Abstract
Objectives
To improve rhamnolipid production and its potential application in removal of crude oil, the recombinant Pseudomonas aeruginosa strain DAB was constructed to enhance yield of rhamnolipids.
Results
Strain DAB had a higher yield of 17.3 g rhamnolipids l−1 in the removal process with crude oil as the sole carbon source than 10 g rhamnolipids l−1 of wild-type strain DN1, where 1% crude oil was degraded more than 95% after 14 days cultivation. These rhamnolipids reduced the surface tension of water from 72.92 to 26.15 mN m−1 with CMC of 90 mg l−1. The predominant rhamnolipid congeners were Rha–C10–C10 and Rha–Rha–C10–C10 detected by MALDI-TOF MS analysis with approx. 70% relative abundance, although a total of 21 rhamnolipid congeners were accumulated.
Conclusion
Increasing the copy number of rhlAB genes efficiently enhanced the production of rhamnolipids by the recombinant P. aeruginosa DAB and thus presents a promising application for the bioremediation process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crude oil is one of the most important and predominant energy resources in the world market. Nevertheless, oil spillage is a major environmental issue with severe health and ecological consequences, since many of these substances are considered to be hazardous priority pollutants (Nikolopoulou et al. 2013; Sajna et al. 2015). Chemically-synthesized surfactants are usually used for clean-up of oil spills. However, because of their toxicity and resistance to degradation, they can cause serious environmental problems (Nikolopoulou et al. 2013). Compared to synthetic chemical surfactants, biosurfactants produced by microbial cells are environmentally compatible, have a low toxicity and are easily biodegraded and thus are more appropriate for bioremediation (Kaczorek and Olszanowski 2011; Zhang et al. 2011).
Rhamnolipids are biosurfactants capable of increasing the bioavailability of hydrophobic chemicals by dissolving and emulsifying these non-hydrophilic hydrocarbons, and their supplementation improved the bioremediation of crude oil contaminated soil with reduction of petroleum hydrocarbon up to 87% (Kaczorek and Olszanowski 2011; Zhang et al. 2011). Pseudomonas aeruginosa is a well-known rhamnolipid producer. It is also a ubiquitous hydrocarbon-degrader (Müller et al. 2011). However, rhamnolipid production has not yet been achieved in a large scale so far, owing to various drawbacks such as low product yields and relatively costly raw materials. Conceivable strategies, such as metabolic engineering, production in heterologous hosts and fermentation approaches have been studied for rhamnolipid production improvement, (Dobler et al. 2016). Nevertheless, there is little information about the investigation on over-expression of the key genes and increase the copy numbers of key genes under some harsh conditions such as contamination of crude oil, although it has been shown that different waste substrates may also be used for production of rhamnolipids, since they are usually less expensive and prove to be beneficial for the environment (Amani et al. 2013; Dobler et al. 2016). Herein, the aim of this study is to investigate rhamnolipid productivity by a recombinant P. aeruginosa with crude oil as the sole carbon source, and to evaluate the possible use of those rhamnolipid congeners as surface actives in biodegradation of crude oil.
Materials and methods
Bacterial strains, media and cultural conditions
Pseudomonas aeruginosa strain DN1 (GeneBank accession No. CP017099) used in this study was originally isolated from petroleum-contaminated soil in Shaanxi, China, and is already known to degrade various hydrocarbons (Dong et al. 2017; Ma et al. 2016). For constructing an engineered strain, the rhamnosyltransferase 1 complex RhlAB with the native operon promoter was amplified from PAO1 genomic DNA (purchased from ATCC) with the primers: rhlAB-F (5′-GAATCGAATTCATGCGGCCGAAAGTCTGT-3′) and rhlAB-R.
(5′-CGGTAAGCTTTCAGGACGCAGCCTTCAGCC-3′), which were designed based on the genomic sequences of PAO1. The underlined sequences represent the recognition sites of restriction enzymes EcoRI and HindIII, respectively. The PCR product of the rhlAB operon was digested with EcoRI and HindIII and cloned into pAK1900 to produce the recombinant plasmid pAK-AB, then it was transferred into P. aeruginosa DN1 by electroporation, giving rise to the recombinant strain DAB (Jansons et al. 1994). The recombinant plasmid, pAK-AB isolated in the LB medium supplemented with carbenicillin (250 mg l−1), was screened by PCR with the primers used for rhlAB amplification and confirmed by double digested analysis of recombinant plasmids pAK-AB. After genotypic and phenotypic analysis, the engineered strain DAB was selected for further production of rhamnolipid.
Lysogeny broth (LB) was used for seed culture of P. aeruginosa strain DN1 and strain DAB. Biosurfactant Production Liquid Medium (BPLM, pH 7.4) was chosen to evaluate surfactant activity (Ma et al. 2016; Nie et al. 2010). The strain DAB was inoculated in the nutrition optimization BPLM media with crude oil as the sole carbon source.
Biodegradation of crude oil
The crude oil used contained ~34% saturated hydrocarbons, ~29% aromaties, ~35% resins and 2% asphaltene. A 10 ml 108 CFU ml−1 seed culture of strain DN1 and strain DAB was prepared for adding into a 500 ml Erlenmeyer flask containing 200 ml BPLM broth supplemented with 1% (v/v) crude oil. Three experimental replicates, including one negative control with autoclaved cells of strains added, were incubated at 30 °C with shaking at 200 rpm. Samples were taken at every 24 h for analysis. Rhamnolipid concentrations were measured using the anthrone method at 620 nm (Abidi et al. 2010). The total mass of hydrocarbon residues was determined as described by Kaczorek and Olszanowski (2011). The final results were calculated with respect to negative control.
Analytical methods
The interfacial tension was carried out against crude oil by using a spinning drop tensiometer (ZL-3000, Zibo, China) as detailed by Cayias et al. (1975). The surface tension and critical micelle concentration (CMC) were measured by a JYW-200A automatic interfacial tension meter (Chengde Jinhe Equipment Manufacture Co., Ltd) using the ring methods. Based on the surface tension measurement, the CMC was then obtained by plotting the surface tension as a function of the serial concentration of rhamnolipids, the surface tension at this point was designated as the CMC value (Bordoloi and Konwar 2008).
Fermentation broth was centrifuged at 8000×g for 20 min at 4 °C. The supernatant was acidified to a ~pH 2–3 with 85% (v/v) phosphoric acid and extracted three times with 0.3 vol. ice-cold chloroform/methanol (2:1 v/v). The organic phases were collected and pooled. The solvent was evaporated in a rotary vacuum evaporator. The residue was further purified twice with dichloromethane and dried with a rotary evaporator to obtain the pure rhamnolipids. These were stored at −20 °C for further analysis.
MALDI-TOF MS was employed to elucidate the structure of rhamnolipids using an Applied Biosystems ABI4700 TOF/TOF mass spectrometer in reflector mode with an accelerating voltage of 20 kV. Samples were mixed in a 1:4 ratio with α-cyano-4-hydroxycinnamic acid (HCCA) in 50% acetonitrile and 0.1% TFA (Nie et al. 2010). 0.5 μl of the sample was applied to the sample plate and air dried.
Results and discussion
Rhamnolipid production by wild strain DN1 and recombinant strain DAB
Rhamnolipids were produced by strains DN1 and DAB under aerobic conditions with crude oil as the sole carbon source. During the initial 24 h of cultivation, 0.5 g rhamnolipids l−1 were produced by strain DAB (Fig. 1). Rhamnolipid accumulation with strain DAB started after 48 h c and continued to increase to 17.3 g l−1 after 14 day cultivation. The wild strain DN1 had a yield of 10 g rhamnolipids l−1 under the same conditions.
Generally, the type of rhamnolipids depends on the bacterial strain, the carbon source used and the process strategy (Abdelmawgoud et al. 2010; Nitschke et al. 2010). The strategy is more effective when the microorganisms are using hydrocarbons as carbon sources, as well as the culture producing the biosurfactant itself was capable of the targeted bioremediation as was the case in this study. Compared to other conceivable strategies for rhamnolipid production improvement, the copy numbers of rhlAB genes through genetic manipulation enhanced the rhamnolipid production by recombinant strain DAB, which stood out from other rhamnolipid-producing Pseudomonas strains because of its capability to use crude oil as the sole carbon source to produce rhamnolipids (Zhao et al. 2015). The results reported here support those observations that hydrophobic substrates showed to be the best choice for rhamnolipid production and emphasize the importance of carbon source in the diversity of rhamnolipid production (Dobler et al. 2016; Müller et al. 2011).
Chemical characterization of rhamnolipids
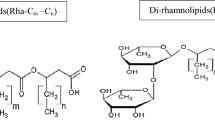
Rhamnolipids were extracted after 14 day cultivation in BPLM media with crude oil as the sole carbon source. The purified rhamnolipids were analyzed by MALDI-TOF MS. Strain DAB accumulated 21 rhamnolipids with 33 different metal ion (Na+ or K+) adducts including dirhamnolipid congeners as well as monorhamnolipid congeners. These are summarized in Table 1 and illustrated in Fig. 2. The parent ions at m/z 527.4 and 673.3 were predominantly 70% relative abundance of the total contents, and could be assigned to singly sodiated monorhamnolipid Rha–C10–C10–Na+ and dirhamnolipid Rha–Rha–C10–C10–Na+, respectively (Fig. 2b, c). Thus, strain DAB was characterized for its capacity to produce diverse components of rhamnolipids with crude oil as the sole carbon source. Among those components were two predominant components, Rha–C10–C10 and Rha–Rha–C10–C10, that played key roles in exhibiting great performance of surface actives and emulsification. This finding was consistent with previous reports for P. aeruginosa SP4 (Sarachat et al. 2010), P. aeruginosa LBI (Nitschke et al. 2010) and P. aeruginosa NY3 (Nie et al. 2010).
Effect on surface tension and interfacial tension of rhamnolipids
CMC indicates the minimum concentration of rhamnolipids necessary for the maximum reduction in surface tension and interfacial tension (IFT). The profile of changes in surface tension and IFT versus rhamnolipid concentration are illustrated in Fig. 3. The rhamnolipid mixture from strain DAB reduced the surface tension of water from 72.92 to 26.15 mN m−1 with the rhamnolipid at 90 mg l−1, and remained nearly unchanged above this concentration. Moreover, the IFT of 1% oil was decreased from 47.36 to 4.59 mN m−1, and the minimum IFT kept at 4.59 mN m−1 when the rhamnolipid was up to 90 mg l−1.
Bioavailability and biodegradability of crude oil
Evaluation of the effectiveness of strains on crude oil removal efficiency was determined by measuring the cell growth and hydrocarbon utilization, and estimated in terms of crude oil component change throughout the experiments by gas chromatography. Figure 4a and b present the degradability of crude oil and the total depletion of the heavy components during the 14 d growth of strain DAB. During the first 24 h, only 0.05% of the crude oil was removed in the absence of rhamnolipids, which could stabilize the hydrocarbon emulsion and improve the bioavailability. Subsequently, there was a marked increase in the degradation rates, which correlated with increased production of rhamnolipids by the DAB strain. After 14 day, nearly all of the contents in crude oil were decreased and the maximum removal rate of 1% crude oil was 95.7% which compared to 70% by the wild-type strain.
Time course of pollutant removal by the engineered strain DAB after inoculation in the optimized medium consisting of BPLM with crude oil as the sole carbon source a GC chromatograms of crude oil; b removal rate versus cultivation time; c fermentation flasks consisting of BPLM with 10% crude oil as the sole carbon source
A possible explanation for this observation is that strain DAB mainly produced the Rha–Rha–C10–C10 type dirhamnolipid and the Rha–C10–C10 type monorhamnolipid, which produced effective emulsions with hydrocarbons, oils, and fats (Abdelmawgoud et al. 2010). Figure 4c showed that mostly crude oil was emulsified to oil droplets, which increased the crude oil availability for strain DAB and secondly involved in the interaction with the cell surface to increase hydrophobic substrates to associate more easily with the bacterial cells. That is, increased removal was caused by enhancement of cell surface hydrophobicity after rhamnolipid production by strain DAB, which facilitated uptake via direct contact between cells and hydrocarbon droplets (Amani et al. 2013).
Conclusion
A recombinant P. aeruginosa strain DAB produced rhamnolipids up to 17.3 g l−1 with crude oil as the sole carbon source. Rha–Rha–C10–C10 dirhamnolipid and Rha–C10–C10 monorhamnolipid, were the principle products. Compared to wild-type strain DN1, the recombinant strain DAB had a greater capacity to emulsify crude oil, thus would have great potential utility in the bioremediation of petroleum contaminated water and soil.
References
Abdelmawgoud AM, Lépine F, Déziel E (2010) Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol 86:1323–1336
Abidi N, Hequet E, Cabrales L (2010) Changes in sugar composition and cellulose content during the secondary cell wall biogenesis in cotton fibers. Cellulose 17:153–160
Amani H, Müller MM, Syldatk C, Hausmann R (2013) Production of microbial rhamnolipid by Pseudomonas Aeruginosa MM1011 for ex situ enhanced oil recovery. Appl Biochem Biotechnol 170:1080–1093
Bordoloi NK, Konwar BK (2008) Microbial surfactant-enhanced mineral oil recovery under laboratory conditions. Colloid Surf B Biointerfaces 63:73–82
Cayias JL, Schechter RS, Wade WH (1975) The measurement of low interfacial tension via the spinning drop technique. ACS Symp 8:234–247
Dobler L, Vilela LF, Almeida RV, Neves BC (2016) Rhamnolipids in perspective: gene regulatory pathways, metabolic engineering, production and technological forecasting. Nat Biotechnol 33:123–135
Dong W, He CQ, Li YP, Huang C, Chen FL, Ma YL (2017) Complete genome sequence of a versatile hydrocarbon degrader, Pseudomonas aeruginosa DN1 isolated from petroleum-contaminated soil. Gene Rep 7:123–126
Jansons I, Touchie G, Sharp R, Almquist K, Farinha MA, Lam JS, Kropinski AM (1994) Deletion and transposon mutagenesis and sequence analysis of the pRO1600 OriR region found in the broad-host-range plasmids of the pQF series. Plasmid 31:265–274
Kaczorek E, Olszanowski A (2011) Uptake of hydrocarbon by Pseudomonas fluorescens (P1) and Pseudomonas putida (K1) strains in the presence of surfactants: a cell surface modification. Water Air Soil Pollut 214:451–459
Ma KY, Sun MY, Dong W, He CQ, Chen FL, Ma YL (2016) Effects of nutrition optimization strategy on rhamnolipid production in a Pseudomonas aeruginosa strain DN1 for bioremediation of crude oil. Biocat Agric Biotechnol 6:144–151
Müller MM, Hörmann B, Kugel M, Syldatk C, Hausmann R (2011) Evaluation of rhamnolipid production capacity of Pseudomonas aeruginosa PAO1 in comparison to the rhamnolipid over-producer strains DSM 7108 and DSM 2874. Appl Microbiol Biotechnol 89:585–592
Nie M, Yin X, Ren C, Wang Y, Xu F, Shen Q (2010) Novel rhamnolipid biosurfactants produced by a polycyclic aromatic hydrocarbon-degrading bacterium Pseudomonas aeruginosa strain NY3. Biotechnol Adv 28:635–643
Nikolopoulou M, Pasadakis N, Norf H, Kalogerakis N (2013) Enhanced ex situ bioremediation of crude oil contaminated beach sand by supplementation with nutrients and rhamnolipids. Mar Pollut Bull 77:37–44
Nitschke M, Costa SG, Contiero J (2010) Structure and applications of a rhamnolipid surfactant produced in soybean oil waste. Appl Biochem Biotechnol 160:2066–2074
Sajna KV, Sukumaran RK, Gottumukkala LD, Pandey A (2015) Crude oil biodegradation aided by biosurfactants from Pseudozyma sp. NII 08165 or its culture broth. Bioresour Technol 191:133–139
Sarachat T, Pornsunthorntawee O, Chavadej S, Rujiravanit R (2010) Purification and concentration of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa SP4 using foam fractionation. Bioresour Technol 101:324–330
Zhang W, Li J, Huang G, Song W, Huang Y (2011) An experimental study on the bio-surfactant-assisted remediation of crude oil and salt contaminated soils. J Environ Sci Health A Tox Hazard Subst Environ Eng 46:306–313
Zhao F, Cui Q, Han S, Dong H, Zhang J, Ma F, Zhang Y (2015) Enhanced rhamnolipid production of Pseudomonas aeruginosa SG by increasing copy number of rhlAB genes with modified promoter. RS Adv 5:70546–70552
Acknowledgements
This research was supported by the National Science Foundation for Young Scientists of China (Grant No. 31000069) and the research project of Shaanxi Provincial Key Laboratory of Biotechnology (16JS108).
Supplementary information
Supplementary Figure 1—Schematic diagram of the construction of recombinant plasmid.
Supplementary Figure 2—Time course of pollutant degradation by the engineered strain DAB and DN1 after inoculation in the optimized medium consisting of BPLM with different PAHs as the sole carbon source (A) naphathalene; (B) phenanthrene; (C) pyrene; (D) fluoranthene.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
He, C., Dong, W., Li, J. et al. Characterization of rhamnolipid biosurfactants produced by recombinant Pseudomonas aeruginosa strain DAB with removal of crude oil. Biotechnol Lett 39, 1381–1388 (2017). https://doi.org/10.1007/s10529-017-2370-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-017-2370-x