by the linear regression equation.
Purification and identification of the active components. TLC and bioautography. Methanolic crude extracts were spotted onto glass plates overlain with 0.2 mm silica gel G (Merck, Germany). The plates were then run in solvent mixtures of chloroform and methanol in various ratios. Visualization was done using UV light (254–312 nm) which reflected the presence of organic compounds on the plate. Bioautography was performed by spraying 0.04 mM alcoholic DPPH solution over the plate. The regions where the purple colour of DPPH was decolourized and converted to yellow indicated the presence of antioxidant compounds.
Column chromatography. The crude solvent extract was subjected to purification via column chromatography. Sample extract was loaded into 170 × 20 mm column containing silica gel (pore size 60 Å,, mesh size 230−400, 30–40 (d10), 55–65 (d50), 95–110 (d90) μm particle size) and eluted with a solvent mixture of chloroform and methanol, gradually decreasing the quantity of chloroform and increasing the quantity of methanol. Twelve fractions were collected. The antioxidant activity of the fractions was determined through DPPH assay. Active fractions were then subjected to HPLC analysis.
HPLC analysis. The active fractions were combined, concentrated, dissolved in methanol and subjected to HPLC for determining the extent of purification. Fractions were loaded on HPLC Column ZORBAX SB-C18, 80, 5 µm, 4.6 × 150 mm (Agilent, USA) with pore size of 170 Å. The solvent phase consisted of acetonitrile and methanol in a gradient of 0.5 : 9.5, 3 : 7, 1 : 1, 7 : 3 and 9.5 : 0.5 (vol/vol) ratio, with flow rate of 1 mL/min. Compounds were detected at 220 nm with the help of UV detector.
GC-MS analysis. The chemical composition of the active fractions was determined through GC-MS analysis. The combined active fractions were diluted and 1 µL of the sample was injected into the ISQ QD single quadrupole gas chromatograph mass spectrometer system with autosampler (Thermo Scientific, Germany) in splitless mode. The GC was equipped with TG-WAXMS GC column (30 m × 0.25 mm × 0.25 µm, Thermo Scientific, Germany). Flow rate of carrier gas helium was set at 1 mL/min. Injector temperature, ion source temperature and MS transfer line temperature were 240, 230 and 240°C, respectively. The MS system had EI mode of ionization with the scanning mode set at m/z of 40.00−400.00. The program was set as follows—initial temperature of 50°C was held for 2 min, after that a ramp of 8°C/min was provided until it reached 230°C with a final hold of 2 min. Thermo Scientific Xcalibur data acquisition and processing software (Germany) was used for analysis of data based on NIST5.0 database.
Chromatogram of the sample was subtracted from the chromatogram of control (EA extract of PDB dissolved in methanol) and analysed for the bioactive compounds present in extract.
Data analysis. All tests were done in triplicate and the results have been depicted as the mean ± standard deviation. The standard curves were prepared with the help of Microsoft Excel 2010 and SigmaPlot version 11.0.
RESULTS
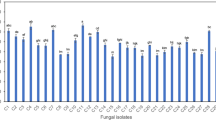
Isolated endophytes and antioxidant activity of their culture broths. Eight culturable fungi have been isolated from the incubated plant fragments. Seven isolates showed varied scavenging activity when tested against DPPH free radical. One particular isolate, coded Dro2, showed the highest scavenging ability. This isolate was chosen for further exploration. Table 1 depicts the antioxidant activity of the culture broths of the endophytic fungal isolates.
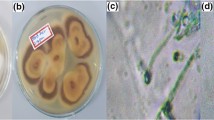
Identification and characterization of the isolate. The isolate showed rapid growth on PDA at 24°C forming hyaline aerial, surface and penetrative hyphae. Light microscopic studies revealed no reproductive structures indicating sterile nature of the strain under the specified culture conditions.
Comparison of the 5.8S rDNA sequence with the NCBI database showed that the closest GenBank match was Mucor irregularis strain Bpf-19 having accession no. MH 397 495. The identity was 97.9% over a query cover of 95%. Our novel strain was named as M. irregularis isolate Dro2 (MH 102381). The phylogeny of this strain with closely related fungal strains, as obtained from NCBI GenBank database matching, was analysed using MEGA 5.1 software by neighbour joining method. The results are shown in Fig. 1.
Extraction optimization. Three different solvents were used for extraction of bioactive compounds from the culture broth of M. irregularis isolate Dro2—EA, CH and BA. The DPPH inhibiting activity of EA, CH and BA extracts was tested. It was found that EA extract showed the maximum inhibition of DPPH free radicals. The results are summarized in Fig. 2.
Antioxidant activity of the EA extract. The antioxidative potential of the isolate was validated by additional antioxidant assays. ABTS assay performed using methanolic 100 µg/mL EA extract demonstrated 89.6% ABTS free radical scavenging activity. Equivalent concentration of ascorbic acid showed 86.1% ABTS radical inhibition. FRAP assay of the same concentration of crude sample revealed its reducing power to be equivalent to 824.0 ± 25.5 µM, compared to 1204.0 ± 17.8 µM of ascorbic acid.
The crude extract showed high DPPH free radical inhibition which increased in a dose-dependent manner, exhibiting IC50 of 53.6 µg/mL. It was significantly lower than the IC50 of positive control (ascorbic acid) calculated (IC50 79.5 µg/mL). One hundred µg/mL concentration of the crude sample approached 98.2% radical scavenging efficiency compared to 74.5% scavenging efficiency of comparable concentration of positive control (Fig. 3).
Partial purification of antioxidants. Thin layer chromatography followed by visualization under UV light revealed several spots containing probable organic compounds (Fig. 4a). Spraying the plate with DPPH solution resulted in discolouration of the purple colour at two spots (Fig. 4b). These two spots correspond to the localised antioxidant compounds and show that separation of the bioactive compounds on the TLC plate is satisfactory. The crude extract when subjected to column chromatography yielded twelve fractions. The twelve fractions were evaluated for antioxidant activity through DPPH assay. Out of these, three fractions showed positive antioxidant activity. Fraction seven exhibited 15.1% radical scavenging ability, fraction 9 showed 15.3%, while the radical inhibition of fraction 10 was 8.6%. These three fractions were combined and subjected to HPLC analysis for testing the extent of purification. Results of HPLC revealed high level of purity in the fractions under detection by UV-detector (220 nm) (Fig. 5). These combined fractions were then subjected to GC-MS analysis for identification of the bioactive compounds present in those fractions.
GC-MS analysis. A variety of organic compounds belonging to various classes were identified in the GC chromatogram (Fig. 6) The presence of compounds such as palmitic acid, myristic acid, 2,4-di-tert-butylphenol confirmed the antioxidant properties of the fungal extract. In addition to these antioxidants, biologically active compounds with diverse effects and applications were also found in the composition. The results are summarized in Fig. 6.
DISCUSSION
Eight morphologically different fungal endophytes were isolated from D. burmannii Vahl. using culture methods. This is the first study involving isolation of endophytic fungi from this carnivorous plant and exploration of antioxidant potential of these isolates. Previously, fungi residing in the roots of Drosera rotundifolia were explored by Quilliam and Jones [14]. They were able to isolate 8 different morphotypes of mycorrhizal and non-mycorrhizal root fungi. Low isolation frequency can be attributed to the skewed nature of culture-dependent isolation process which favours fast-growing fungi. The delicate tissues of this plant might not have been able to withstand the surface sterilization process, contributing further to fewer endophytes being isolated.
In this work, one objective was to bioprospect fungal endophytes which can be cultured for their bioactive metabolites. Focus was on the secreted metabolome instead of intracellular metabolites since extraction and recovery of secreted metabolites from the culture media is more feasible and large amounts of desired products can be obtained in less time. The culture media of the endophytic isolates were analysed for the presence of secreted antioxidant compounds. Among 8 endophytic fungal isolates 7 (87.5%) showed positive radical scavenging activity with their culture broths and 75% isolates showed DPPH radical scavenging activity of >40% demonstrating noteworthy antioxidant potential.
The isolates differed from each other morphologically. The isolate coded Dro2 was recognized as having the highest antioxidant activity. Dro2 produced sterile mycelia and did not show any sporulation. Molecular identification techniques were employed for naming this fungal endophytic isolate. It was found to be a morphotype of M. irregularis (previously named Rhizomucor variabilis) and was named ‘M. irregularis isolate Dro2’. M. irregularis has been rarely reported as an endophyte, there being only 2 previous studies reporting it as an endophyte of Moringa stenopetala [15] and of Rhizophora stylosa [16].
Optimization of extraction using different solvents was done with EA, CH and BA. DPPH scavenging assay of the respective solvent extracts, when compared to scavenging activity of culture media control extracts, showed that EA was the optimum solvent for extraction of antioxidant compounds from the culture broth. EA extract scavenged 81.9 ± 0.5% free radicals, whereas BA and CH extracts showed 30.5 ± 2.5 and 20.5 ± 2.9% radical inhibition, respectively, indicating weak antioxidant activity. Differential affinities of the antioxidant compounds towards the disparate organic solvents, as a function of polarity, lead to this huge difference in antioxidant activity. This also indicates presence of multifarious compounds possessing antioxidant activity, which were extracted by either of the solvents and contributed to its radical scavenging action.
ABTS and FRAP assays were performed using the EA extract, with ascorbic acid kept as the positive control. The sample crude extract (100 µg/mL) exhibited greater ABTS scavenging activity compared to ascorbic acid. However, FRAP of the sample was slightly lower than that of the standard antioxidant. Comparisons drawn between the standard curves of DPPH radical inhibition as a function of concentration again proved Dro2 crude extract to be superior to the standard antioxidant. In general, the sample showed better DPPH inhibition than ABTS inhibition. This is in contrast with the results obtained by Pan et al. [12] where they reported crude extracts of 5 different endophytic strains of Fusarium sp. to be more capable of scavenging ABTS radical than DPPH radical. The results reflect competent antioxidant potential of the endophytic isolate.
The chromatographic technique was applied for separation and purification of the bioactive compounds. Bioautography performed using DPPH visualized the presence and separation of antioxidant compounds of the crude extract. HPLC analysis of active fractions obtained from column chromatography demonstrated the presence of bioactive compounds in high abundance.
GC-MS analysis of the active fractions revealed assorted biologically active compounds constituting the semi-purified bioactive crude extract. As depicted in Fig. 6, the sample extract contains several antioxidant compounds, pertaining to both primary and secondary metabolites. The fatty acids—tetradecanoic (myristic) and hexadecanoic (palmitic) acids have established antioxidatory potential [17]. Myristic acid as an antioxidant was also extracted from the endophytic fungus Psathyrella candolleana [18]. Another antioxidant compound found in this study in the extract in significant abundance was 2,4-di-tert-butylphenol. This compound has shown high antifungal and antioxidatory potential, as reported by Varsha et al. [19]. Pan et al. [20] also reported 2,4-di-tert-butylphenol as a constituent of antioxidant extract of Fusarium trincinctum CBY11, an endophyte isolated from Fritillaria cirrhosa. 7-Hydroxy-3-(1,1-dimethylprop-2-enyl)coumarin detected by the gas chromatogram is also postulated to have antioxidant activity [21]. This compound also acts as an anti-quorum sensing molecule [22]. Qin et al. [23] obtained a coumarin analogue from endophytic Aspergillus fumigatus which acts as an antioxidant and increases host’s resistance to drought.
The compound found in highest amount in GC-MS analysis was indole derivative, tryptophol, known as growth factor in roots and leaves of plants [24]. This is the first report of tryptophol being synthesized in vitro by an endophytic fungus. Besides its importance in regulation of plant physiological processes, tryptophol has also been explored as a compound with potential antibacterial properties [25].
In addition, numerous other compounds with diverse biological activities have been detected (Table 2). Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- and pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenyl methyl) have been shown to possess antifungal properties [26, 27]. Several studies have reported its production by endophytic actinobacteria and bacteria [26–28]. 4-Quinoliamine and tetradecanoic acid have established antiplasmodial and larvicidal properties [29, 30].
Quilliam and Jones [14] in their research hypothecated that the endophytes of Drosera sp. might play critical functional roles such as conferring abiotic stress tolerance to the host plant and regulating nutrient signalling between root and shoot. Studies by several authors [31–33] have revealed that endophytes do indeed relieve their hosts of abiotic stress, such as metal, salt and drought stresses, by upregulating the antioxidant system and synthesis of antioxidant compounds and enzymes.
In 2015, Ghate et al. [34] investigated the antioxidant potential of D. burmannii and stated that the plant extract is capable of alleviating iron-induced oxidative stress.
Endophytic microorganisms have been shown to be producing a variety of bioactive compounds as a part of their contribution to the mutualistic relationship they share with their host plants [35]. In multiple instances, an endophyte has been seen to be producing compounds similar to its host’s metabolites as a result of years of co-evolution. The endophytic community of D. burmannii might be contributing to the antioxidant property demonstrated by the plant extract. The diverse bioactive compounds produced by the endophytic fungal isolate can be speculated to help the host plant in thriving in a nutrient-deficient habitat.
REFERENCES
Pham-Huy, L.A., He, H., and Pham-Huy, C., Int. J. Biomed. Sci., 2009, vol. 4, no. 2, pp. 89–96.
Tyrell, R.M., Biochem. Soc. Symp., 1995, vol. 61, pp. 47–53.
Halliwell, B., Antioxidants in Disease Mechanisms and Therapy, August, J.T., Murad, F., Anders, M.W., Coyle, J.T., and Packer, L., Eds., Cambridge: Academic, 1996, vol. 38.
Ahuja, N., Singh, H.P., Batish, D.R., and Kohli, R.K., Pestic. Biochem. Phys., 2015, vol. 118, pp. 64–70.
Strobel, G., Daisy, B., Castillo, U., and Harper, J., J. Nat. Prod., 2004, vol. 67, no. 2, pp. 257–268.
Woropong, J., Strobel, G.A., Ford, E.J., Li, J.Y., Baird, G., and Hess, W.M., Mycotaxon, 2001, vol. 79, pp. 67–79.
Blois, M.S., Nature, 1958, vol. 181, pp. 1199–1200.
Murray, M.G. and Thompson, W.F., Nucleic Acids Res., 1980, vol. 9, no. 19, pp. 4321–4325.
Pan, F., Hou, K., Gao, F., Hu, B., Chen, Q., and Wu, W., Phytomedicine, 2014, vol. 21, no. 8–9, pp. 1104–1109.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., and Kumar, S., Mol. Biol. Evol., 2011, vol. 28, no. 19, pp. 2731–2739.
Rajurkar, N.S. and Hande, S.M., J. Pharm. Sci., 2011, vol. 73, no. 2, pp. 146–151.
Pan, F., Su, T.J., Cai, S.M., and Wu, W., Sci. Rep., 2017, vol. 7, art. 42008.
Mensor, L.L., Menezes, F.S., Leitão, G.G., Reis, A.S., dos Santos, T.C., Coube, C.S., and Leitão, S.G., Phytother. Res., 2001, vol. 15, no. 2, pp. 127–130.
Quilliam, R.S. and Jones, D.L., Mycorrhiza, 2010, vol. 20, pp. 341–348.
Akone, S., Daletos, G., Lin, W., and Proksch, P., Z. Naturforsch. C, 2016, vol. 71, no. 1–2, pp. 15–19.
Gao, S.S., Li, X.M., Williams, K., Proksch, P., Ji, N.Y., and Wang, B.G., J. Nat. Prod., 2016, vol. 79, no. 8, pp. 2066–2074.
Krishnamoorthy, K. and Subramaniam, P., Int. Sch. Res. Notices, 2014, vol. 2014 567409.
Pan, Y., Zheng, W., and Yang, S., Nat. Prod. Res., 2019, vol. 21, pp. 1–4.
Varsha, K.K., Devendra, L., Shilpa, G., Priya, S., Pandey, A., and Nampoothiri, K.M., Int. J. Food Microbiol., 2015, vol. 211, pp. 44–50.
Pan, F., Su, T.J., Deng, K.L., and Wu, W., Mycosystema, 2017, vol. 36, no. 6, pp. 752–765.
Al-Majedy, Y., Al-Amiery, A., Kadhum, A.A., and BakarMohamad, A., Syst. Rev. Pharm., 2017, vol. 8, no. 1, pp. 24–30.
Shastry, R.P. and Aman, M., Curr. Bioact. Compd., 2019, vol. 15, p. 1.
Qin, W., Liu, C., Jiang, W., Xue, Y., Wang, G., and Liu, S. BMC Microbiol., 2019, vol. 19, p. 50.
Palmieri, A. and Petrini, M., Nat. Prod. Rep., 2018, vol. 36, no. 3, pp. 490–530.
Gos, F.M.W.R., Savi, D.C., Shaaban, K.A., Thorson, J.S., Aluizio, R., Possiede, Y.M., et al., Front. Microbiol., 2017, vol. 8, art. 1642.
Sanjenbam, P., Gopal, J.V., and Kannabiran, K., Appl. Biochem. Microbiol., 2014, vol. 50, no. 5, pp. 429–499.
Awla, H.K., Kadir, J., Othman, R., Rashid, T.S., and Wong, M.Y., Am. J. Plant Sci., 2016, vol. 7, pp. 1077–1085.
Sheoran, N., ValiyaNadakkakath, A., Munjal, V., Kundu, A., Subaharan, K., Venugopal, V., et al., Microbiol. Res., 2015, vol. 173, pp. 66–78.
Kaschula, C.H., Egan, T.J., Hunter, R., Basilico, N., Parapini, S., Taramelli, D., et al., J. Med. Chem., 2002, vol. 45, pp. 3531–3539.
Sivakumar, R., Jebanesan, A., Govindarajan, M., and Rajasekar, P., Asian Pac. J. Trop. Med., 2011, vol. 4, no. 9, pp.706–710.
Lata, R., Chowdhury, S., Gond, S.K., and White, J.F. Jr., Lett. Appl. Microbiol., 2018, vol. 66, no. 4, pp. 268–276.
Lubna, Asaf, S., Hamayun, M., Khan, A.L., Waqas, M., Khan, M.A., Jan, R., et al., Plant Physiol. Biochem., 2018, vol. 128, pp. 13–23.
Zhu, L., Li, T., Wang, C., Zhang, X., Xu, L., Xu, R., and Zhao, Z., Environ. Sci. Pollut. Res. Int., 2018, vol. 25, no. 35, pp. 35232–35241.
Ghate, N.B., Chaudhuri, D., Das, A., Panja, S., and Mandal, N., PLoS One, 2015, vol. 10, no. 5, art. e0128221. https://doi.org/10.1371/journal.pone.0128221
Strobel, G. and Daisy, B., Microbiol. Mol. Biol. Rev., 2003, vol. 67, no. 4, pp. 491–502.
Funding
Authors are grateful to the Council of Scientific and Industrial Research, India, for financial support in the form of junior research fellowship to SM.
Author information
Authors and Affiliations
Contributions
Conceptualization, experiments, data collection and analysis were performed by S. Mandal. S. Maity helped in chromatography, HPLC and GC-MS studies. Manuscript was prepared by S. Mandal. Insightful comments were received from S. Maity and D. Banerjee which helped in drafting the final version of the manuscript. All the research work was supervised by D. Banerjee.
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Mandal, S., Maity, S. & Banerjee, D. Antioxidative Compounds from the Secreted Metabolome of Strain ‘Mucor irregularis Isolate Dro2’—an Endophyte of the Carnivorous Plant Drosera burmannii. Appl Biochem Microbiol 57 (Suppl 1), S88–S97 (2021). https://doi.org/10.1134/S0003683821100069
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683821100069