Abstract
The modification of flows in lotic ecosystems can have dramatic effects on abiotic and biotic processes and change the structure of basal trophic levels. In high-gradient streams, most of the biota are benthic, and decreased flow may homogenize and reduce benthic current velocity, potentially changing stream ecosystem function. Grazing by macroinvertebrates is an important component of stream function because grazers regulate energy flow from primary producers to higher trophic levels. We conducted an experiment to examine how macroinvertebrate grazers facilitated or removed algal biomass across a gradient of benthic current velocity (0–40 cm s−1). We chose three grazers (Drunella coloradensis, Cinygmula spp., and Epeorus deceptivus) from a montane stream and conducted our experiment using 24 artificial stream channels that had three treatments: no grazers (control), single-grazer, and combined-grazer treatments. In the absence of grazers, algal biomass increased with benthic current velocity. Grazer treatments differed from the control in that more algal biomass was removed at higher velocities, whereas algal accrual was largely facilitated at low velocities. The transition from facilitation to removal ranged from 4.5 to 5.9 cm s−1 for individual grazer treatments and occurred at 11.7 cm s−1 for the combined-grazer treatment. Our data suggest that velocity plays a significant role in the facilitation and removal of algae by macroinvertebrate grazers. Additionally, the patterns revealed here could have general implications for algal accrual in systems where flow is reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is difficult to contemplate a pattern or process in streams that is not either directly or indirectly related to flow (Hart and Finelli 1999). Flow regulates energy transfer rates (Feminella and Hawkins 1995), resource and consumer distributions (Downes et al. 1993; Wellnitz et al. 2001), and the functional contributions by species to ecosystem processes (Cardinale et al. 2005; Dewson et al. 2007a). The manipulation of stream flow by humans continues to increase worldwide (Dynesius and Nilsson 1994; Poff et al. 2007), and our demand for freshwater is unlikely to abate in the near future (e.g., Vörösmarty et al. 2000). Given this continued need, it is critical that we understand how decreased discharge will affect stream ecosystem functioning at scales both large and small (Dewson et al. 2007a).
One important ecosystem process affected by flow at small-scales is the removal of benthic algae by stream grazers. Grazers in general and macroinvertebrate grazers in particular play a pivotal role in channeling algal-based nutrients and energy through stream food webs (Liess and Hillebrand 2004). When stream discharge is reduced by way of human activity, current velocity across the streambed changes and this can affect interactions between grazers and their algal food resources. In stony-bottomed streams, reduction in flow makes benthic current slower and velocity ranges narrower (Hart and Finelli 1999; Dewson et al. 2007b, c). High-velocity habitats favored by rheophilic grazers are eliminated, and reduced shear may alter periphytic mat structure and physiognomy (Biggs and Hickey 1994). These changes affect the dynamics of algal accrual and removal as well as the functional contributions that grazers make to these processes.
Grazers can influence algal accrual either negatively or positively (see reviews by Feminella and Hawkins 1995; Liess and Hillebrand 2004). Positive effects have only recently received attention in lotic systems (Halpern et al. 2007; Holomuzki et al. 2010), but may be quite common and subject to the effects of benthic current. For example, grazer foraging can facilitate algal growth by dislodging light-blocking sediment from periphytic mats (Pringle et al. 1993; Haglund and Hillebrand 2005). This process is influenced by water velocity, since downstream transport of fine particulates is more likely to occur in fast current, whereas fine sediments typically accumulate in slow current (Malmqvist et al. 2001; Yamada and Nakamura 2002). Current velocity may also enable facilitation by shaping the periphytic assemblage (Biggs et al. 1998; Villeneuve et al. 2010; Passy and Larson 2011). For example, filamentous macroalgae proliferate under slow current and are often colonized by epiphytic diatoms (Bergy et al. 1995). Macroinvertebrate grazers feeding on these epiphytes often leave the underlying filaments intact and thereby benefit the host alga by removing competitors for light and nutrients (Dodds 1991; Dudley 1992). Similarly, by clearing away the senescent over-story layers of periphyton that accumulate in the absence of high-velocity shear, grazers may stimulate periphytic production and growth in underlying layers (Wellnitz and Poff 2006, 2012).
Although benthic current has important direct and indirect effects on algal dynamics, its influence has traditionally been examined within discrete velocity ranges. This approach helps to uncover velocity-specific mechanisms, but at the cost of discerning landscape-dependent processes. In naturally heterogeneous streams, grazers are exposed to gradients of current that create spatial variation in habitat and resources (Palmer et al. 2000; Wellnitz et al. 2001; Opsahl et al. 2003). This variation can influence grazer patch residence times, movement patterns, consumption rates (Poff et al. 2003; Hoffman et al. 2006; Oldmeadow et al. 2010), and ultimately, the functional contributions that grazers make to algal removal and accrual.
We hypothesized that grazer foraging would have a net positive effect on algal accrual at lower velocities (i.e. facilitate algal growth) and a negative effect at higher velocities (i.e. removal of algal biomass). We recognized that two processes could contribute to this pattern in natural streams: (1) different grazer effects (e.g., removal or facilitative processes) within specific ranges of current and (2) different grazer residence times within those specific ranges. Although additive effects between these processes are likely, synergistic effects are also possible. For example, Wellnitz and Poff (2006) demonstrated that a grazer’s influence on algal accrual is affected not just by current velocity, but the duration of grazing within a given velocity. Believing that synergistic effects were important, we designed an experiment in which grazers could choose both the velocity-specific location in which to forage and the time spent foraging there. Our goals were to (1) identify how velocity affects the facilitation and removal of algae by macroinvertebrate grazers along a gradient of benthic current, (2) whether this pattern was linear or nonlinear, (3) identify the transition point, if any, between facilitation and removal along the current velocity gradient, and (4) observe whether it differed among grazer species.
Materials and methods
Experimental design
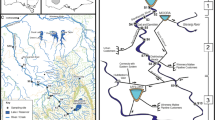
We constructed an array of 24 flow-through channels that created similar gradients of benthic current across which grazers could freely forage and feed (Fig. 1). The array was assembled on an un-shaded gravel bank adjacent to Copper Creek, a high-gradient mountain stream at the Rocky Mountain Biological Laboratory in Gothic, Colorado, USA (38 57 29N, 106 59 06W) and received water directly from the stream. Stream water was filtered using a knitted polyester drain sock to remove debris and stream macroinvertebrates and then directed into a 500 L Rubbermaid® stock tank located 6 m above the array to provide hydraulic head pressure. An overflow back to the stream on the stock tank ensured the stream water temperature did not change before entry into the channels by allowing continuous replenishment. Water was gravity-fed into the array through plumbing that supplied water to 24 adjustable valves. Each valve was attached to feeder hose that supplied water to a channel, and adjusting valves allowed water speed to be controlled so similar gradients of benthic current could be created across channel substrates (Fig. 1). The high water turnover rate in the channels kept temperature between them and Copper Creek within 1 °C.
The experimental channels used to evaluate the pattern of grazer-mediated algal accrual and removal. Each channel was 60 cm in diameter, had a water depth 15 cm, and contained approximately 900 cm2 of substrate. Water was siphoned from Copper Creek (in background) 100 m upstream and filtered before entering a cattle tank reservoir that fed the channel array. The reservoir was positioned 6 m above the channels on a nearby hill to create head pressure and valves manipulated channel flows. Standpipes fitted with 1-mm mesh allowed water to drain back into the stream. The inset photo shows the 2.5 × 2.5 cm ceramic tiles that were colonized with stream algae and placed in channels to establish the current gradient
Benthic algae were grown on square (2.5 × 2.5 cm) ceramic tiles attached to stream cobbles with silicon adhesive. The ceramic tiles provided a standardized surface and were small enough to characterize velocity, but large enough to support a quantifiable amount of algal biomass (Lamberti and Resh 1985). Tiles and their attached cobbles were colonized in Copper Creek for 21 days and then transferred to the array, six tiles to a channel, along with 2 additional cobbles (dry and from the stream bank) to provide substrate area approximating that enclosed by a Surber sampler, that is, 900 cm2. Tiles and cobbles were allowed to acclimate to channel conditions for 3 days before grazers were added. Current velocity was measured directly from the top of all experimental tiles with a Schiltknecht MiniAir20 velocity probe, which had a spatiotemporal resolution of 10 mm and 1 Hz. Cobbles with tiles were numbered with respect to their position in the channels, and the tiles were checked daily to maintain velocities set at the beginning of the experiment. Summary values (mean, SD, minimum, maximum) for the experimental channel velocity gradients that were measured on tiles are shown in Table 1.
Grazers
We chose three of the four most abundant mayfly grazers (because caddisflies were rare, and there were no snails in Copper Creek): Drunella coloradensis Dodds, Cinygmula spp. McDonnough, and Epeorus deceptivus McDonnough. Baetis spp. were also abundant, but were undergoing a period of emergence, which made their use in the experiment problematic. Grazer species were applied to channels separately or in combination with each treatment replicated five times (4 treatments × 5 replicates = 20 channels). The remaining four channels were used as controls and had no grazers. Grazer densities were determined using a Surber sampler (30 × 30 cm with 500-μm mesh) to survey Copper Creek and scale natural densities so grazer biomass could be calculated and held constant among experimental treatments. The combined-grazer treatment contained three Drunella, eight Cinygmula, and eight Epeorus. The single species treatments had 8 (wt = 2.76 g), 23 (wt = 2.61 g), and 20 (wt = 2.91 g) individuals for Drunella, Cinygmula, and Epeorus, respectively. If during the course of the experiment, a grazer was found dead or to have emerged, it was replaced immediately with a conspecific from Copper Creek.
Quantifying algal biomass
At the end of the 10-day experiment, ceramic tiles were sampled to measure chlorophyll a and estimate algal biomass. Epilithic algae were removed from the entire tile by scraping with a razor blade and rinsing with a wash bottle. The resulting slurry was filtered onto a glass fiber filter, placed in aluminum foil, and stored in a cooler for transfer to the laboratory. Chlorophyll was extracted the same day by placing filters in 90 % buffered ethanol following Biggs and Kilroy (2000) and an Opti-sciences GFC-1 fluorometer quantified chlorophyll a. No measurements of chlorophyll a were taken from the cobbles included in the experimental channels. We were unable to estimate chlorophyll a on 11 of 144 tiles due to dropping them or accidental removal of epilithon.
Data analyses
Our a priori goal was to keep velocities consistent across the tile surfaces among our treatments. We used one-way analysis of variance (ANOVA) to test whether velocities differed between grazer treatments by averaging the tile velocities for each of the 24 channels. After averaging chlorophyll a values for each channel, we examined whether algal biomass differed among our control and grazer treatments with one-way ANOVA and used post hoc Tukey’s honest significant difference tests to examine pairwise comparisons if ANOVAs were significant at or below the α-level of 0.05. These analyses were conducted in R (version 2.15.3; R Development Core Team 2013).
Generalized linear mixed models (GLMMs) were used to analyze how velocity affected chlorophyll a within our grazer treatments. The GLMMs were particularly useful given our split-plot design, and ‘channel’ was included as the random effect in the GLMMs (i.e. velocity was nested within channels). To identify whether grazers facilitated or removed algae, we used GLMM coefficients from the control to produce a linear equation (chlorophyll a = 10.90 + 0.92 × velocity) and used that to calculate predicted chlorophyll a values for the benthic current velocities observed in the grazer treatments. We then calculated the proportion of algae removed (PAR) as follows:
The PAR allowed us to observe and test how grazers facilitate or remove algae along a benthic velocity continuum compared to what was predicted by the control. In this way, we could account for the effect velocity had on the periphytic assemblage in the absence of grazers. The limits of these observations are theoretically 1 to −∞ (i.e., 100 % removal to facilitated growth), although negative values are biologically constrained. We also used GLMMs to analyze the PAR data. One of our goals was to also identify the transition point between facilitation and removal. We defined this as the velocity at which the fitted GLMM model crossed the velocity axis where PAR = 0. The estimated coefficients from the GLMMs were considered statistically significant if the associated probability values were ≤0.05. The GLMMs were conducted using SAS software (version 9.2, Copyright© 2009 SAS Institute Inc., Cary, NC, USA).
Results
Average velocity did not differ among the grazer treatments (F 4, 18 = 1.09, p = 0.39), and the parametric assumptions of normality (p = 0.16) and constant variance (p = 0.92) for ANOVA were met. Periphytic algae on control tiles showed accumulations of fine silt that were largely absent from grazed tiles; however, ANOVA revealed that algal biomass did not differ among the experimental treatments (F 4, 18 = 2.04, p = 0.13). The ANOVA model for algal biomass also passed the assumptions of normality (p = 0.50) and constant variance (p = 0.90).
Algal biomass responded differently to velocity between control and grazer treatments. Chlorophyll a increased with velocity in the control channels, showed no relationship to velocity in the Drunella, Cinygmula, and combined-grazer treatments, and decreased with velocity in the Epeorus treatment (Fig. 2). The control GLMM indicated a significant positive effect of velocity on chlorophyll a in the absence of grazers (coefficient = 0.92, t 14 = 2.77, p = 0.01). In the Epeorus treatment, chlorophyll a decreased with velocity (GLMM: coefficient = −0.28, t 19 = −2.53, p = 0.02). The GLMMs for Drunella (t 20 = 0.42, p = 0.67), Cinygmula (t 22 = −0.31, p = 0.76), and combined-grazer treatments (t 18 = −1.43, p = 0.16) did not show a positive or negative effect of velocity of on chlorophyll a across the velocity gradient.
Patterns of chlorophyll a for all grazer treatments. Generalized linear mixed models indicated chlorophyll a increased with velocity in the control treatment (i.e. no grazers) and decreased with velocity in the Epeorus treatment. Algal biomass in the other grazer treatments had no discernible relationship with current velocity (see text for GLMM model details)
Facilitation and removal of algae by grazers
The patterns of facilitation and removal by grazers in this experiment differed along the gradient of benthic current velocity. We observed a gradual change from facilitation to almost complete removal in the PAR data (i.e., the transition from the negative values below dashed line to positive values above dashed line in Fig. 3). In general, grazers facilitated algal growth at low benthic current velocities (<15 cm s−1) and almost completely removed algae at higher benthic velocities (>20 cm s−1). The transition point between facilitation and removal, however, varied among treatments.
Generalized linear mixed models for the combined-grazer and single-grazer treatments. Dashed line indicates where the proportion of algal biomass removed (PAR) was zero. Negative values indicate facilitation, and positive values are indicative of removal. The quadratic and linear models (indicated in lower left of graphs) were statistically significant for all grazing treatments (see text for details)
The combined-grazer treatment was unique from the single-grazer treatments in that greater negative values were observed (Fig. 3). Overall, the GLMM indicated that velocity had a significant effect on grazing (t 18 = −4.39, p < 0.001). The pattern in the PAR data was nonlinear. A quadratic function was fit to the data, and the partial quadratic coefficient was significant (t 18 = −3.48, p = 0.007). The transition from facilitation to removal occurred at 11.7 cm s−1. It should be noted that one of the flow-through channels for this treatment flooded due to debris accumulation and some of the grazers were lost. Therefore, we decided to eliminate this channel (6 chlorophyll a measurements) from the analysis, although the data did conform to the pattern shown in Fig. 3a.
The grazing pattern in the Drunella treatment was markedly different from the combined-grazer treatment. Though Drunella facilitated and removed periphytic algae, this pattern was positive and linear, rather than curvilinear (Fig. 3b). There was a strong effect of velocity on grazing by Drunella (GLMM: t 24 = 4.53, p < 0.001), and the transition from facilitation to removal occurred at 5.9 cm s−1.
The Cinygmula treatment exhibited a similar pattern of facilitation and removal of periphytic algae as the Drunella treatment (Fig. 3c). The pattern was a linear increase from facilitation to removal along the gradient of benthic current velocity. The PAR data for the Cinygmula treatment were more variable than the other treatments, but the effect of velocity on grazing was significant (GLMM: t 28 = 2.58, p = 0.015). The transition from facilitation to removal occurred at 4.6 cm s−1.
The Epeorus treatment exhibited a nonlinear pattern of facilitation and removal across the current velocity gradient resembling the combined-grazer treatment (Fig. 3d). The quadratic term added to the GLMM was significant (t 22 = −2.85, p = 0.009), as was the main effect of velocity on Epeorus grazing (t 28 = 4.34, p < 0.001). Similar to the other single-grazer treatments, the transition from facilitation to removal occurred at 4.5 cm s−1. One tile became dislodged and overturned with the silicon adhesive facing upward so this tile was eliminated from the analysis.
Discussion
Our data suggest benthic current velocity affects the facilitation and removal of algal biomass by stream grazers. The nature of the transitions between facilitation and removal occurred at or below velocities of 11.7 cm s−1 for all grazer treatments. In two of four grazer treatments, the pattern of facilitation and removal was nonlinear and linear in the remaining two. This suggests that for some macroinvertebrate species, the transition from facilitation and removal may be gradual and for others, more abrupt.
The grazers in Copper Creek are adapted to fast flow environments, and their effectiveness in removing algae in fast benthic current was not surprising, nor was their facilitation of algal biomass in slow current. What was surprising was the extent to which facilitation occurred and the higher velocity of the transition point for the combined-grazer treatment, which most closely approximated the natural grazing community. Collectively, these grazers caused a doubling in algal biomass at lower velocities (i.e. below 11.7 cm s−1) compared to the single-grazer treatments, and the transition point was fast enough to be common in stream riffles (e.g., Wellnitz et al. 2001). This suggests that facilitation of algal accrual by grazers is not restricted to stream margins, pools, and other regions of homogeneous slow current, but may contribute to mid-channel patterns. This being the case, current-mediated grazer function may be an important process for creating algal heterogeneity across the streambed.
It is noteworthy that we did not observe any differences in algal biomass between the grazing treatments and controls. This is not surprising in view of the fact that grazers were simultaneously facilitating and removing algae. However, it does highlight the importance of scaling when examining benthic processes in general and algal–grazer interactions in particular. Our experiment was designed to test grazer functionality across a continuum of benthic current by maintaining the same velocity gradient across treatments; by achieving this, we effectively held the average velocity in channels constant. Therefore, if benthic current was profiled at the channel scale (900 cm2)—which is the scale of resolution commonly used to assess streambed communities (i.e., the area of a Surber sampler)—one might fail to detect a velocity-dependent effect. Moreover, one could not observe the shifting functional roles of facilitation and removal played by the grazers in this system or the degree to which they were functionally distinct or redundant (Wellnitz and Poff 2001). It is only at the scale of periphyton patches on cobbles (i.e. <10 cm2) that the patterns emerge.
The precise mechanism driving algal facilitation in our experiment is difficult to identify. Although elucidating this process was not an objective of our study, we speculate that facilitation resulted from the time grazers spent in fast versus slow current and the removal of fine sediments that resulted from their foraging. Brooks et al. (2005) showed that the abundance and community composition of macroinvertebrates can vary markedly with the hydraulic conditions of microhabitats in streams. Streambed densities of the mayfly species used in our experiment generally have a positive relationship to benthic current in Copper Creek; the highest densities of Ephemerellidae (Drunella) and Heptageniidae (Cinygmula, Epeorus) were observed at average velocities of 42.6 and 36.5 cm s−1, respectively (T. Wellnitz, unpublished data). It is reasonable to assume that these rheophilic grazers spent a disproportionate amount of time foraging in faster flows in our experimental channels and removed more algae there. By contrast, the time grazers spent foraging at slow velocities may have been insufficient for removing large quantities of algae, yet adequate enough to dislodge fine sediments from periphytic mats. Silt and fine sediments can block light and slow nutrient diffusion to benthic algae, and macroinvertebrates are capable of dislodging sediments from benthic substrates (Zanetell and Peckarsky 1996; Ledger and Hildrew 1998; Wellnitz et al. 2010). Evidence that this occurred comes from the observation that the fine silt layer seen on control treatment tiles was largely absent from grazer treatment tiles. We propose that the transition in grazer function from facilitation to removal of algae was a result of this time-dependent process. Foraging of brief duration had mostly positive effects (sediment removal), whereas longer duration foraging had mostly negative effects (increased consumptive and non-consumptive removal of periphytic algae), and this may have created the patterns of facilitation and removal we observed.
Functional traits of the mayflies may also have contributed to the processes of facilitation and removal. The three species used in our experiment showed distinctive trait suites that might have influenced their contributions to algal dynamics. For example, Epeorus and Cinygmula have brushing and prognathous mouthparts as opposed to blade-like and hypognathous mouthparts of Drunella, and mouthpart structure and function is known to affect algal removal (Karouna and Fuller 1992; Holomuzki et al. 2006). It is likely the efficacy of these traits may vary with different velocities, given macroinvertebrates are strongly influenced by hydraulic conditions (e.g., Poff et al. 2003; Brooks et al. 2005). Reduced drift behavior at slow velocities may also influence grazer performance and functionality. For example, Poff et al. (2003) speculated that the mayfly Baetis bicaudatus in their grazing experiments foraged less efficiently in slow velocities because Baetis may have had to swim rather than drift between algal patches. The grazers used in our study drift, but Epeorus (McIntosh et al. 2002) and Cinygmula (Peckarsky 1996) appear to be more frequent drifters compared to Drunella (e.g., Dahl and Peckarsky 2002).
The shift in grazer functional roles from slow to fast benthic current velocities is striking and has implications for past and present modifications to lotic systems. The contribution of lotic grazers to algal removal may be altered as benthic current becomes constrained by decreased stream flow, which can result from human activities such as damming, water abstraction, and reduced precipitation from climate change. It is difficult to predict what implications the patterns of facilitation and removal shown here may have on a system-wide scale and higher trophic levels, yet it is possible altered stream flows could produce an alternative stable state (e.g., Dodds et al. 2010). However, results presented here suggest reduced flow fields may change the role of macroinvertebrate grazers and we would expect these effects to cascade to higher trophic levels (Feminella and Hawkins 1995). Thus, for streams in which similar patterns occur, benthic community structure and function may change with decreasing flow (e.g., Walters and Post 2011).
References
Bergy EA, Boettiger CA, Resh RH (1995) Effects of water velocity on the architecture and epiphytes of Cladophora glomerata (Chlorophyta). J Phycol 31:264–271
Biggs BJF, Hickey CW (1994) Periphyton responses to a hydraulic-gradient in a regulated river in New Zealand. Freshw Biol 32:49–59
Biggs BJF, Kilroy C (2000) Stream periphyton monitoring manual. NIWA, Christchurch
Biggs BJF, Goring DG, Nikora VI (1998) Subsidy and stress responses of stream periphyton to gradients in water velocity as a function of community growth form. J Phycol 34:598–607
Brooks AJ, Haesler T, Reinfelds I, Williams S (2005) Hydraulic microhabitats and the distribution of macroinvertebrate assemblages in riffles. Freshw Biol 50:331–344
Cardinale BJ, Palmer MA, Ives AR, Brooks SS (2005) Diversity-productivity relationships in streams vary as a function of the natural disturbance regime. Ecology 86:716–726
Dahl J, Peckarsky BL (2002) Induced morphological defenses in the wild: predator effects on a mayfly, Drunella coloradensis. Ecology 83:1620–1634
Dewson ZS, James ABW, Death RG (2007a) Stream ecosystem functioning under reduced flow conditions. Ecol Appl 17:1797–1808
Dewson ZS, James ABW, Death RG (2007b) Invertebrate community responses to experimentally reduced discharge in small streams of different water quality. J N Am Benthol Soc 26:754–766
Dewson ZS, James ABW, Death RG (2007c) A review of the consequences of decreased flow for instream habitat and macroinvertebrates. J N Am Benthol Soc 26:401–415
Dodds WK (1991) Community interactions between the filamentous alga Cladophora glomerata (L) Kuetzing, its epiphytes and epiphyte grazers. Oecologia 85:572–580
Dodds WK, Clements WH, Gido K, Hilderbrand RH, King RS (2010) Thresholds, breakpoints and nonlinearity in freshwaters as related to management. J N Am Benthol Soc 29:988–997
Downes BJ, Lake PS, Schreiber ESG (1993) Spatial variation in the distribution of stream invertebrates—implications of patchiness for models of community organization. Freshw Biol 30:119–132
Dudley TL (1992) Beneficial effects of herbivores on stream macroalgae via epiphyte removal. Oikos 65:121–127
Dynesius M, Nilsson C (1994) Fragmentation and flow regulation of river systems in the northern 3rd of the world. Science 266:753–762
Feminella JW, Hawkins CP (1995) Interactions between stream herbivores and periphyton: a quantitative analysis of past experiments. J N Am Benthol Soc 14:465–509
Haglund AL, Hillebrand H (2005) The effect of grazing and nutrient supply on periphyton associated bacteria. FEMS Microbiol Ecol 52:31–41
Halpern BS, Silliman BR, Olden JD, Bruno JP, Bertness MD (2007) Incorporating positive interactions in aquatic restoration and conservation. Front Ecol Environ 5:153–160
Hart DD, Finelli CM (1999) Physical-biological coupling in streams: the pervasive effects of flow on benthic organisms. Annu Rev Ecol Syst 30:363–395
Hoffman AL, Olden JD, Monroe JB, Poff NL, Wellnitz T, Wiens JA (2006) Current velocity and habitat patchiness shape stream herbivore movement. Oikos 115:358–368
Holomuzki JR, Lowe RL, Ress JA (2006) Comparing herbivory effects of stream macroinvertebrates on microalgal patch structure and recovery. N Z J Mar Freshw Res 40:357–367
Holomuzki JR, Feminella JW, Power ME (2010) Biotic interactions in freshwater benthic habitats. J N Am Benthol Soc 29:220–244
Karouna NK, Fuller RL (1992) Influence of four grazers on periphyton communities associated with clay tiles and leaves. Hydrobiologia 245:53–64
Lamberti GA, Resh VH (1985) Comparability of introduced tiles and natural substrates for sampling lotic bacteria, algae and macroinvertebrates. Freshw Biol 15:21–30
Ledger ME, Hildrew AG (1998) Temporal and spatial variation in the epilithic biofilm of an acid stream. Freshw Biol 40:655–670
Liess A, Hillebrand H (2004) Invited review: direct and indirect effects in herbivore periphyton interactions. Arch Hydrobiol 159:433–453
Malmqvist B, Wotton RS, Zhang YX (2001) Suspension feeders transform massive amounts of seston in large northern rivers. Oikos 92:35–43
McIntosh AR, Peckarsky BL, Taylor BW (2002) The influence of predatory fish on mayfly drift: extrapolating from experiments to nature. Freshw Biol 47:1497–1513
Oldmeadow DF, Lancaster J, Rice SP (2010) Drift and settlement of stream insects in a complex hydraulic environment. Freshw Biol 55:1020–1035
Opsahl RW, Wellnitz T, Poff NL (2003) Current velocity and invertebrate grazing regulate stream algae: results of an in situ electrical exclusion. Hydrobiologia 499:135–145
Palmer MA, Swan CM, Nelson K, Silver P, Alvestad R (2000) Streambed landscapes: evidence that stream invertebrates respond to the type and spatial arrangement of patches. Landscape Ecol 15:563–576
Passy SI, Larson CA (2011) Succession in stream biofilms is an environmentally driven gradient of stress tolerance. Microb Ecol 62:414–424
Peckarsky BL (1996) Alternative predator avoidance syndromes of stream-dwelling mayfly larvae. Ecology 77:1888–1905
Poff NL, Wellnitz T, Monroe JB (2003) Redundancy among three herbivorous insects across an experimental current velocity gradient. Oecologia 134:262–269
Poff NL, Olden JD, Merritt M, Pepin DM (2007) Homogenization of regional river dynamics by dams and global biodiversity implications. Proc Nat Acad Sci USA 104:5732–5737
Pringle CM, Blake GA, Covich AP, Buzby KM, Finley A (1993) Effects of omnivorous shrimp in a montane tropical stream: sediment removal, disturbance of sessile invertebrates and enhancement of understory algal biomass. Oecologia 93:1–11
Villeneuve A, Montuelle B, Bouchez A (2010) Influence of slight differences in environmental conditions (light, hydrodynamics) on the structure and function of periphyton. Aquat Sci 72:33–44
Vörösmarty CJ, Green P, Salisbury J, Lammers RB (2000) Global water resources: vulnerability from climate change and population growth. Science 289:284–288
Walters AW, Post DM (2011) How low can you go? Impacts of a low-flow disturbance on aquatic insect communities. Ecol Appl 21:163–174
Wellnitz T, Poff NL (2001) Functional redundancy in heterogeneous environments: implications for conservation. Ecol Lett 4:177–179
Wellnitz T, Poff NL (2006) Herbivory, current velocity and algal regrowth: how does periphyton grow when the grazers have gone? Freshw Biol 51:2114–2123
Wellnitz T, Poff NL (2012) Current-mediated periphytic structure modifies grazer interactions and algal removal. Aquat Ecol 46:521–530. doi:10.1007/s10452-012-9419-7
Wellnitz T, Poff NL, Cosyleon G, Steury B (2001) Current velocity and spatial scale as determinants of the distribution and abundance of two rheophilic herbivorous insects. Landscape Ecol 16:111–120
Wellnitz T, Troia M, Ring M (2010) Does ambient substrate composition influence consumer diversity effects on algal removal? Hydrobiologia 652:15–22
Yamada H, Nakamura F (2002) Effect of fine sediment deposition and channel works on periphyton biomass in the Makomanai river, northern Japan. River Res Appl 18:481–493
Zanetell BA, Peckarsky BL (1996) Stoneflies as ecological engineers—hungry predators reduce fine sediments in stream beds. Freshw Biol 36:569–577
Acknowledgments
We thank Megan Ring and Katie Weber for their assistance in the field and the Rocky Mountain Biological Laboratory for logistical support and allowing us access to Copper Creek. We also thank Matt Whiles, Heidi Rantala, and two reviewers for comments that helped improve earlier versions of our manuscript, and David Glover for assistance with SAS software. Funding was provided by the Office of Research and Sponsored Programs at the University of Wisconsin—Eau Claire, and a United States National Science Foundation CAREER Grant to T. W. (DEB-0642512).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Michael T. Monaghan.
Rights and permissions
About this article
Cite this article
Hintz, W.D., Wellnitz, T. Current velocity influences the facilitation and removal of algae by stream grazers. Aquat Ecol 47, 235–244 (2013). https://doi.org/10.1007/s10452-013-9438-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-013-9438-z