Abstract
The effects of grazing and climate change on primary production have been studied widely, but seldom with mechanistic models. We used a Bayesian model to examine the effects of extreme weather and the invertebrate grazer community on epilithic algal biomass dynamics over 10 years (from January 2004 to August 2013). Algal biomass and the invertebrate grazer community were monitored in the upstream drainage of the Dajia River in Taiwan, where extreme floods have been becoming more frequent. The biomass of epilithic algae changed, both seasonally and annually, and extreme flooding changed the growth and resistance to flow detachment of the algae. Invertebrate grazing pressure changes with the structure of the invertebrate grazer community, which, in turn, is affected by the flow regime. Invertebrate grazer community structure and extreme flooding both affected the dynamics of epilithic algae, but in different ways. Awareness of the interactions between algal communities and grazers/abiotic factors can help with the design of future studies and could facilitate the development of management programs for stream ecosystems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In freshwater ecosystems, epilithic algae are one of the major primary producers and are an important source of organic matter in food webs [1, 2]. The distribution, structure, and dynamics of epilithic algal communities strongly affect the energy flow and structure of aquatic food webs [1, 2]. At the same time, these communities are strongly affected by environmental and biotic variables, including grazer activity, the hydrologic regime, light intensity, nutrient concentration, and water temperature (e.g., [3, 4]). The diverse responses of epilithic algal communities to ecological changes are attributed to differences in sensitivity and tolerance to environmental changes (e.g., [5, 6]). The responsiveness of epilithic algae to different environmental changes has been widely used to study their effect on ecological processes, such as primary production, secondary production, and nutrient retention [7, 8]. As one major component of complex biofilm communities, epilithic algae provide crucial nutrients and physical habitats to biofilm bacteria [1]. In doing so, the algae play a key role in regulating the growth and composition of biofilm bacteria communities [1], which are closely linked to overall ecosystem productivity [9]. The profound effects of epilithic algae on ecological processes may make them useful for a variety of management applications in freshwater ecosystems [10, 11]. Understanding the driving forces governing the dynamics of the algal communities in natural habitats can provide information for answering fundamental ecological questions and making sound management decisions.
Hydrodynamics affect ecosystem function and community structure in lotic ecosystems [12]. The flow regime and hydrologic disturbances heavily influence algal communities [1, 13]. Changes in the magnitude and predictability of hydrologic disturbances threaten biodiversity and ecosystem function in lotic ecosystems worldwide [14]. For instance, flow regulation reduces hydrologic dynamics, which leads to reduced spatial variability of stream algal assemblages [15]. Unpredicted flooding affects algal resilience in ecosystems in which flow variation is low [16]. In addition to rising temperatures, global climate change likely accounts for the increase in the frequency, severity, and unusual timing of extreme weather events [17]. Changes in patterns of precipitation and the increase in extreme weather events will increase the severity, variability, and frequency of both floods and droughts, which will change existing ecological processes and community structure in river ecosystems (e.g., [18, 19]). These changes will probably impact the dynamics of stream algae. Many studies have addressed natural hydrological disturbance (e.g., normal flooding) on epilithic algae using mechanistic modeling and observational surveys (e.g., [20–22]), but there is limited research about the impacts of extreme weather events (e.g., extreme flooding or extended drought) on the ecology of stream periphyton using long-term data and mathematical models. It is important to document and understand the effects of hydrologic extremes on algal dynamics and their role in the bottom-up and top-down effects of these extreme events. In Taiwan, rivers are strongly impacted by floods during monsoonal periods (from May to October). We have long-term data from high-elevation streams that have experienced more extreme flooding than usual in recent years due to abnormally strong typhoons. These events have allowed us to examine their effects on the biomass dynamics of stream algae over a 10-year period.
Grazing by vertebrates and invertebrates affects the biomass, productivity, and community structure of stream algae [23, 24]. Grazing by macroinvertebrates transfers energy from primary producers to higher trophic levels and has been shown to be a very important process affecting stream algae [25–27]. Hydrodynamics regulates grazer–algae interactions (e.g., [28–30]). Flooding strongly affects the dynamics of stream algae, both directly and indirectly. For example, during floods, algae can be removed directly by the force of the water and by abrasion from suspended sediments [31]. In addition, flooding also affects the invertebrate grazer community, which affects algal dynamics [1, 21]. The structure of the grazer community can affect the grazing pressure on stream algae. For instance, grazer species appear to remove stream algae differentially, and competition among grazers could affect total grazing pressure [29, 32, 33]. Differences in the ability to remove algae are likely associated with differences in grazer size, mobility, and mouthpart morphology, and higher grazer diversity could achieve functional complementarity, which enhances grazing efficiency on algal communities with complex composition and architecture [28, 30]. Grazing has been included in a few mechanistic models (e.g., [1, 34]), but we are not aware of any study that integrates the community structure of invertebrate grazers into the model framework to improve the understanding of algal dynamics.
In this study, we evaluated the effects of grazer community structure and extreme flooding on epilithic algal dynamics in the river ecosystems using a numerical model of algal dynamics based on previous mechanistic models (e.g., [1, 22, 34]). To achieve our objectives, the model has additional functions describing (1) the constraints imposed by hydrological extremes on algal attributes (e.g., flow-induced detachment and growth rate) and (2) grazing pressure, which is affected by the structure of the grazer community. Data from long-term monitoring (a 10-year period from January 2004 to August 2013) of temporal variation in the biomass of epilithic algae and the key factors associated with this variation in a monsoonal Taiwan stream were used to test two hypotheses: (1) extreme flooding changes the resistance and resilience attributes of the epilithic algal community by changing its taxonomic and functional composition during recovery periods, and (2) the higher the structural similarity among invertebrate grazer communities, the more similar their grazing pressure on epilithic algae.
Materials and Methods
Study Area
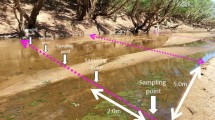
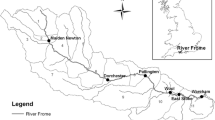
The upstream drainage of the Dajia River in Central Taiwan contains two major streams, Chichiawan Stream and Yousheng Stream (Fig. 1), and has a catchment area of 105 km2, an elevation range of 1700–2100 m, and average annual rainfall of 2071 mm. The streams have short, steep channels and are heavily impacted by floods during monsoonal periods (from May to October), in which rainfall averaged 1365 mm and the annual maximum flow rate averaged 118 m3 s−1 [18]. Our study was conducted in the 50-m section of Chichiawan Stream. This section is bordered by riparian forest and the streambed is dominated by pebbles and rubble in winter and boulders in summer.
Sample Collection
From January 2004 to August 2013, epilithic algal samples were collected on 52 separate occasions (every 69 days, on average) from 5 to 15 randomly selected cobbles in the riffle and run section. Each patch of algae was scraped from the upper surface of each cobble with a toothbrush, then suspended in 50–100 mL of local stream water filtered through a glass fiber filter, and stored in cool dark conditions until laboratory analysis. In the laboratory, the algal samples were centrifuged (3500 rpm) for 10 min to concentrate them using a centrifuge (Kubota KN-70). A subsample was filtered using a glass fiber filter (Whatman GF/F 47mm), and chlorophyll a (Chl a) was extracted from the filter in 90 % acetone for 24 h at 4 °C in the dark. The acetone, with extracted chlorophyll a, was placed in a spectrophotometer (Hitachi U-2001) and the absorbance was measured. The concentration of chlorophyll a [35] was determined using the following equations [36]:
where Chl_a is the concentration of chlorophyll a (in milligrams Chl a per liter) and A w is the absorbance of the sample minus the absorbance of the blank at wavelength w. Algal biomass was calculated using the following equation:
where Bio is algal biomass (in milligrams Chl a per square meter), Vol1 is the volume (in liters) of acetone used for extraction, Vol2 is the volume (in liters) of stream water in which the scraped algae was suspended, Vol3 is the volume (in liters) of the subsample, and Area is the area (in square meters) of the patch of algae on the cobble.
From December 2003 to August 2013, grazer samples were collected on 51 separate occasions (every 71 days, on average) along the same reach and usually during the same month as the algal samples. The samples were taken from six random locations in the riffle and run using a Surber sampler (with an area of 30.48 × 30.48 cm and a mesh size of 250 μm). In the laboratory, we used taxonomic keys [37–39] to identify all macroinvertebrates to the lowest taxonomic level possible, usually genus. The structure of the grazer community at each sample time was determined by counting the number of individuals in each taxon and integrating the abundance of all grazer taxa.
The daily discharge was obtained from a Taiwan Power Company gauging station located 7 km downstream of the study site. Daily photosynthetically active radiation was obtained using a value conversion (with a ratio of 0.46) of global daily radiation measured by a meteorological station 60 km SE of the study site. For 2005–2009, the daily water temperature was provided by a meteorological station at the study site. For 2003–2004 and 2010–2013, the daily water temperature was interpolated with a nonlinear regression model [40] of water and air temperatures using our local data from a meteorological station located 5 km downstream of the study site (R 2 = 0.86).
Model Development and Bayesian Inference
We developed a mechanistic model to describe the key drivers governing the dynamics of epilithic algal biomass based on a previously developed model using a differential equation [22]. Furthermore, a multivariate Gaussian process was added to describe algae removal by invertebrate grazing activity in the differential equation in our study. Our ordinary differential equation models a dynamic balance between environment-dependent growth and abiotic/biotic detachment, as follows:

where B is epilithic algal biomass (in milligrams Chl a per square meter) and t is the time (in days). In this formula, parts 1a to 1d describe algal growth, while parts 2a and 2b describe algal detachment. Part 1a describes exponential increase in algal biomass, where μ max is the maximum instantaneous growth rate (per day) at the reference temperature T 0 (20 °C). Part 1b describes the limitation of the algal growth, where K inv is the inverse half-saturation coefficient (in square meters per milligram), β is the coefficient of temperature dependence (per degree Celsius), and T is the mean daily temperature (in degree Celsius). Part 1c describes the effect of temperature on algal growth, and 1d describes the effect of light. I is the daily integrated light intensity (in photons per square meter) and K I is the light half-saturation coefficient (in photons per square meter).
Parts 2a and 2b describe algal detachment due to water flow and grazing activity, respectively. Q is the measured discharge of the stream (in cubic meters per second), C det is the flow detachment coefficient (in seconds per cubic meter per day), GF is grazer feeding efficiency (in square meters per day), and B 0 is the minimum algal biomass required for the algae to recover after detachment (in milligrams Chl a per square meter).
The log-transformed feeding activity (GF) across the sampled grazer communities has a multivariate distribution, with mean 0 and a covariance matrix ∑. We model the covariance as a function of the community dissimilarity between the sampled grazer community i and j as follows:
where the parameter α 0 controls the variance of community-specific grazing activity (at BC i,j = 0), which has a semi-Cauchy distribution with a location of 0 and a scale of 2.5. BC i,j is the Bray–Curtis dissimilarity between community i and j calculated as community dissimilarity. Daily grazer feeding activity was estimated using linear interpolation from the feeding activity of the sampled communities. This function is based on the concept that biological attributes can be modeled as Gaussian processes with a covariance matrix determined by ecological dissimilarity [41]. With a time step of one day, the Euler method was used to solve the ordinary differential equation.
In the Bayesian framework, we performed Markov chain Monte Carlo (MCMC) sampling to estimate parameters and quantify uncertainty. MCMC is primarily used for numerical approximations of Bayesian inference. In our Bayesian model, the log-transformed epilithic algal biomass is normally distributed, with mean (i.e., the log-transformed value of the predicted biomass) and variance (S algae), where S algae has a semi-Cauchy distribution with a location of 0 and a scale of 2.5. We placed semi-Cauchy distributions on β, K inv, and C det with a location of 0 and a scale of 2.5. We chose uniform distributions on μ max, B 0, and K I, each with a specific range (μ max = 0–8; B 0 = 0–0.01; K I = 0–8) determined by our local conditions and previous studies (e.g., [1, 21, 22]).
To model the response of algae to flooding, for the next year (from November to October), the parameters maximum instantaneous growth rate (μ max) and flow detachment coefficient (C det) were each given a value for post-extreme flooding and for post-normal flooding scenarios. Based on a previous study in the same watershed [42], we defined extreme flooding as an annual maximum discharge exceeding 200 m3 s−1, a discharge rate that had never been recorded (since 1967) prior to the start of this study in 2004. A Bayesian inference framework ‘Stan’ [43] and R package ‘RStan’ [44] were used together to perform our Bayesian modeling. We ran three MCMC chains, each with 100,000 iterations, and the first 50,000 iterations of each chain were discarded as burn-in. For each chain, we collected one sample every 50 iterations, 1000 samples in total, to build the posterior distribution of each model parameter.
Results
Environmental Variation
The daily average temperature varied seasonally from 4 °C in the winter (December to February) to 18 °C in the summer (June to August). Daily active radiation also varied seasonally, ranging from 0 to 60 photons m−2 (Fig. 2). During this study, the stream underwent notable seasonal changes, including periods of low flow in the dry season (May to October) and high flow in the wet season (November to April), and extreme floods of 259, 610, 428, 317, and 311 m3 s−1 occurred in 2004, 2005, 2007, 2008, and 2012, respectively (Fig. 3). In addition, there were an extended flood-free period in 2003 and normal floods in the other years.
Algal and Grazer Dynamics
The biomass of epilithic algae fluctuated seasonally, exhibiting annual peaks during the dry season and low values during the wet season. The greatest algal biomass (74 mg Chl a m−2) occurred after the extended flood-free period in 2003, while the second highest amount (73 mg Chl a m−2) occurred in early 2012, after three successive years of normal flooding (2009–2011; Fig. 3). Much like the biomass of algae, grazer density peaked in the winter (December to February), dropped steeply in the spring (March to May), and was almost absent during the summer (June to August). Moreover, grazer density was high (up to 2087 individuals m−2) during the flood-free period after normal flooding, but decreased rapidly (down to 13 individuals m−2) after periods of extreme flooding. Mayflies (Rhithrogena ampla, Heptageniidae) and/or chironomid flies usually become more dominant after extreme floods (Fig. 4).
Model Evaluation
The Bayesian model explained 83.5 % of deviance (R 2 = 0.835) in the seasonal and annual changes of the epilithic algal biomass. The model performed perfectly because the Nash–Sutcliffe efficiency coefficient value (NSE = 0.831) is larger than 0.75 [45]. We found that extreme flooding altered algal growth (μ max) and flow detachment (C det). After extreme floods, the maximum growth rate of algal assemblages decreased because of lower algal growth (μ max) (2.973 after extreme vs. 3.105 after normal floods), and the algal assemblages showed higher resistant to flow detachment because flow detachment (C det) became lower (0.037 after extreme vs. 0.045 after normal floods). In our model, the community structure of the invertebrate grazers affected grazing activity and, thus, algal dynamics. Simulated invertebrate grazing activity varied seasonally and annually and changed in response to changes in flow discharge. Grazing activity was usually higher during the dry season than during high-flow periods in the wet season, and it varied less and had lower peaks during the three successive years of normal flooding (2009–2011). We did not find notable differences in grazer activity during low-flow periods following extreme and normal floods (Fig. 5).
Discussion
In this study, we modeled the effects of hydrological extremes and the grazing community on algal dynamics and explored the mechanisms behind changes in the structure and function of epilithic communities. By including the effects of hydrological extremes and the grazer community, we improved on previous mechanistic models (e.g., [21, 22]). In the sections below, we discuss how algal communities were affected by extreme flooding and how changes in grazer community structure were linked to different rates of algal removal.
Algal Responses to Hydrodynamics
We found that hydrology was the major factor controlling the dynamics of epilithic algae and that flooding could explain seasonal changes in algal biomass. Along with floods, high flows, which can arise from both natural and anthropogenic hydrologic events, imposed high shear forces on algal mats and rapidly decreased algal biomass [46]. In addition, water velocity is key, facilitating algal removal when high and algal accrual when low [47].
In our study, typhoon- and rainstorm-induced flooding led to very low biomass during the monsoon season, while the peaks of algal biomass occurred during the dry season. Other studies also found that the hydrologic regime affected the spatiotemporal changes in epilithic algal biomass [1, 16, 21]. Although hydrology can explain the general pattern of seasonal changes, the composition of algal communities changes with time, so responses to seasonal hydrodynamics may vary. This could account for some of the unexplained variation in our model. These changes in algal biomass could be associated with changes in the structure of the algal community as it transitioned from stable low flows to variable high-flow periods [1, 16, 21]. Thus, seasonal change in the composition of algal communities could affect their resistance and resilience to annual hydrologic disturbances [1].
A variety of factors explain algal dynamics in monsoonal Asia, where high algal detachment by strong flow and high algal growth resulting from intense light and warm water occur simultaneously in the summer. In our model, increasing temperature and light increase algal growth rates, so algal growth should be high during the warm, long days of summer (June to August). In our study, the destructive forces of flooding overwhelmed the generative effects of high light levels and warm water. The higher growth rates of algae could not compensate for the huge detachments caused by extreme floods. Similarly, one study found that seasonal variation in neither light nor temperature substantially affected the biomass of epilithic algae during wet seasons [48]. However, other studies have found that, in some streams, light and/or nutrient availability can override the effects of flooding [20, 21].
In our study, low flow during autumn (September to November) allowed for a rapid increase in algal biomass, which peaked in the winter (December to February). Another study found similar results [49]. In low-flow years, an extended flood-free period allowed for additional increases in epilithon biomass and higher seasonal peaks [49]. In addition, the growth and biomass accumulation of stream algae during non-flooding periods could be high because moderate discharge reduced limitations on algal growth rate by decreasing mat thickness, which increased light and nutrient availability [50]. During late winter and early spring, prior to the beginning of the monsoon season in Taiwan in April or May, stream flow continued to decrease and water temperature and light intensity increased. However, algal biomass remained level or declined after the winter peaks. Negative biomass-dependent effects on growth rate, included in our model, could explain the leveling off or decrease in algal biomass. In addition, unlike moderate flow, low flow does not provide more colonists to disturbed areas and, thus, could not increase productivity and biodiversity [51].
In other studies in which algal communities were affected by hydrologic disturbances, the resilience of the communities is closely related to the flow regime [16, 52]. Unpredictable extreme flooding that exceeds historical norms and peaks could result in a shift toward a persistent benthic algal community characterized by an increase in the relative abundance of adaptable species [16, 18]. Based on our modeling, after extreme flooding, the relative abundance of algae species with high flow resistance should increase compared to algae with low flow resistance. Because algae with high flow resistance could survive extreme flooding and start their recovery during periods of stable low flows before less resistant species, the structure of epilithic algal communities changed and the relative abundance of species with high flow resistance increased. The trade-off for species of algae more resistant to detachment during extreme floods may be lower maximum growth rates.
Effect of Grazing Pressure on Algal Dynamics
During this study, algal dynamics were affected by changes in grazing pressure, which were caused by changes in grazer dynamics. At times, grazer abundance and biomass were high in our study stream. As in some other studies [1, 21, 27], we found that grazer activity was especially intense during low-flow periods and could destabilize algal mats. A previous study in our study stream found that, after wet season floods, macroinvertebrate abundance and biomass gradually increased and then remained high until late spring [42]. Thus, during low-flow recovery periods, the high biomass and density of invertebrate grazers can lead to increased grazing pressure on algae. However, the effects of grazing on algal biomass were minor and algal biomass still accumulated and peaked in the winter because it increased rapidly during low-flow periods.
Water velocity could affect the removal of algae by stream grazers because grazers remove algae at the highest rates in a fast benthic current [29]. In this study, however, the high grazing efficiency could not compensate for the low grazer density, and grazing pressure on algae was low during high-flow periods. The structure and architecture of the algal community might affect grazing pressure [28]. In the summer, filamentous green algae are more abundant in our study streams than in the winter, but only small amounts of these large algae are consumed by grazers [27]. Interestingly, variation in the structure and architecture of the algal community can explain why the effects of grazing were visible only during the winter and spring when the algal community might be dominated by diatoms rather than by filamentous green algae.
In addition to grazer dynamics, invertebrate grazing activity is also affected by grazer community structure [1, 21]. Interestingly, although grazer biomass and abundance varied greatly between years, grazing activity varied much less. Opportunistic species can rapidly overcome the effects of flooding through rapid reproduction. For example, under the unstable conditions caused by unpredictable and severe flooding, opportunistic macroinvertebrates can dominate the stream invertebrate community [53]. Previous studies in our watershed have demonstrated that extreme flooding changed the structure of the macroinvertebrate community by causing a decline in the relative abundance of large K-selected species [18, 42]. Flooding can also change community structure and functional organization [18, 42]. This change in the structure of the grazer community following extreme flooding could explain the higher rates of algal removal during the periods after floods. After extreme floods, stronger grazing pressure might be exerted by the remaining small r-selected generalists (e.g., Chironomidae), which consume more algal biomass per unit grazer biomass than large grazers [33]. However, large scraping caddisflies graze more algal biomass than mayflies [54]. In our study, Heptageniidae mayflies, which have traits that enable them to resist flooding, became more dominant, and the abundance of flood-susceptible scraping caddisflies was low during the periods after extreme floods [18]. Thus, we did not find a notable difference in grazing pressure after extreme and normal floods because of structural changes in the grazer community.
Conclusions
We used a Bayesian model of algal biomass dynamics that combines growth and detachment processes to model the seasonal and annual changes in algal biomass in response to fluctuations in the environment, especially flooding and invertebrate grazing, over a 10-year period. Our principal findings were: (1) flow regime was the primary driver of algal dynamics; (2) extreme flooding can affect the composition of epilithic algal communities during the recovery period, altering the growth and hydrological resistance of the algal community; and (3) feeding activity was governed by community structure of the invertebrate grazers. Including extreme flooding and grazer activity in this study boosted our understanding of the relationships between ecological variables and variation in the biomass of epilithic algae. Extreme floods in monsoonal and Mediterranean streams with notable seasonal flow dynamics probably directly affect algal biomass dynamics and also have top-down effects on algal dynamics by affecting their grazers. Awareness and understanding of these interactions and their effects is crucial for future studies and management programs for stream ecosystems, especially when considering the effects of climate change and weather scenarios.
References
Graba M, Sauvage S, Majdi N, Mialet B, Moulin FY, Urrea G, Buffan-Dubau E, Tackx M, Sabater S, Sanchez-Perez JM (2014) Modelling epilithic biofilms combining hydrodynamics, invertebrate grazing and algal traits. Freshw Biol 59:1213–1228. doi:10.1111/Fwb.12341
Smith VH, Tilman GD, Nekola JC (1999) Eutrophication: impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ Pollut 100:179–196. doi:10.1016/S0269-7491(99)00091-3
Schneck F, Schwarzbold A, Melo AS (2013) Substrate roughness, fish grazers, and mesohabitat type interact to determine algal biomass and sediment accrual in a high-altitude subtropical stream. Hydrobiologia 711:165–173. doi:10.1007/s10750-013-1477-x
Uehlinger U, Robinson CT, Hieber M, Zah R (2010) The physico-chemical habitat template for periphyton in alpine glacial streams under a changing climate. Hydrobiologia 657:107–121. doi:10.1007/s10750-009-9963-x
Hlúbiková D, Novais MH, Dohet A, Hoffmann L, Ector L (2014) Effect of riparian vegetation on diatom assemblages in headwater streams under different land uses. Sci Total Environ 475:234–247. doi:10.1016/j.scitotenv.2013.06.004
Bere T, Tundisi JG (2011) Influence of ionic strength and conductivity on benthic diatom communities in a tropical river (Monjolinho), São Carlos-SP, Brazil. Hydrobiologia 661:261–276. doi:10.1007/s10750-010-0532-0
Sabater S, Guasch H, Romani A, Munoz I (2002) The effect of biological factors on the efficiency of river biofilms in improving water quality. Hydrobiologia 469:149–156. doi:10.1023/A:1015549404082
Jardine TD, Pettit NE, Warfe DM, Pusey BJ, Ward DP, Douglas MM, Davies PM, Bunn SE (2012) Consumer-resource coupling in wet–dry tropical rivers. J Anim Ecol 81:310–322. doi:10.1111/j.1365-2656.2011.01925.x
Ford TE, Lock MA (1987) Epilithic metabolism of dissolved organic-carbon in boreal forest rivers. Fems Microbiol Ecol 45:89–97. doi:10.1111/j.1574-6968.1987.tb02344.x
Corcoll N, Bonet B, Leira M, Guasch H (2011) Chl-a fluorescence parameters as biomarkers of metal toxicity in fluvial biofilms: an experimental study. Hydrobiologia 673:119–136. doi:10.1007/s10750-011-0763-8
Murdock JN, Shields FD, Lizotte RE (2013) Periphyton responses to nutrient and atrazine mixtures introduced through agricultural runoff. Ecotoxicology 22:215–230. doi:10.1007/s10646-012-1018-9
Poff NL (1992) Why disturbances can be predictable: a perspective on the definition of disturbance in streams. J N Am Benthol Soc 11:86–92. doi:10.2307/1467885
Biggs BJF, Smith RA (2002) Taxonomic richness of stream benthic algae: effects of flood disturbance and nutrients. Limnol Oceanogr 47:1175–1186
Poff NL, Olden JD, Merritt DM, Pepin DM (2007) Homogenization of regional river dynamics by dams and global biodiversity implications. Proc Natl Acad Sci U S A 104:5732–5737. doi:10.1073/pnas.0609812104
Ponsatí L, Acuña V, Aristi I, Arroita M, García-Berthou E, Dv S, Elosegi A, Sabater S (2015) Biofilm responses to flow regulation by dams in Mediterranean rivers. River Res Appl 31:1003–1016. doi:10.1002/rra.2807
Stanish LF, Nemergut DR, McKnight DM (2011) Hydrologic processes influence diatom community composition in Dry Valley streams. J N Am Benthol Soc 30:1057–1073. doi:10.1899/11-008.1
IPCC (2013) Fifth assessment report. Intergovernmental Panel on Climate Change, World Meteorological Organization, Geneva
Chiu M-C, Kuo M-H (2012) Application of r/K selection to macroinvertebrate responses to extreme floods. Ecol Entomol 37:145–154. doi:10.1111/j.1365-2311.2012.01346.x
Chiu M-C, Kuo M-H, Hong S-Y, Sun Y-H (2013) Impact of extreme flooding on the annual survival of a riparian predator, the Brown Dipper Cinclus pallasii. Ibis 155:377–383. doi:10.1111/Ibi.12035
Izagirre O, Elosegi A (2005) Environmental control of seasonal and inter-annual variations of periphytic biomass in a North Iberian stream. Ann Limnol-Int J Lim 41:35–46. doi:10.1051/Limn/2005004
Tsai JW, Chuang YL, Wu ZY, Kuo MH, Lin HJ (2014) The effects of storm-induced events on the seasonal dynamics of epilithic algal biomass in subtropical mountain streams. Mar Freshw Res 65:25–38. doi:10.1071/Mf13058
Uehlinger U, Buhrer H, Reichert P (1996) Periphyton dynamics in a floodprone prealpine river: evaluation of significant processes by modelling. Freshw Biol 36:249–263. doi:10.1046/j.1365-2427.1996.00082.x
Hillebrand H (2009) Meta-analysis of grazer control of periphyton biomass across aquatic ecosystems. J Phycol 45:798–806. doi:10.1111/j.1529-8817.2009.00702.x
Holomuzki JR, Feminella JW, Power ME (2010) Biotic interactions in freshwater benthic habitats. J N Am Benthol Soc 29:220–244. doi:10.1899/08-044.1
Alvarez M, Peckarsky BL (2013) The influence of moss on grazers in high-altitude streams: food, refuge or both? Freshw Biol 58:1982–1994. doi:10.1111/Fwb.12185
Effenberger M, Diehl S, Gerth M, Matthaei CD (2011) Patchy bed disturbance and fish predation independently influence the distribution of stream invertebrates and algae. J Anim Ecol 80:603–614. doi:10.1111/j.1365-2656.2011.01807.x
Lin HJ, Peng TR, Cheng IC, Chen LW, Kuo MH, Tzeng CS, Tsai ST, Yang JT, Wu SH, Sun YH, Yu SF, Kao SJ (2012) Trophic model of the subtropical headwater stream habitat of Formosan landlocked salmon Oncorhynchus formosanus. Aquat Biol 17:269–283. doi:10.3354/Ab00481
Wellnitz T, Poff NL (2012) Current-mediated periphytic structure modifies grazer interactions and algal removal. Aquat Ecol 46:521–530. doi:10.1007/s10452-012-9419-7
Hintz WD, Wellnitz T (2013) Current velocity influences the facilitation and removal of algae by stream grazers. Aquat Ecol 47:235–244. doi:10.1007/s10452-013-9438-z
Hoffman AL, Olden JD, Monroe JB, Poff NL, Wellnitz T, Wiens JA (2006) Current velocity and habitat patchiness shape stream herbivore movement. Oikos 115:358–368. doi:10.1111/j.2006.0030-1299.14675.x
Francoeur SN, Biggs BJF (2006) Short-term effects of elevated velocity and sediment abrasion on benthic algal communities. Hydrobiologia 561:59–69. doi:10.1007/s10750-005-1604-4
Poff NL, Wellnitz T, Monroe JB (2003) Redundancy among three herbivorous insects across an experimental current velocity gradient. Oecologia 134:262–269. doi:10.1007/s00442-002-1086-2
Alvarez M, Peckarsky BL (2005) How do grazers affect periphyton heterogeneity in streams? Oecologia 142:576–587. doi:10.1007/s00442-004-1759-0
McIntire CD, Gregory SV, Steinman AD, Lamberti GA (1996) Modeling benthic algal communities: an example from stream ecology. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology, freshwater benthic ecosystems. Academic, San Diego, pp 670–702
Lobban CS, Chapman DJ, Kemer BP (1988) Experimental phycology: a laboratory manual. Cambridge University Press, Cambridge
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, B, c1 and c2 in higher-plants, algae and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Kang S-C (1993) Ephemeroptera of Taiwan (excluding Baetidae). PhD dissertation, National Chung Hsing University
Kawai T, Tanida K (2005) Aquatic insects of Japan: manual with keys and illustrations. Tokai University Press, Tokyo
Merritt RW, Cummins KW, Berg MB (2008) An introduction to the aquatic insects of North America. Kendall/Hunt, Dubuque
Mohseni O, Stefan HG, Erickson TR (1998) A nonlinear regression model for weekly stream temperatures. Water Resour Res 34:2685–2692. doi:10.1029/98wr01877
Bradburd GS, Ralph PL, Coop GM (2013) Disentangling the effects of geographic and ecological isolation on genetic differentiation. Evolution 67:3258–3273. doi:10.1111/Evo.12193
Chiu M-C, Kuo M-H, Sun Y-H, Hong S-Y, Kuo H-C (2008) Effects of flooding on avian top-predators and their invertebrate prey in a monsoonal Taiwan stream. Freshw Biol 53:1335–1344. doi:10.1111/j.1365-2427.2008.01968.x
Stan Development Team (2014) Stan modeling language: user’s guide and reference manual, version 2.5.0
Stan Development Team (2014) RStan: the R interface to Stan, version 2.5. http://mc-stan.org/rstan.html
Krause P, Boyle DP, Bäse F (2005) Comparison of different efficiency criteria for hydrological model assessment. Adv Geosci 5:89–97. doi:10.5194/adgeo-5-89-2005
Uehlinger U (1991) Spatial and temporal variability of the periphyton biomass in a prealpine river (Necker, Switzerland). Arch Hydrobiol 123:219–237
Horner RR, Welch EB, Seeley MR, Jacoby JM (1990) Responses of periphyton to changes in current velocity, suspended sediment and phosphorus concentration. Freshw Biol 24:215–232. doi:10.1111/j.1365-2427.1990.tb00704.x
Boulêtreau S, Garabétian F, Sauvage S, Sánchez-Pérez J-M (2006) Assessing the importance of a self-generated detachment process in river biofilm models. Freshw Biol 51:901–912. doi:10.1111/j.1365-2427.2006.01541.x
Boulêtreau S, Izagirre O, Garabétian F, Sauvage S, Elosegi A, Sánchez-Pérez J-M (2008) Identification of a minimal adequate model to describe the biomass dynamics of river epilithon. River Res Appl 24:36–53. doi:10.1002/Rra.1046
Jasper S, Bothwell ML (1986) Photosynthetic characteristics of lotic periphyton. Can J Fish Aquat Sci 43:1960–1969
Jørgensen SE, Patten BC, Straskraba M (2000) Ecosystems emerging: 4. Growth. Ecol Modell 126:249–284. doi:10.1016/S0304-3800(00)00268-4
Tang T, Niu SQ, Dudgeon D (2013) Responses of epibenthic algal assemblages to water abstraction in Hong Kong streams. Hydrobiologia 703:225–237. doi:10.1007/s10750-012-1362-z
Wallace JB (1990) Recovery of lotic macroinvertebrate communities from disturbance. Environ Manag 14:605–620. doi:10.1007/Bf02394712
Feminella JW, Power ME, Resh VH (1989) Periphyton responses to invertebrate grazing and riparian canopy in 3 northern California coastal streams. Freshw Biol 22:445–457. doi:10.1111/j.1365-2427.1989.tb01117.x
Acknowledgments
We thank three anonymous referees who commented on the manuscript. Our research was supported by research grants from Shei-Pa National Park, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chiu, MC., Kuo, MH., Chang, HY. et al. Bayesian Modeling of the Effects of Extreme Flooding and the Grazer Community on Algal Biomass Dynamics in a Monsoonal Taiwan Stream. Microb Ecol 72, 372–380 (2016). https://doi.org/10.1007/s00248-016-0791-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0791-z