Abstract
Rhamnolipids are biosurfactants with interesting physico-chemical properties. However, the main obstacles towards an economic production are low productivity, high raw-material costs, relatively expensive downstream processing, and a lack of understanding the rhamnolipid production regulation in bioreactor systems. This study shows that the sequenced Pseudomonas aeruginosa strain PAO1 is able to produce high quantities of rhamnolipid during 30 L batch bioreactor cultivations with sunflower oil as sole carbon source and nitrogen limiting conditions. Thus PAO1 could be an appropriate model for rhamnolipid production in pilot plant bioreactor systems. In contrast to well-established production strains, PAO1 allows knowledge-based systems biotechnological process development combined with the frequently used heuristic bioengineering approach. The maximum rhamnolipid concentration obtained was 39 g/L after 90 h of cultivation. The volumetric productivity of 0.43 g/Lh was comparable with previous described production strains. The specific rhamnolipid productivity showed a maximum between 40 and 70 h of process time of 0.088 gRL/gBDMh. At the same time interval, a shift of the molar di- to mono-rhamnolipid ratio from 1:1 to about 2:1 was observed. PAO1 not only seems to be an appropriate model, but surprisingly has the potential as a strain of choice for actual biotechnological rhamnolipid production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycolipids, consisting of one or two l-rhamnose moieties and one or two β-hydroxyalkanoic acid residues, are termed rhamnolipids (RL). Various microorganisms, mainly Pseudomonas aeruginosa, are able to produce rhamnolipids (Jarvis and Johnson 1949; Manso Pajarron et al. 1993; Häußler et al. 1998; Andrä et al. 2006; Gunther 2007). Rhamnolipids are biosurfactants of special interest for cosmetic, pharmaceutical and detergent manufacturers, and already proved their potential in enhanced oil recovery (Wang et al. 2007), bioremediation (Nguyen et al. 2008), and biodegradation (Zhang et al. 2005). They show excellent biodegradability, low toxicity, thus environmental friendliness, and can be produced by using renewable or waste resources (Giani et al. 1997; Rahman et al. 2002; Wei et al. 2005; Marsudi et al. 2008), while showing comparable physico-chemical properties to synthetic surfactants (Syldatk et al. 1985b). Rhamnolipids were first described by Jarvis and Johnson (1949). The main rhamnolipid producing bacteria applied are Pseudomonads, especially strains of the species P. aeruginosa. Rhamnolipid production in P. aeruginosa is quorum sensing (QS) regulated (Ochsner and Reiser 1995). In 2000, the complete genome of P. aeruginosa PAO1 (Stover et al. 2000), a QS model organism, was published and thus PAO1 became the best studied P. aeruginosa strain (Venturi 2006; Potvin et al. 2008; Williams and Camara 2009). However, today there is still a lack of understanding the rhamnolipid regulation in pilot plant bioreactor systems on genetic, protein, and metabolic level. Two main types of rhamnolipids (Fig. 1) are typically secreted when P. aeruginosa is growing on water immiscible substrates (Lang and Trowitzsch-Kienast 2002). The most frequently mono- and di-rhamnolipids consist of β-hydroxydecanoic acid residues, namely α-l-rhamnopyranosyl-3-hydroxydecanoyl-3-hydroxydecanoate (Rha-C10-C10) and α-l-rhamnopyranosyl-(1-2)-α-l-rhamnopyranosyl-3-hydroxydecanoyl-3-hydroxydecanoate (Rha-Rha-C10-C10), respectively. In a patent Giani et al. (1997) claimed to achieve 74-112 g/L of rhamnolipids. No verification of these claims on laboratory scale was reported since then. Typical obstacles encountered during rhamnolipid production include, excessive foaming, insufficient rhamnolipid yields, expensive raw materials and relatively high costs for downstream processing (Banat et al. 2000). The fact that many different strains have been used and the quantification of rhamnolipids is mostly done by indirect carbohydrate analysis (Chandrasekaran and BeMiller 1980), leads to confusing comparisons in literature. Mainly heuristic approaches of process development were used in the last decades but according to Lee et al. (2005) up-to-date process development should include systems biotechnological approaches (e.g., prote-, metabol-, transcriptomics). As many of the tools are established for PAO1, this strain should be an appropriate model for rhamnolipid processes. The intention of this study was to investigate whether P. aeruginosa PAO1 produces high enough quantities of rhamnolipids to be used as a model for pilot plant scale bioreactor process development. Additionally, the introduction of a platform batch process for further knowledge-based systems biotechnological and bioengineering approaches, rather than heuristics, was pursued.
Materials and methods
Chemicals
Sunflower oil (Bellasan®, Aldi Süd, Rastatt, Germany) was of food grade. The di-rhamnolipid (Rha-Rha-C10-C10) standard was a gift from former Hoechst AG (Hoechst, Germany) and the mono-rhamnolipid (Rha-C10-C10) standard for high performance liquid chromatography (HPLC) analysis was prepared as described before by Trummler et al. (2003). For rhamnolipid derivatization triethylamine and 4-bromophenacylbromide were used (Sigma-Aldrich Laborchemikalien GmbH, Seelze, Germany) like described by Schenk et al. (1995). Other chemicals were from Carl Roth GmbH (Karlsruhe, Germany). All chemicals were of analytical grade.
Microorganism
The bacterial strain P. aeruginosa PAO1 (Holloway et al. 1979; Stover et al. 2000) was used for all experiments described below. The strain P. aeruginosa PAO1 was kindly provided by Frank Rosenau, Forschungszentrum Jülich (Jülich, Germany). Glycerol stocks were prepared of a culture in Lysogeny Broth (Bertani 1951) from the exponential growth phase and stored at −80 °C until needed.
Culture methods
Media
Lysogeny broth (LB) was used for pre-cultivation step one. For the seed culture, a nitrogen-limited medium consisting of 125 g/L sunflower oil and a Ca-free mineral salt solution with 1.5 g/L NaNO3, 0.05 g/L MgSO4 × 7 H2O, 0.1 g/L KCl, containing a 0.1 M sodium phosphate buffer at pH 6.5, was used. A total of 1 mL/L of trace element solution was added. The trace element solution contained 2.0 g/L sodium citrate × 2 H2O, 0.28 g/L FeCl3 × 6 H2O, 1.4 g/L ZnSO4 × 7 H2O, 1.2 g/L CoCl2 × 6 H2O, 1.2 g/L CuSO4 × 5 H2O, and 0.8 g/L MnSO4 x H2O.
Nitrogen-limited production medium consisted of a Ca-free mineral salt solution with 15.0 g/L NaNO3, 0.5 g/L MgSO4 × 7 H2O, 1.0 g/L KCl and as a phosphate source 0.3 g/L K2HPO4. As sole carbon source sunflower oil with a starting concentration of 250 g/L was used and 1 mL/L of the above mentioned trace element solution was added. Mineral salt solutions, phosphate sources, and sunflower oil were autoclaved separately for all experiments. Trace elements were filter-sterilized through a 0.22 µm membrane filter (Carl Roth GmbH, Karlsruhe, Germany). Production medium was adjusted to pH 6.5 and controlled during cultivation using 4 M NaOH or 4 M H3PO4, respectively.
Preparation of seed culture
All shake flask cultures were incubated in a shake incubator chamber (Multitron II, HT Infors, Bottmingen, Switzerland). First 25 mL of LB in a 100-mL baffled shake flask were inoculated with a total volume of 100 µL from the glycerol stock solution of P. aeruginosa PAO1 and incubated for 24 h at 37 °C, 120 rpm. Seed cultures containing 200 mL medium were cultivated in a 1-L baffled shake flask and inoculated using a total of 5 mL from the 24-h LB culture. This culture was incubated for at least 24 h at 37 °C, 120 rpm. The seed culture was used to inoculate with a resulting OD580 of 0.06 in the aqueous phase at the start of the bioreactor cultivation.
Production of rhamnolipids using P. aeruginosa PAO1 in 30 L bioreactor scale
A 42-L stirred tank, with a nominal capacity of 30 L, was used for all cultivations (Biostat® Cplus, Sartorius Stedim Biotech, Göttingen, Germany). The bioreactor was equipped with an integrated process control system for temperature, pH, pO2, airflow and foam level regulation (MFCS/win 2.1, Sartorius BBI, Göttingen, Germany). The foaming was controlled by a modified foam breaker in the headspace of the bioreactor, which was automatically activated when the foam level reached the 35 L scale of the bioreactor (approximately 15 L of foam). Stirrer speed was set fix at 400 rpm, temperature at 37 °C and dissolved oxygen was set at minimum 5% and controlled by variation of the aeration rates between 0.1 L/(L min) and 3.3 L/(L min), related to the aqueous phase of the production medium (15 L). For the cultivation, 19 L of the production medium was used. The trace element solution was added at cultivation times of 0, 20, 40, 70, and 120 h, as described before (Giani et al. 1997). Exhaust gas was continuously analyzed (EGAS-L gas analyzer, Sartorius BBI, Göttingen, Germany). The cultivation was performed as a triplicate. Online parameters (exhaust gas, pH control, etc.) were monitored for 165 h.
Analytical methods
Sampling and processing
Samples were taken for offline analysis of biodrymass (BDM), sunflower oil and RL concentrations. Therefore, the culture suspension was mixed vigorously with n-hexane 1:1 (v/v) and centrifuged (4,600 g, 4 °C, 30 min) for separation of cells, aqueous and n-hexane phase. The n-hexane phase was used for gravimetric determination of sunflower oil concentrations, after evaporation of n-hexane. It was confirmed by HPLC (cf. below), that no rhamnolipids were extracted by the n-hexane extraction step. The cell pellet was washed once in 0.9% NaCl solution (4,600 g, 4 °C, 30 min), dried till constant weight and BDM was quantified gravimetrically. An aliquot of the aqueous phase was acidified with 85% phosphoric acid 1:100 (v/v) to adjust a pH of about 2-3, leading to precipitation of the rhamnolipids. Rhamnolipids were extracted twice with ethyl acetate 1:1.25 (v/v). Appropriate amounts of ethyl acetate extracts were evaporated and used for rhamnolipid quantification by HPLC as described below. Offline analysis was continued till 100 h of process time for all triplicates and till 165 h for one of the cultivations.
Data analysis
Bacterial growth was fitted using a logistic equation (BDMfit) with four parameters in a scientific data analysis and graphing software (Sigma Plot 9.0, Systat, San Jose, USA). The used equation was:
The four parameters are the following: y 0 indicates the BDMinoc after inoculation; a indicates the maximum additional BDMadd produced in addition to BDMinoc (\( {\hbox{BD}}{{\hbox{M}}_{\max }} = a + {y_0} \)); x 0 indicates the process time when 1/2BDMadd is reached. The fourth parameter b in the four-parameter equation is a shape parameter and difficult to explain biologically (Erkmen and Alben 2002). Derivation of the fitting was used for determination of, specific (μfit) and maximum (μfit,max), growth rates.
HPLC-UV/vis quantification of rhamnolipids
Rhamnolipid standard solutions of 1 g/L in 0.1 M sodium buffer solution pH 6.5 were used for calibration. Phenacyl esters of rhamnolipids for HPLC analysis were obtained as described before by Schenk et al. (1995) with minor changes. The analysis was performed with a standard HPLC device (Agilent 1100 Series, Agilent, Waldbronn, Germany) equipped with a 15 cm reverse phase column (Supelcosil® LC-18, Supelco, Deisenhofen, Germany) at 30 °C. Mobile phase solutions were solution A with 5% methanol and solution B with 95% methanol in ultrapure water, respectively. To achieve separation, a gradient of solution B from 80% to 100% was used according to the following protocol: from t = 0 to t = 17 min increase of solution B from 80% to 100%. Holding 100% solution B up to t = 25 min and decrease to 80% solution B until t = 30 min. Holding 80% solution B for 5 min more to equilibrate. The flow rate was 0.4 mL/min and rhamnolipids were monitored at 254 nm. Retention times were (21.5 ± 0.1) min for Rha-Rha-C10-C10 and (22.2 ± 0.1) min for Rha-C10-C10.
Results
Triplicate production of rhamnolipids in a 30 L bioreactor system
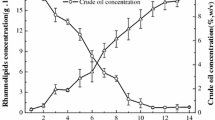
The cultivation of P. aeruginosa PAO1 in a 30 L bioreactor system was performed three times. Logistic growth of the bacterial culture was determined (Fig. 2). A logistic equation with four parameters was used to fit the bacterial growth (BDMfit, Eq. 1). The maintained parameters were the following: BDMinoc was 0.65 g/L; BDMadd was 15.63 g/L; time (1/2BDMadd) was 31.41 h and the shape factor b was −2.68. Thus, BDMmax was 16.28 g/L. After 24 h of cultivation rhamnolipid concentrations of 0.65 g/L were quantified. Rhamnolipid production continued up to 90 h of cultivation and reached a maximum of approximately 39 g/L. No further increase of the total rhamnolipid concentration (cRL) was observed from 90 h of process time. After 40-45 h of cultivation, the mechanical foam control was activated automatically due to excessive foaming. Visually, the foam was uncongested at the beginning while getting more and more compact with progress of the cultivation, leading to a heterogeneously composed broth of a 35 L dense airy emulsion at the end of the process. The sunflower oil was not degraded completely by the microorganisms resulting in a resting concentration of about 50 g/L. Although BDM and rhamnolipid concentration were stable after 90 h, still a small decrease of sunflower oil was observed.
Production of rhamnolipids with P. aeruginosa PAO1 in a 30 L pilot plant bioreactor with sunflower oil as sole carbon source. Measured biodrymass (BDM, square), rhamnolipid (cRL, circle), and sunflower oil (triangle) concentrations are given as averages of cultivation triplicates. The dashed line represents a logistic fitting of the biodrymass concentration (BDMfit) based on Eq. 1
From the BDMfit results, the specific growth rate (µfit) lapse was determined. Figure 3 shows values for specific growth (µcalc), based on the measured data, together with µfit and the specific rhamnolipid productivity (PRL). The relatively high standard deviations derive from the error propagation of the BDM, t, and cRL data of Fig. 2. The maximum specific growth rate (μfit,max) determined by the deviation was 0.133 h−1 at 11 h of cultivation. This reflects a minimum cell division (t d) time of 5.21 h. The maximum rhamnolipid production was not growth related, what is in accordance to literature data and a typical behavior of secondary metabolites (Sobéron-Chávez et al. 2005). The maximum of the PRL was calculated for between 40 and 70 h of cultivation (max. PRL = 0.088 gRL/gBDMh) in the transition towards stationary phase. However, small quantities of rhamnolipid were already produced in the early growth phase. The production process was finished after 90 h and all below calculated rates are therefore related to this process length. A volumetric productivity (PV) of 0.43 g/Lh, which is comparable with the best-reported productivities (Table 1), was achieved. The overall specific productivity per final BDM was 2.4 gRL/gBDMh, the product yield per consumed sunflower oil (YP/S) was 0.23 gRL/goil and in total 8.17 gRL/h were produced.
Calculated averaged specific growth rate (μcalc, blank square) and rhamnolipid productivities (PRL, filled square) of a triplicate cultivation of P. aeruginosa PAO1 in a 30 L pilot plant bioreactor using sunflower oil as sole carbon source. Dashed line represents the μfit course derived from the logistic fitting in (Fig. 2)
Molar ratio of di-rhamnolipid to mono-rhamnolipid
Figure 4a shows the molar di-rhmanolipid (Rha-Rha-C10-C10)/mono-rhamnolipid (Rha-C10-C10) ratio over the time course of the cultivations. While starting at about 1:1 the molar di- to mono-rhamnolipid ratio shifts towards 1.5:1 during the phase of maximum rhamnolipid productivity between 40 and 70 h of cultivation. In later stages, even a ratio close to 2:1 was observed. After 90 h of cultivation, the total rhamnolipid concentration stayed on a level of about 60 mM. Rha-C10-C10 decreased about 3-4 mM, while Rha-Rha-C10-C10 showed an increase of a similar amount (Fig. 4b), respectively.
Investigation of the molar variation of mono-rhamnolipid (Rha-C10-C10) and di-rhamnolipid (Rha-Rha-C10-C10) derived from three 30 L bioreactor cultivations of P. aeruginosa PAO1 with sunflower oil as sole carbon source. a Lapse of molar ratios Rha-Rha-C10-C10: Rha-C10-C10 (di-RL:mono-RL (blank square)). b Course of averaged rhamnolipid concentrations for Rha-C10-C10 (blank circle) and Rha-Rha-C10-C10 (filled circle)
Exhaust gas analysis
During the cultivations, oxygen and carbon dioxide partial pressures were analyzed in the exhaust gas and recorded by the process control system. Figure 5a shows the calculated oxygen uptake rate (OUR) and carbon evolution rate (CER) which increased during the logistic growth phase. Both showed a distinct peak within the time interval of maximum rhamnolipid production. The specific OUR and CER (Fig. 5b) per BDM were calculated with the BDMfit results from the logistic fitting described above (Fig. 2) by dividing OUR and CER through BDMfit, respectively. The specific rates showed a maximum in the growth phase but also allowed to distinguish a small peak during the max. PRL. The respective CER/OUR ratios (respiratory quotient or RQ) allowed that four different phases of microbial metabolism could be distinguished (Fig. 5c). The relatively high standard deviations are due to error propagation of averaged exhaust gas data. Higher RQs of about 0.66-0.76 were observed at the growth phase (I), the second phase (II) was characterized by lower RQs of 0.6. The third phase (III) showed RQs of between 0.63 and 0.66 decreasing to a phase (IV) with average RQs of 0.62.
Results of exhaust gas analysis for the cultivation of P. aeruginosa PAO1 with sunflower oil as the sole carbon source. a OUR (straight) and CER (dashed) courses; b BDM related spec. OUR (straight) and spec. CER (dashed) courses; c respective respiratory quotients (RQ = CER/OUR). Indicated phases are: I—maximum growth, II—maximum PRL, III—end of growth and decrease of rhamnolipid production, and IV—stationary phase
Discussion
It was shown that PAO1 has the potential to produce rhamnolipids in high enough quantities to match with well-established production strains like DSM 2874 (Syldatk et al. 1985a, b; Matulovic 1987; Trummler et al. 2003), DSM 7107 and DSM 7108 (Giani et al. 1997). Inquiries with PAO1 aiming to overproduce rhamnolipids were mostly restricted to shake flask (Wang et al. 2007) or small bioreactor scale, and did not surpass 2.2 g/L (Ochsner et al. 1994, 1995; Ochsner and Reiser 1995; Rahim et al. 2001). Although intensive research was done on QS regulation (Dockery and Leener 2001; Chen et al. 2004; Wagner et al. 2006; Williams and Camara 2009), the rhamnolipid production was studied and optimized with mostly heuristic approaches during the last decades. In comparison to shake flask experiments, the rhamnolipid concentrations in bioreactor experiments are up to 100-fold higher. A main finding under the investigated experimental set-up was that P. aeruginosa PAO1 produces high quantities of rhamnolipids in batch culture of up to 39 g/L with a PV of 0.43 g/Lh. Additionally, the OUR and the CER could directly be associated with PRL, both showing peaks between 40 and 60 h of process time. Thus exhaust gas analysis will allow online observation and could support future investigations and development of rhamnolipid production. The complete sequenced and annotated genome of PAO1 (Stover et al. 2000) gives the possibility to use the up-to-date systems biotechnological tools like microarray, mass spectrometric, and in-silico modeling techniques. Basic research groups working in the field of QS regulation have already established many of these technologies for PAO1 (Wagner et al. 2003; Nouwens et al. 2003; Wagner et al. 2004). During PAO1 experiments, it was possible to distinguish different process stages (Fig. 5) by exhaust gas analysis, which now could be investigated by the help of process analytical technologies like transcriptome, proteome, and metabolome analysis. Comparative experiments of PAO1 wild-type and already available mutant strains should lead to new insights about rhamnolipid production in bioreactor cultivations. Among the main issues must be the regulation of rhamnolipid production, oil degradation, lipase activity, assimilation of substrate, and rhamnolipid feed-back, which all leads to a characteristic, nearly Gaussian distribution, of the specific productivity in the pilot plant bioreactor system (Fig. 3).
The analysis of the single rhamnolipids by HPLC instead of the commonly used total or indirect quantification (Chandrasekaran and BeMiller 1980; Pinzon and Ju 2009) lead to important results like the transition from mono- to di-rhamnolipids (Fig. 4a), the difference in final mono- and di-rhamnolipid concentrations and the potential extracellular conversion of Rha-C10-C10 to Rha-Rha-C10-C10 (Fig. 4b). These findings indicate a more complex rhamnolipid mechanism of regulation then thought before (Sullivan 1998). The importance of sigma and other transcriptional factors has already been discussed (Rahim et al. 2001; Medina et al. 2003; Potvin et al. 2008) for rhamnolipid regulation. However, their characteristic devolution during batch cultivation should reveal important information about the regulation on genetic level.
After 40 h of cultivation mostly di-rhamnolipids were synthesized, leading to nearly four times more l-rhamnose incorporated in di-rhamnolipids than in mono-rhamnolipids (1 mol di-rhamnolipid equals 2 mol of l-rhamnose). There was still enough l-rhamnose for this step of synthesis catalyzed by RhlC (Rahim et al. 2001), or rhamnosyltransferase II, while the production pathway towards Rha-C10-C10 must have been limited or suppressed somewhere. Additional metabolome analyzes could show whether there is a limitation in the respective precursor pools for rhamnolipid production. Such information on genomic and metabolomic level should give the possibility to integrate the findings in a systems bio(techno)logic network (Wagner et al. 2004; Wang et al. 2006).
The present results suggest PAO1 being used as a model organism also for the biotechnological production of rhamnolipids. Advantages would be an enhancement of the comparability of experimental results throughout different laboratories and the possibility of closer cooperation between engineering and basic research groups. New insights will be applicable to the established high production strains like DSM 7107, DSM 7108 and DSM 2874. After all, it has to be mentioned that the established batch cultivation was not optimized, thus leaving space for higher productivities and yields with PAO1 in future studies. PAO1 not only seems to be an appropriate model, but surprisingly has the potential as a strain of choice for actual biotechnological rhamnolipid production. With further research and application of culture and process development strategies, and additional incorporation of exhaust gas analysis into an advanced process control, economic rhamnolipid production should be in not-so-distant future.
References
Andrä J, Rademann J, Howe J, Koch M, Heine H, Zähringer U, Brandenburg K (2006) Endotoxin-like properties of a rhamnolipid exotoxin from Burkholderia (Pseudomonas) plantarii: immune cell stimulation and biophysical characterization. Biol Chem 387:301–310
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508
Bertani G (1951) Studies on lysogenisis. 1. The mode of phage II liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300
Chandrasekaran EV, Bemiller JN (1980) Constituent analysis of glycosaminoglycans. In: Whistler RL, Bemiller JN (eds) Methods in carbohydrate chemistry. Academic, New York
Chen F, Chen C, Riadi L, Ju L (2004) Modeling rhl quorum sensing regulation on rhamnolipid production by Pseudomonas aeruginosa. Biotechnol Prog 20:1325–1331
Dockery JD, Keener JP (2001) A mathematical model for quorum sensing in Pseudomonas aeruginosa. Bull Math Biol 63:95–116
Erkmen O, Alben E (2002) Mathematical modelling of citric acid production and biomass formation by Aspergillus niger in undersized semolina. J Food Eng 52:161–166
Giani C, Wullbrandt D, Rothert R, Meiwes J (1997) Pseudomonas aeruginosa and its use in a process for the biotechnological preparation of l-rhamnose. German Patent, US005658793A
Gunther NW (2007) Processes for the production of rhamnolipids. US Patent, 7,202,063 B1
Häußler S, Nimtz M, Domke T, Wray V, Steinmetz I (1998) Purification and characterization of a cytotoxic exolipid of Burkholderia pseudomallei. Infect Immun 66:1588–1593
Hembach T (1994) Untersuchungen zur mikrobiellen Umsetzung von Maiskeimöl zu Rhamnolipid. University Press Hohenheim, Stuttgart, Germany, PhD Thesis
Holloway BW, Krishnapillai V, Morgan AF (1979) Chromosomal genetics of Pseudomonas. Microbiol Rev 43:73–102
Jarvis FG, Johnson MJ (1949) A glyco-lipide produced by Pseudomonas aeruginosa. J Am Chem Soc 71:4124–4126
Lang S, Trowitzsch-Kienast W (2002) Biotenside. B. G. Teubner, Stuttgart
Lee SY, Lee DY, Kim TY (2005) Systems biotechnology for strain improvement. Trends Biotechnol 23:349–358
Linhardt RJ, Bakhit R, Daniels L, Mayerl F, Pickenhagen W (1989) Microbially produced rhamnolipid as a source of rhamnose. Biotechnol Bioeng 33:365–368
Manso Pajarron A, De Koster CG, Heerma W, Schmidt M, Haverkamp J (1993) Structure identification of natural rhamnolipid mixtures by fast atom bombardment tandem mass spectrometry. Glycoconj J 10:219–226
Marsudi S, Unno H, Hori K (2008) Palm oil utilization for the simultaneous production of polyhydroxyalkanoates and rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 78:955–961
Matulovic U (1987) Verfahrensentwicklung zur Herstellung grenzflächenaktiver Rhamnolipide mit immobilisierten Zellen von Pseudomonas spec. DSM 2874. Thesis: Technische Universität Braunschweig, Germany
Medina G, Juarez K, Diaz R, Sobéron-Chávez G (2003) Transcriptional regulation of Pseudomonas aeruginosa rhlR, encoding a quorum-sensing regulatory protein. Microbiology (UK) 149:3073–3081
Nguyen TT, Youssef NH, Mcinerney MJ, Sabatini DA (2008) Rhamnolipid biosurfactant mixtures for environmental remediation. Water Res 42:1735–1743
Nouwens AS, Beatson SA, Whitchurch CB, Walsh BJ, Schweizer HP, Mattick JS, Cordwell SJ (2003) Proteome analysis of extracellular proteins regulated by the las and rhl quorum sensing systems in Pseudomonas aeruginosa PAO1. Microbiology (UK) 149:1311–1322
Ochsner UA, Reiser J (1995) Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA 92:6424–6428
Ochsner UA, Koch A, Fiechter A, Reiser J (1994) Isolation and characterization of a regulatory gene affecting rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. J Bacteriol 176:2044–2054
Ochsner UA, Reiser J, Fiechter A, Witholt B (1995) Production of Pseudomonas aeruginosa rhamnolipid biosurfactants in heterologous hosts. Appl Environ Microbiol 61:3503–3506
Pinzon NM, Ju LK (2009) Analysis of rhamnolipid biosurfactants by methylene blue complexation. Appl Microbiol Biotechnol 82:975–981
Potvin E, Sanschagrin F, Levesque R (2008) Sigma factors in Pseudomonas aeruginosa. Fems Microbiol Rev 32:38–55
Rahim R, Ochsner UA, Olvera C, Graninger M, Messner P, Lam JS, Sobéron-Chávez G (2001) Cloning and functional characterization of the Pseudomonas aeruginosa rhlC gene that encodes rhamnosyltransferase 2, an enzyme responsible for di-rhamnolipid biosynthesis. Mol Microbiol 40:708–718
Rahman KSM, Rahman TJ, Mcclean S, Marchant R, Banat IM (2002) Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low-cost raw materials. Biotechnol Prog 18:1277–1281
Schenk T, Schuphan I, Schmidt B (1995) High-performance liquid-chromatographic determination of the rhamnolipids produced by Pseudomonas aeruginosa. J Chromatogr 693:7–13
Soberón-Chávez G, Lépine F, Déziel E (2005) Production of rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 68:718–725
Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV (2000) Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964
Sullivan ER (1998) Molecular genetics of biosurfactant production. Environ Microbiol 9:263–269
Syldatk C, Lang S, Matulovic U, Wagner F (1985a) Production of four interfacial active rhamnolipids from n-alkanes or glycerol by resting cells of Pseudomonas species DSM 2874. Z Naturforsch [C] 40:61–67
Syldatk C, Lang S, Wagner F, Wray V, Witte L (1985b) Chemical and physical characterization of four interfacial-active rhamnolipids from Pseudomonas spec. DSM 2874 grown on n-alkanes. Z Naturforsch [C] 40:51–60
Trummler K, Effenberger F, Syldatk C (2003) An integrated microbial/enzymatic process for production of rhamnolipids and L-(+)-rhamnose from rapeseed oil with Pseudomonas sp DSM 2874. Eur J Lipid Sci Technol 105:563–571
Venturi V (2006) Regulation of quorum sensing in Pseudomonas. Fems Microbiol Rev 30:274–291
Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH (2003) Microarray analysis of Pseudomonas aeruginosa quorum sensing regulons: effects of growth phase and environment. J Bacteriol 185:2080–2095
Wagner VE, Gillis RJ, Iglewski BH (2004) Transcriptome analysis of quorum sensing regulation and virulence factor expression in Pseudomonas aeruginosa. Vaccine 22(Suppl 1):S15–S20
Wagner VE, Frelinger JG, Barth RK, Iglewski BH (2006) Quorum sensing: dynamic response of Pseudomonas aeruginosa to external signals. Trends Microbiol 14:55–58
Wang QZ, Wu CY, Chen T, Chen X, Zhao XM (2006) Integrating metabolomics into a systems biology framework to exploit metabolic complexity: strategies and applications in microorganisms. Appl Microbiol Biotechnol 70:151–161
Wang QH, Fang XD, Bai BJ, Liang XL, Shuler PJ, Goddard WA, Tang YC (2007) Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnol Bioeng 98:842–853
Wei YH, Chou CL, Chang JS (2005) Rhamnolipid production by indigenous Pseudomonas aeruginosa J4 originating from petrochemical wastewater. Biochem Eng J 27:146
Williams P, Camara M (2009) Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol 12:182–191
Zhang GL, Wu YT, Qian XP, Meng Q (2005) Biodegradation of crude oil by Pseudomonas aeruginosa in the presence of rhamnolipids. J Zhejiang Univ SCI 6B:725–730
Acknowledgment
We want to thank the Fachagentur für nachwachsende Rohstoffe e.V. (FNR) for funding the project. Thanks go to Dr. Frank Rosenau for kindly providing the strain P. aeruginosa PAO1 and Siegfried Almstedt for construction and installation of a new non-commercially available mechanical foam breaker.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Müller, M.M., Hörmann, B., Syldatk, C. et al. Pseudomonas aeruginosa PAO1 as a model for rhamnolipid production in bioreactor systems. Appl Microbiol Biotechnol 87, 167–174 (2010). https://doi.org/10.1007/s00253-010-2513-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2513-7