Abstract
Background
Pediatric patients with inflammatory bowel disease (IBD) are at increased risk of gadolinium deposition given the potential need for multiple contrast-enhanced magnetic resonance enterography (MRE) exams over their lifetime.
Objective
To determine whether gadolinium-based contrast agents are necessary in assessing active bowel inflammation on MRE in pediatric patients with known or suspected IBD.
Materials and methods
We conducted a retrospective study of 77 patients (7–18 years; 68.8% male) with known (n=58) or suspected (n=19) IBD and endoscopy with biopsy performed within 30 days of MRE without and with contrast evaluated bowel and non-bowel findings. During three visual analysis sessions, two radiologists reviewed pre-, post-, and pre-/post-contrast MRE images. A third radiologist independently reviewed 27 studies to assess inter-reader reliability. We used Cohen kappa (κ), Fleiss kappa, (κF), McNemar test, and sensitivity and specificity to compare MRE readings to combined endoscopic/histopathological findings (the reference standard).
Results
The pre- and pre-/post-contrast-enhanced MRE vs. combined endoscopic/histopathological results had moderate agreement (85.7%; κ 0.713, P<0.001; P-value 0.549). Compared to combined endoscopy/histopathology, pre- vs. pre-/post-contrast sensitivity (67%, confidence interval [CI] 0.53–0.79 vs. 67%, CI 0.53–0.79) and specificity (80%, CI 0.59–0.92 vs. 68%, CI 0.46–0.84) varied little (κ 0.42, P<0.001 and κ 0.32, P=0.003, respectively). The three readers had moderate agreement (85.2%; κ 0.695, P=0.001; P-value 0.625). More penetrating complications were identified following contrast administration (P-value 0.04).
Conclusion
Use of a contrast agent does not improve the detection of active inflammation in the terminal ileum and colon compared to non-contrast MRE, although use of a contrast agent does aid in the detection of penetrating disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD) includes Crohn's disease, ulcerative colitis and indeterminate colitis. People with IBD typically undergo many imaging studies and other diagnostic procedures throughout their lifetime to diagnose and monitor the disease course, as well as to assess the treatment response. Approximately 25% of people with IBD are diagnosed during childhood, and the incidence of pediatric IBD is increasing in the United States [1, 2].
The current preferred imaging study for pediatric IBD is magnetic resonance enterography (MRE). It has largely replaced diagnostic techniques such as fluoroscopy and CT because it does not utilize ionizing radiation, a particularly important consideration in pediatric patients, who have a longer life expectancy than adults and who are especially vulnerable to the theoretical carcinogenic effects of ionizing radiation. MRE is also useful for its excellent soft-tissue resolution and for its utility for functional imaging through cine acquisitions [3].
The standard MRE protocol for evaluating potential or known IBD includes fat-saturated and non-fat-saturated T2- and T1-weighted sequences and diffusion-weighted imaging. The T1-weighted fat-saturated images are acquired both prior to and after gadolinium-based contrast agent (GBCA) administration [2].
However, several disadvantages are associated with GBCAs. Acquiring post-contrast images lengthens the examination time, which increases the likelihood of patient motion artifact and extends sedation time for children who require sedation to undergo the examination. An intravenous catheter must be placed in order to administer the contrast material, which can be a source of anxiety and discomfort for pediatric patients. Adverse events can also occur after contrast administration, and these range from minor to severe allergic reactions and can result in death [4]. In addition, while rare in pediatric patients, there is the potential for the development of nephrogenic systemic fibrosis (NSF), a potentially fatal disease process related to the use of GBCAs in people with underlying renal disease or impairment [5].
Further, multiple studies in the last several years have shown that gadolinium is deposited in the brain, bones and skin of people with normal renal function who have received GBCAs [6,7,8,9,10,11,12,13,14,15]. The exact mechanism by which this occurs and its significance are unknown. Currently, there is no evidence to support that gadolinium deposition is harmful in people with normal renal function; however, the long-term effects are unknown. Concern regarding the possible deleterious effects of gadolinium deposition in the pediatric population is increasing because children have a longer life expectancy than adults, thereby potentially resulting in greater gadolinium deposition.
Pediatric patients with IBD represent a population that undergoes multiple contrast-enhanced MREs. Given the known disadvantages of GBCA administration and potential risks associated with gadolinium deposition, a more selective and judicious approach to the administration of GBCAs should be considered in these children. Therefore, the purpose of this study was to determine the diagnostic accuracy of non-contrast MRE in detecting active bowel inflammation in pediatric patients with known or suspected IBD, in order to evaluate whether intravenous administration of a GBCA is necessary.
Materials and methods
Patients
Our institutional review board approved this retrospective study and waived the need for informed consent. Eligible patients included those 0–18 years of age with a diagnosis of or suspected Crohn's disease, ulcerative colitis or indeterminate colitis who had undergone gastrointestinal endoscopy with biopsy or bowel resection showing active bowel inflammation and who also had MRE without and with contrast agent within 30 days. Potential patients were identified through a search of our electronic medical record (Epic, Verona, WI) using a combination of procedure order code IMG 2248 (unique Epic identifier for MR enterography) and various distinct Epic codes used for colonoscopy (SHX504, SHX503, SHX502, SHX1191, HM4, GI7, GI6, GI52 and GI49).
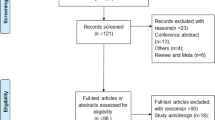
Utilizing these search methods through the electronic medical record, we identified 83 patients and included 77 in the study. Of the 6 who were excluded, one was excluded because of motion artifact on the MRE exam, one because no endoscopy record was found, and four because the entire colon was not examined. Four children were included in the analysis but had altered anatomy as a result of surgical resection (three with ileocecectomy and one with colectomy). Eleven of the included children had endoscopies that did not reach the terminal ileum.
Endoscopy and histopathology analysis
All endoscopies were performed by experienced pediatric gastroenterologists, and biopsy specimens were reviewed and interpreted by pediatric pathologists. Endoscopy reports and images, patients’ clinical charts, and biopsy results from the endoscopies were reviewed by pediatric gastroenterologists.
Presence of inflammation was determined using an aggregate assessment by pediatric gastroenterologists (T.L.R., a third-year fellow, and D.R.P., a board-certified pediatric gastroenterologist with 3 years of post-fellowship experience) combining endoscopic and histopathological report findings. We used the aggregate result as the reference standard for the MRE visual analysis sessions.
Combined endoscopic and histopathological findings were considered positive if they met one of the following criteria: (1) The endoscopy report described moderate erythema or inflammation or presence of ulcer, erosion or friability; (2) The endoscopic photographs showed clear friability or pre-biopsy bleeding, ulcer, erosion or clear disruption of surface mucosa; (3) The endoscopy report described mild erythema, inflammation or edema, and there was any degree of corresponding inflammation in the histopathology report for that area; or (4) There was moderate or severe inflammation or presence of necroinflammatory debris (ulcer) on the histopathology report, and the endoscopy was visually normal.
The combined endoscopic and histopathological findings were considered negative for the presence of active inflammation if they met the following criteria: (1) The endoscopy and histopathology reports were normal; or (2) The histopathological report described mild or non-graded inflammation or mild histological alterations, and the endoscopy photographs and report were normal in that segment. These are findings known to be associated with bowel cleansing preparations and other nonspecific factors associated with the procedure itself. Additional exclusion criteria were ulcerations on endoscopy from causes other than IBD (e.g., angioectasia in one case) and presence of crypt abscesses and infiltrative inflammatory cells on histopathology report without presence of additional necessary criteria for active inflammation.
Magnetic resonance enterography protocol
All children undergoing a MRE had to be nil per os (NPO) for at least 4 h prior to obtaining the scan. Children were given oral VoLumen (Bracco Diagnostics, Monroe, NJ) or Breeza (Beekley Medical, Bristol, CT) prior to the exam to distend the bowel. Just prior to scanning, children were also given 1 mg of intramuscular glucagon to slow intestinal motility. All MREs were performed on a 1.5-T Signa HDXT 23.0 scanner (GE Healthcare, Milwaukee, WI). Details of our MRE protocol are shown in Table 1. All children were awake and cooperative for their examination and were scanned in the supine position. Standard screening of patients included a history of renal disease, contrast allergy and other contraindications to MRI. Our institution underwent a change in contrast agents in late December 2015. MREs obtained in 2014 and 2015 utilized gadopentetate dimeglumine (Magnevist; Bayer Healthcare Pharmaceuticals, Berlin, Germany) while MREs obtained after 2015 utilized gadoterate meglumine (Dotarem; Guerbet, Villepinte, France). Both Magnevist and Dotarem were administered using a weight-based dose of 0.1 mmol/kg through an automatic injector. The maximum dose that a patient received was 20 mL of Magnevist. We also transitioned from using VoLumen to Breeza in December 2015.
Image analysis
Two board-certified attending radiologists reviewed the MRE examinations (Reader 1, S.G.F., a pediatric radiologist with 4 years of experience interpreting MREs; and Reader 2, J.J.B., a body radiologist with 16 years of experience interpreting MREs) in three consensus visual analysis sessions, which were modeled on the study by Quaia et al. [16]. The readers were blinded to the children’s endoscopy, operative and histopathological findings. During the first visual analysis session, the readers analyzed the non-contrast MRE sequences (T1, T2, fast imaging employing steady-state acquisition [FIESTA] and diffusion-weighted imaging/apparent diffusion coefficient [DWI/ADC] sequences). During the second session, they analyzed the contrast-enhanced MRE sequences only (T1 fat-saturated dynamic contrast-enhanced and delayed contrast-enhanced sequences). During the third session the readers analyzed all the MRE sequences (both non-contrast and contrast-enhanced sequences). They did not assess degree of bowel distension in this study. There was a washout period of 4 weeks between each session. The order of case presentation was not altered at each session. A third board-certified attending radiologist (Reader 3, T.Y.T., a pediatric radiologist with 4 years of experience interpreting MREs) was available to review any studies in which there was disagreement between the two radiologists regarding interpretation of findings during the same session. However, there were no disagreements between the two readers during the consensus readings.
The third reader also independently reviewed a random selection of 27 MRE examinations included in the study to assess inter-reader variability. This reader was also blinded to the endoscopy, operative and histopathological findings of the children in the study.
The intestines were divided into five sections on MRE: terminal ileum, right colon (cecum and ascending colon), transverse colon, left colon (descending colon and sigmoid colon) and rectum. The remainder of the gastrointestinal tract was not evaluated on MRE because there was no reference standard with which to compare the results. Readers assessed each section of bowel for the following: abnormal bowel wall thickening (bowel wall measuring >3 mm in thickness), bowel wall edema (abnormal increased T2 signal in the bowel wall), bowel wall diffusion restriction, abnormal bowel wall enhancement, and skip lesions (i.e. more than one single loop). If at least two of these features were present in a section of bowel, it was considered actively inflamed [2, 16,17,18,19,20]. The length of bowel disease was also recorded. Non-bowel findings associated with IBD were also assessed, including: creeping fat, vasa recta hyperemia, abdominopelvic lymphadenopathy, and penetrating complications. The latter included the presence of a fistula (indicated by tethering of bowel loops), a sinus tract, phlegmon (an inflammatory mass) or abscess (a well-defined fluid collection).
Statistical analysis
We used descriptive statistics to report the counts and percentages of patient characteristics as well as the mean and standard deviation (SD) of age.
Analyses of the intestines were divided into five sections: terminal ileum, right colon (cecum and ascending colon), transverse colon, left colon (descending colon and sigmoid colon), and the rectum as well as overall terminal ileum to rectum and colon to rectum (excluding terminal ileum).
We calculated percentage agreement, Cohen kappa statistic (κ) and McNemar tests to assess inter-reader agreement pairwise among the pre-contrast, post-contrast, and pre-/post-contrast assessments as well as Fleiss kappa (κF) among all three assessments. Cohen kappa adjusts the percentage agreement for agreement from chance alone and agreement was graded as poor (κ value<0.20), fair (>0.20 and<0.40), moderate (>0.40 and<0.60), good (>0.60 and<0.80) or very good (>0.8 up to 1). Fleiss kappa is similar to Cohen kappa but allows for measuring agreement between more than two raters and utilizes the same grading scale as Cohen kappa.
We analyzed Cohen kappa, sensitivity and specificity for the pre-contrast, post-contrast, and pre-/post-contrast assessments with respect to the combined endoscopic and histopathological results. We used the McNemar test and Fleiss kappa to examine differences pairwise and overall among pre-contrast, post-contrast, and pre-/post-contrast assessments of categorical features (bowel wall thickening, bowel wall signal abnormality, diffusion restriction, skip lesions, creeping fat, vasa recta hyperemia, lymphadenopathy and penetrating disease). Finally, we computed percentage agreement, Cohen kappa and McNemar test for the subset of subjects who had an independent third reviewer of the pre-/post-contrast results on the individual sections of the bowel as well as overall (terminal ileum to rectum, and colon to rectum). A two-sided P<0.05 was considered statistically significant. Analysis was done on SPSS v25 (IBM, Armonk, NY); Fleiss kappa was computed in R: A Language and Environment for Statistical Computing (R Core Team, Vienna, Austria); and sensitivity, specificity and confidence intervals of kappa were calculated via VassarStats (Poughkeepsie, NY).
Results
The demographic and clinical characteristics of the 77 patients included are summarized in Table 2. Patients ranged from 7 years to 18 years of age (mean [SD] = 13.8 [2.8] years). Fifty-eight of the 77 patients (75.3%) had known IBD, while 19 had suspected disease. Active inflammation was found in 52 patients (67.5%) on endoscopy/biopsy.
Initially we examined the agreement of MRE and the reference standard of combined endoscopic and histopathological results in identifying areas of active inflammation. Table 3 summarizes the agreement as well as the sensitivity and specificity of active inflammation on MRE compared to combined endoscopy and histopathology. Sensitivity of pre-contrast to combined endoscopy and histopathology versus pre-/post-contrast to combined endoscopy and histopathology was the same (67%; CI 0.53–0.79, P<0.001). Specificity was better on the pre-contrast images to combined endoscopy and histopathology compared to pre-/post-contrast images to combined endoscopy and histopathology (80%; CI 0.59–0.92; P<0.001 vs. 68%; CI 0.46–0.84; P=0.003). The sensitivity of MRE, whether with contrast agent or without, was poor in every section of bowel; the specificity was higher on the pre-contrast images.
The agreement of MRE with and without contrast agent is shown in Table 4. In the combined analysis of all five sections of the bowel (terminal ileum, right colon, transverse colon, left colon and rectum), there was moderate agreement among the three assessments (κF=0.57, P<0.001) and between the pre-contrast and pre-/post-contrast MRE readings (85.7%; κ 0.71, P<0.001; McNemar P-value 0.55). We also analyzed each of the five bowel sections for agreement. Agreement, according to Cohen kappa, was lowest in the transverse colon (97.4%; κ 0.49, P<0.001; McNemar P-value 1.00) and highest in the right colon (94.8%; κ 0.75, P<0.001; McNemar P-value 0.625). Moderate agreement was seen in the terminal ilium (81.8%; κ 0.63, P<0.001; McNemar P-value 0.79).
We evaluated findings indicative of macroscopic active inflammation on MRE (bowel wall thickening, bowel wall edema, diffusion restriction) and skip lesions. Table 5 shows the results for the McNemar test comparing the differences in pre-contrast exams, post-contrast exams, and pre-/post-contrast exams in assessing these features of inflammation. The addition of a contrast agent did not statistically improve the ability to identify any of the factors indicative of active inflammation (Fig. 1). There was good agreement in the identification of terminal ileum wall thickening among the three assessments (κF=0.63, P<0.001) and this was statistically similar among the pairs of pre- vs. pre-/post-contrast (McNemar P-value 0.77), pre- vs. post-contrast (McNemar P value 0.42) and post- vs. pre-/post-contrast exams (McNemar P-value 0.18). Near-perfect identification of terminal ileum bowel wall edema was also seen in the pre- vs. pre-/post-contrast exams (29 vs. 30 exams; McNemar P-value 1.00). There was no significant difference in the identified areas of diffusion restriction in the terminal ileum (26 vs. 23; McNemar P-value 0.45). Bowel wall thickening and edema were also identified similarly in the colon and rectum on pre- vs. pre-/post-contrast exams. More areas of wall thickening and edema were found in the left colon on the pre-contrast exams compared to the pre-/post-contrast exams, but it was not statistically significant (P=0.22 and P=0.45, respectively). With the exception of colon to rectum bowel wall thickening (pre- vs. post-contrast agent) all pairwise comparisons among pre-, post-, and pre-/post-contrast exams were non-significant, indicating agreement. According to the Fleiss kappa, the majority of the agreements among the three assessments were moderate to strong, with the transverse colon bowel wall thickening being poor (κF=0.18, P=0.006) and rectal bowel wall thickening being fair (κF=0.35, P<0.001).
Identification of factors of active inflammation. a Axial T2-W half-acquisition single-shot fast spin echo (SSFSE) MR image without contrast agent in a 14-year-old boy with Crohn's disease. Image shows wall thickening (10 mm) and edema of the terminal ileum (arrows) with pseudosacculation (*) and adjacent free pelvic fluid. b Axial T1-W fast spoiled gradient recalled acquisition in the steady state (FSPGR) fat-saturated post-contrast MR image shows enhancement of the thick-walled terminal ileum (arrows) and pseudosacculation (*)
Post-contrast imaging helped to identify more penetrating complications than pre-contrast imaging alone (3 exams pre- vs. 10 exams pre-/post-contrast; McNemar P-value 0.04). These findings are summarized in Table 6 and demonstrated in Fig. 2. Pre-/post-contrast exams identified more other non-bowel findings, as well, although the difference was not statistically significant. For example, imaging with a contrast agent identified more vasa recta hyperemia (9 pre- vs. 13 pre-/post; McNemar P-value 0.39) and more lymphadenopathy (19 pre vs. 25 pre/post; McNemar P-value 0.18). Creeping fat was identified in equal measure on both pre- and pre-/post-contrast exams (18 exams; McNemar P-value 1.0).
Identification of penetrating complications. a Coronal T2-W half-acquisition single-shot fast spin echo (SSFSE) MR image without contrast agent in a 12-year-old girl with Crohn's disease status post subtotal colectomy with ileoproctostomy shows pseudopolyp formation in the ileum near the anastomosis with prominent perirectal fat and perirectal lymphadenopathy. There is a focal area of T2 hyperintensity (arrow) adjacent to the anus that appears to be related to wall thickening in this region. b However, post-contrast coronal dynamic liver acquisition with volume acceleration (LAVA) MR image demonstrates peripheral enhancement consistent with a small abscess (arrow)
The independent reader (Reader 3) interpreted a random selection of 27 of the 77 exams (35.1%). Table 7 summarizes the inter-reader agreement between the consensus visual analysis sessions (performed by Reader 1 and Reader 2) and Reader 3. Overall, good agreement was seen among the joint readers and independent reader, with 85.2% agreement (κ 0.70, P=0.001; McNemar P-value 0.63). Agreement was almost perfect in the right colon (100.0%; κ 1.0, P<0.001; McNemar P-value 1.00) and lowest with good agreement in the left colon (96.3%; κ 0.65, P<0.001; McNemar P-value 1.00).
Discussion
The results of our study show that the use of a GBCA does not improve the detection of active bowel inflammation in the terminal ileum, colon and rectum on MRE. Overall moderate agreement (85.7%; κ 0.71, P<0.001; McNemar P-value 0.55) between the pre-contrast readings and pre-/post-contrast readings in all evaluated segments of bowel (from terminal ileum to rectum) when using combined endoscopic and histopathological results as the reference standard suggests that a contrast agent often does not contribute to the accurate diagnosis of active inflammation. In other words, non-contrast images are often sufficient for diagnosing active inflammation in the terminal ileum and colon.
The administration of a contrast agent did not significantly affect sensitivity or specificity; pre-contrast MRE and post-contrast MRE demonstrated similar sensitivities and specificities for detecting active bowel inflammation. Interestingly, we observed that the sensitivity of MRE, whether without or with a contrast agent, is poor when using combined endoscopic and histopathological results as the reference standard. This held true for all segments of evaluated bowel, and the sensitivity and specificity did not significantly differ on the pre-contrast images alone (67% and 80%) from the combined pre-/post-contrast images (67% and 68%) compared to the combined endoscopic and histopathological results. Our results indicate that endoscopy with biopsy is better at diagnosing active inflammation than MRE. This might differ from previously reported values of sensitivity and specificity of MRE in the literature because of variations in the reference standards used. There are no standard criteria for interpreting combined endoscopic and histopathological results. Some studies have used both endoscopic and histopathological results while others have only compared imaging findings to histopathological results. We used both histopathological and endoscopic results in a method similar to that described by Quaia et al. [16], although we did not use the same reporting standards of the Crohn's disease endoscopic index of severity criteria or the histological acute inflammatory score. This approach is also the most consistent with typical clinical practice in which physicians use many sources of data (history, physical exam, endoscopic appearance, pathology reports, etc.) in making the diagnosis of IBD. There is no gold standard test recognized for IBD. The reduced sensitivity and specificity of MRE in our study do not, however, indicate that MRE is ineffective in evaluating active inflammation in these children. Endoscopy with biopsy can only evaluate the bowel mucosa and cannot evaluate the other layers of the bowel wall or detect extraluminal abnormalities, whereas MRE can. It is also difficult to evaluate large areas of the small bowel by endoscopy. Therefore, MRE and endoscopy are complementary methods of evaluating IBD.
We studied the diagnostic performance of pre-contrast MRE and pre-/post-contrast MRE in assessing various features of active bowel inflammation from the terminal ileum to the rectum. The administration of a contrast agent did not significantly improve the ability to diagnose any features of active bowel inflammation, such as bowel wall thickening, bowel wall edema or diffusion restriction, with the exception of the left colon. These features were found less frequently in the left colon on the pre-/post-contrast images, possibly because with the addition of contrast agent the findings of wall thickening and edema were interpreted to be artifactual because the left colon is particularly prone to poor distension, which decreases the ability to detect active inflammation. In addition, our results showed that the administration of a contrast agent does not significantly improve the detection of the non-bowel findings of creeping fat, vasa recta hyperemia or lymphadenopathy. On the other hand, administration of a contrast agent did significantly improve the detection of penetrating complications, which include fistulas, sinus tracts, phlegmon and abscesses. Interestingly, the number of children found to have lymphadenopathy and penetrating disease was similar between the pre- and post-contrast imaging sessions compared to the pre-/post-contrast imaging sessions, although the difference was not statistically significant. The findings support the conclusion that identification of penetrating disease is increased with use of both pre- and post-contrast images.
These findings are concordant with similar studies in the literature, such as the one performed by Quaia et al. [16] and the one by Lanier et al. [21]. The former study included adults and the latter included pediatric patients. Both sets of authors concluded that intravenous GBCAs are likely not necessary in the detection of active bowel inflammation in the setting of IBD. In both studies, there was no significant difference in the diagnostic accuracy of non-contrast versus contrast-enhanced imaging. Additionally, the study by Lanier et al. [21] noted that perianal penetrating complications were better assessed by contrast-enhanced imaging. This is in agreement with our study, in which penetrating complications were much more frequently detected with the administration of GBCAs. Most patients undergo optimization of medical management, whether they have penetrating disease or not. Therefore, not identifying penetrating disease in children whose disease is controlled or whose symptoms are improving would not significantly alter the management of these children [22]. If a child’s symptoms do not improve or worsen despite escalation of medical therapies, or if there are signs of obstruction, then surgical exploration might be warranted. In these cases a contrast-enhanced study would be indicated because not identifying these factors might delay the child’s care.
In addition, the assessment of inter-reader variability showed moderate agreement between the joint readers and the independent reader in our study (85.2%, κ 0.70, P=0.001; McNemar P-value 0.63) for pre-/post-contrast imaging. This is in concordance with the studies by Quaia et al. [16] and Lanier et al. [21], both of which demonstrated very good inter-reader agreement.
We acknowledge that this study has several limitations. It is a retrospective study with a relatively small sample size of 77 patients. Bowel endoscopy and biopsy, which were used as the reference standard, only evaluate the bowel mucosa; they cannot detect inflammation of the other layers of the bowel wall or evaluate outside the bowel. In addition, endoscopy does not evaluate the bowel proximal to the terminal ileum, so our conclusions cannot be applied to bowel proximal to the terminal ileum. Inflammatory bowel disease (especially Crohn's disease) can involve two or more bowel segments with normal intervening bowel (skip lesions), potentially leading to sampling error in the endoscopic analysis. On the other hand, MRE cannot be used to evaluate microscopic or superficial mucosal disease. Therefore, MRE and endoscopy with biopsy act in a complementary fashion in assessing different aspects of inflammatory bowel disease. Finally, inter-reader agreement was evaluated for only pre-/post-contrast imaging rather than for both pre-contrast imaging and pre-/post-contrast imaging. Therefore, we cannot evaluate whether the presence of a GBCA affects inter-reader agreement (i.e. whether contrast agent increases inter-reader agreement).
Conclusion
The use of intravenous GBCAs does not improve the detection of active inflammation in the terminal ileum, colon or rectum in the setting of known or suspected IBD compared to non-contrast MRE, although use of a contrast agent does aid in the detection of penetrating disease. Therefore, in most children being worked up for possible IBD, or in those with quiescent disease, non-contrast MREs should be considered for routine evaluation, whereas contrast-enhanced imaging might be reserved for evaluating those with acutely worsening symptoms or symptoms that are not improving with treatment.
References
Dillman JR, Ladino-Torres MF, Adler J et al (2011) Comparison of MR enterography and histopathology in the evaluation of pediatric Crohn's disease. Pediatr Radiol 41:1552–1558
Mollard BJ, Smith EA, Dillman JR (2015) Pediatric MR enterography: technique and approach to interpretation — how we do it. Radiology 274:29–43
Foti PV, Farina R, Coronella M et al (2015) Crohn’s disease of the small bowel: evaluation of ileal inflammation by diffusion-weighted MR imaging and correlation with the Harvey-Bradshaw index. Radiol Med 120:585–594
Dillman JR, Trout AT, Davenport AS (2018) Allergic-like contrast media reaction management in children. Pediatr Radiol 48:1688–1694
Fraum TJ, Ludwig DR, Bashir MR, Fowler KJ (2017) Gadolinium-based contrast agents: a comprehensive risk assessment. J Magn Reson Imaging 46:338–353
Flood TF, Stence NV, Maloney JA, Mirsky DM (2017) Pediatric brain: repeated exposure to linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR imaging. Radiology 282:222–228
Kanda T, Ishii K, Kawaguchi H et al (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Kanda T, Osawa M, Oba H et al (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275:803–809
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Miller JH, Hu HH, Pokorney A et al (2015) MRI brain signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics 136:1637–1640
Radbruch A, Weberling LD, Kieslich P et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
Roberts DR, Holden KR (2016) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 38:331–336
White GW, Gibby WA, Tweedle MF (2006) Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (Prohance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investig Radiol 41:272–278
Xia D, Davis RL, Crawford JA, Abraham JL (2010) Gadolinium released from MR contrast agents is deposited in brain tumors: in situ demonstration using scanning electron microscopy with energy dispersive X-ray spectroscopy. Acta Radiol 51:1126–1136
Quaia E, Sozzi M, Gennari AG et al (2016) Impact of gadolinium-based contrast agent in the assessment of Crohn’s disease activity: is contrast agent injection necessary? J Magn Reson Imaging 43:688–697
Moy MP, Sauk J, Gee MS (2016) The role of MR enterography in assessing Crohn’s disease activity and treatment response. Gastroenterol Res Pract 2016:8168695
Seo N, Park S, Kim K et al (2016) MR enterography for the evaluation of small-bowel inflammation in Crohn's disease by using diffusion-weighted imaging without intravenous contrast material: a prospective noninferiority study. Radiology 278:762–772
Smolinski S, George M, Dredar A et al (2014) Magnetic resonance enterography in evaluation and management of children with Crohn’s disease. Semin Ultrasound CT MR 35:331–348
Towbin AJ, Sullivan J, Denson LA et al (2013) CT and MR enterography in children and adolescents with inflammatory bowel disease. Radiographics 33:1843–1860
Lanier MH, Shetty AS, Salter A, Khanna G (2018) Evaluation of noncontrast MR enterography for pediatric inflammatory bowel disease assessment. J Magn Reson Imaging 48:341–348
Ruemmele FM, Veres G, Kolho KL et al (2014) Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis 8:1179–1207
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Farmakis has received research grants from Guerbet, LLC, and GE Healthcare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, S.J., Ratchford, T.L., Buchanan, P.M. et al. Diagnostic accuracy of non-contrast magnetic resonance enterography in detecting active bowel inflammation in pediatric patients with diagnosed or suspected inflammatory bowel disease to determine necessity of gadolinium-based contrast agents. Pediatr Radiol 49, 759–769 (2019). https://doi.org/10.1007/s00247-019-04369-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-019-04369-6