Abstract
Fontan fenestration decreases central venous pressure and preserves cardiac output while decreasing systemic oxygen saturation. Transcatheter fenestration closure increases oxygen saturation, though the persistence of this increase and the long-term incidence of adverse outcomes such as death and heart transplant remain unknown. We describe immediate and long-term clinical and adverse outcomes following fenestration closure. Catheterization, echocardiogram, and clinic reports were reviewed following transcatheter Fontan fenestration closure. Data were reported as n (%) and median (IQR). Continuous variables were compared using Wilcoxon ranked sum test. 51 patients had fenestration closure 0.9 (0.7–1.5) years following extracardiac Fontan operation. Most (84%) were closed with Amplatzer Septal Occluders. Systemic O2 saturation immediately increased from 87 (83–89) to 95 (94–97)%, P < 0.05. Cardiac index decreased from 4 (3–5) to 2.9 (2.6–3.5) L/min/m2. Fontan pressure and pulmonary vascular resistance were not significantly changed. Clinical follow-up duration for all patients was 7.3 (range 1.3–16) years. Oxygen saturation at last follow-up was 94.5 (92–97)% and did not decrease over time (P < 0.05). One patient (2%) developed protein losing enteropathy, 1 (2%) had heart transplant, and 1 (2%) patient died 9.4 years following fenestration closure. No patient required fenestration re-creation following closure. Transcatheter Fontan fenestration closure leads to sustained increases in systemic oxygen saturation and a low incidence of adverse outcomes such as death and transplant. Further study comparing fenestration closure to non-closure and longer follow-up duration are required to determine if there is a survival benefit to fenestration closure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since the initial description in 1971, total cavo-pulmonary anastomosis (commonly referred to as the Fontan operation) has considerably increased the lifespan of patients with single ventricle physiology, by relying on moderately increased systemic venous pressure and appropriately low pulmonary vascular resistance to provide pulmonary blood flow and preload to the systemic ventricle [1, 2]. Multiple modifications have been made to the original technique to improve outcomes, including interposition of an extracardiac synthetic conduit from the inferior vena cava to the right pulmonary artery (extracardiac Fontan) [3]. In an effort to decrease post-operative morbidity and mortality in high risk patients, fenestrations were introduced between the systemic and pulmonary venous atria to reduce central venous pressure and preserve cardiac output, at the expense of systemic oxygen saturation [4]. Appropriately sized and positioned fenestrations could be closed with a transcatheter device post-operatively after the patient had improved clinically [5, 6]. While short- and medium-term outcomes following fenestration closure have been described, long-term outcomes in patients following elective transcatheter fenestration closure have not been reported previously. We describe immediate and long-term clinical and adverse outcomes following transcatheter Fontan fenestration closure with the Amplatzer septal occluder, Gore Helex septal occluder, and Gore Cardioform septal occluder.
Materials and Methods
Study Population
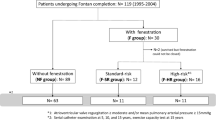
The study sample included all patients who had transcatheter closure of an extracardiac Fontan fenestration from 1/1/2003 to 12/31/2016 at the University of Rochester Medical Center, identified through the institutional electronic pediatric cardiac catheterization database. There were no specified exclusion criteria. Referral for fenestration closure was at the discretion of the primary cardiologist and was not proscribed by institutional protocol. The study protocol was approved by the local Institutional Review Board.
Procedural Details
A complete right and left heart catheterization was completed under general anesthesia for all patients. Fenestration test occlusion was accomplished with a Berman angiographic catheter and hemodynamic measurements repeated after occlusion for 10 min. The decision to proceed with permanent fenestration occlusion was at the discretion of the interventional cardiologist. Occlusion devices included the Amplatzer Septal Occluder (Abbott, Chicago, IL), Gore Helex Septal Occluder and Cardioform Septal Occluder (W.L. Gore & Associates, Flagstaff, AZ). The occlusion device size was based on fenestration diameter documented in the operative note—balloon sizing was not routinely performed. Amplatzer septal occluder diameter was selected such that the device waist was equal to or 1 mm larger than the fenestration. When Gore septal occluders were used, the smallest size device available was generally used. Occlusion devices were always deployed under fluoroscopic guidance. Additional guidance was provided by transesophageal echocardiography (TEE) at the discretion of the interventional cardiologist. Residual fenestration shunt was evaluated by angiography and TEE if this modality was utilized during device deployment.
Data Collection
Catheterization reports, echocardiogram reports, and outpatient clinic notes were retrospectively reviewed for all patients with transcatheter closure of an extracardiac Fontan fenestration from 1/1/2003 to 12/31/2016. Clinical data obtained from the catheterization reports included primary cardiac diagnosis, age and weight at Fontan surgery, diameter of the extracardiac Fontan graft, diameter of the Fontan fenestration, and age and weight at fenestration closure. Hemodynamic data included baseline systemic oxygen saturation, cardiac index, Qp:Qs, mean pressure in the Fontan circuit, and pulmonary vascular resistance. Catheterization data were re-collected for subsequent cardiac catheterizations.
Clinical data obtained from the most recent outpatient clinic evaluation and echocardiogram included age, weight, systemic arterial saturation by pulse oximetry, the presence of arrhythmia, protein losing enteropathy, plastic bronchitis, cirrhosis, or history of paradoxical embolism, age at death (if applicable), and age at heart transplant (if applicable).
Statistical Analysis
Continuous variables were reported as mean (± standard deviation), median (range), or median (interquartile range). Categorical variables were reported as n (%). Continuous variables were compared using the Wilcoxon rank sum test. Freedom from death or heart transplant was evaluated by Kaplan Meier analysis. A P value < 0.05 was considered statistically significant. Statistical analysis was carried out using the R Statistical Programming Language (R Foundation for Statistical Computing, Vienna, Austria).
Results
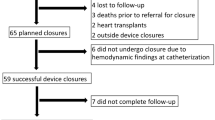
Fifty-one patients (43% female) had transcatheter Fontan fenestration closure at a median age of 5.7 (IQR 5.1–7) years old and median weight 19.3 (IQR 17.1–21.8) kg. The duration from extracardiac Fontan operation to fenestration closure was 0.9 (0.7–1.5) years. Most of the patients had hypoplastic left heart syndrome (27%), double inlet left ventricle (18%), or heterotaxia/other single ventricle physiology (15%). The median extracardiac conduit diameter was 16 (range 13–20) mm and median fenestration diameter 4.5 (range 3–5) mm (Table 1).
With test occlusion of the fenestration, median systemic oxygen saturation increased from 87% (IQR 83–89%) to 95% (IQR 94–97%), P < 0.05. Cardiac index decreased from 4 (IQR 3–5) L/min/m2 to 2.9 (IQR 2.6–3.5) L/min/m2, P < 0.05. Qp:Qs increased from 0.65 (IQR 0.56–0.79) to 1 (IQR 1–1), P < 0.05. Mean Fontan pressure did not change significantly [baseline 13 (IQR 11.5–14) mmHg to test occlusion 13 (IQR 12.3–15) mmHg, P = 0.05], and pulmonary vascular resistance did not change significantly [baseline 1.8 (IQR 1.2–2.2) WU × M2 to test occlusion 1.9 (IQR 1.6–2.5) WU × M2, P = 0.24] (Table 2).
Forty-three (84%) patients had fenestration closure with the Amplatzer septal occluder, 7 (14%) with the Gore Helex septal occluder, and 1 (2%) with the Gore Cardioform septal occluder. Thirty-six (71%) patients had a tiny residual angiographic shunt through the device at the end of the procedure. Among the 32 patients who had concurrent transesophageal echocardiogram, 7 (22%) had a tiny residual shunt through the device by color Doppler at the end of the procedure. Four (8%) patients had concomitant procedures including 1 (2%) with veno-venous collateral closure, 2 (4%) with aorto-pulmonary collateral closure, and 1 (2%) with pulmonary artery angioplasty. There was no procedural mortality with fenestration closure.
Ten (20%) patients had follow-up cardiac catheterization after transcatheter fenestration closure. The median duration from fenestration closure to follow-up cardiac catheterization was 7 (IQR 2.8–11.7) years. Compared to the pre-fenestration closure (baseline) hemodynamics, the systemic oxygen saturation remained significantly greater compared to baseline [92% (IQR 80–94%) vs 87% (IQR 84–89%), P < 0.05]. Cardiac index remained lower compared to baseline [2.3 (1.8–2.6) L/min/m2 vs. 4 (IQR 3–5) L/min/m2, P < 0.05]. While pulmonary vascular resistance was not significantly changed [most recent catheterization 1.9 (IQR 1.5–2.9) WU × M2 vs. baseline 1.8 (IQR 1.2–2.2) WU × M2, P = 0.32], the mean Fontan pressure had increased at the follow-up catheterization [16 (IQR 13–18.5) mmHg vs. 13 (IQR 11.5–14) mmHg, P < 0.05] (Table 3). Five (10%) patients had additional procedures in addition to the diagnostic catheterization. One (2%) patient with a 5 mm fenestration closed with a 15 mm Gore Helex Septal Occluder had a persistent residual fenestration that was successfully closed with a 4 mm Amplatzer septal occluder 2.7 years following the initial closure attempt. One (2%) patient had persistent hypoxemia, and had veno-venous collateral closure and pulmonary artery angioplasty 3 years following fenestration closure. One (2%) patient had closure of aorto-pulmonary collaterals, 1 (2%) patient had pulmonary artery stent implantation, and 1 (2%) patient had a concomitant liver biopsy.
The median duration from transcatheter fenestration closure to most recent follow-up was 7.3 (range 1.3–16) years. At last follow-up, the oxygen saturation by pulse oximetry [94.5% (IQR 92–97%)] was not significantly changed from the systemic oxygen saturation immediately following fenestration closure [95% (IQR 94–97%), P < 0.05]. One (2%) patient developed atrial flutter and non-sustained ventricular tachycardia 4.2 years following fenestration closure. Two (4%) patients developed protein losing enteropathy 1.7 and 1.8 years following fenestration closure. No patient developed plastic bronchitis. One (2%) patient had a heart transplant for Fontan failure 4.1 years after fenestration closure, and one (2%) patient expired 9.4 years after fenestration closure (Table 4). No patients had creation of a new fenestration to decompress the Fontan circuit.
Discussion
This study demonstrates that an immediate increase seen in oxygen saturation following transcatheter Fontan fenestration closure was sustained in long-term follow-up of up to 16 years, with a low incidence of adverse outcomes such as death and transplant. However, in those patients who had an interval diagnostic catheterization between fenestration closure and most recent outpatient follow-up, the mean Fontan pressure had increased.
Although the Fontan operation has increased longevity substantially for patients with single ventricle physiology, there was considerable morbidity and mortality early in the experience with this surgery. The adoption of a staged approach, directing superior vena cava flow to the pulmonary arteries prior to completing the total cavo-pulmonary connection halved surgical mortality from 16 to 8% in one series [7]. To further decrease morbidity and mortality, fenestrations between the systemic and pulmonary venous atria were introduced during the Fontan operation, to allow for some right to left shunting and preserve cardiac output at the expense of systemic oxygen saturation in the immediate post-operative period, when there might be increased pulmonary vascular resistance secondary to pulmonary edema or atelectasis, and impaired systemic ventricle systolic or diastolic function [5]. Though initially utilized for patients determined to be high risk for Fontan operation, the use of fenestration increased over time in the Fontan Cross-Sectional Study, such that from 1992 to 2002 80% of Fontan procedures included fenestration. [8]. One of the major benefits of Fontan fenestration has been reduced post-operative pleural drainage [4]. Patients at our center had transcatheter Fontan fenestration closure approximately 1 year post-operatively, following a period of outpatient stability, with the goal of improving systemic oxygen saturation and decreasing the risk for paradoxical embolism.
Previous studies had described the short- and medium-term outcomes following transcatheter fenestration closure. While systemic oxygen saturation increases immediately following fenestration closure, this increase is not enough to offset the decrease in cardiac index, leading to decreased systemic oxygen transport and increased oxygen extraction ratio, potentially worsening exercise capacity [9]. Despite this, the incidence of morbidity and mortality has been low in short- and medium-term follow-up [10, 11]. Our study adds to this growing body of knowledge, with median follow-up nearly twice as long as that which has been previously reported, demonstrating that increased oxygen saturation is sustained, and with minimal adverse outcomes.
However, the increased mean Fontan pressure on interval catheterization following fenestration closure raises concerns for increased Fontan associated morbidity over time. Various modes of Fontan failure, including Fontan associated liver disease, protein losing enteropathy, and plastic bronchitis, have been associated with persistently elevated central venous pressure [12,13,14,15]. In some cases, transcatheter creation of a fenestration has led to an improvement in symptoms associated with Fontan failure [16,17,18]. While no patients in our study required creation of a fenestration following transcatheter closure, it is possible that some may benefit from decompression of the Fontan circuit over a longer period of follow-up.
There are several limitations to our study. Our study is retrospective and is prone to the inherent biases of such studies. Of particular concern, selection bias may confound the results as the decision to proceed with fenestration closure, and the timing of closure were not proscribed by institutional protocol. The absence of a similar patient cohort that did not undergo fenestration closure prevents comparison of long-term outcomes between patients who had transcatheter fenestration versus those who did not. Additionally, there was variation in closure technique, with utilization of multiple types of closure devices. There were very few patients with non-Amplatzer septal occluder devices used for fenestration closure, and hence comparison of fenestration closure efficacy between devices was not possible.
Conclusions
Transcatheter Fontan fenestration closure effectively and durably increases systemic oxygen saturation with a low incidence of adverse events in long-term follow-up. However, given the relationship between elevated central venous pressure, Fontan associated liver disease, protein losing enteropathy, and plastic bronchitis, even longer term follow-up is needed to determine the risk for very late adverse events following fenestration closure.
References
Rychik J et al (2019) Evaluation and management of the child and adult with Fontan circulation: a scientific statement from the American Heart Association. Circulation 140(6):e234–e284
Fontan F, Baudet E (1971) Surgical repair of tricuspid atresia. Thorax 26(3):240–248
Marcelletti C et al (1990) Inferior vena cava-pulmonary artery extracardiac conduit. J Thorac Cardiovasc Surg 100(2):228–232
Lemler MS et al (2002) Fenestration improves clinical outcome of the Fontan procedure: a prospective, randomized study. Circulation 105(2):207–212
Bridges ND, Lock JE, Castaneda AR (1990) Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation 82(5):1681–1689
Zahn E et al (1998) Transcatheter closure of an extracardiac fontan fenestration. Ann Thorac Surg 66:260–262
Jacobs ML, Norwood Jr WI (1994) Fontan operation: influence of modifications on morbidity and mortality. Ann Thorac Surg 58(4):945–951. (discussion 951–952)
Atz AM et al (2011) Late status of Fontan patients with persistent surgical fenestration. J Am Coll Cardiol 57(24):2437–2443
Hijazi ZM et al (1992) Hemodynamic evaluation before and after closure of fenestrated Fontan. An acute study of changes in oxygen delivery. Circulation 86(1):196–202
Goff DA et al (2000) Clinical outcome of fenestrated Fontan patients after closure: the first 10 years. Circulation 102(17):2094–2099
Webb MK et al (2020) Extracardiac Fontan fenestration device closure with amplatzer vascular plug II and septal occluder: procedure results and medium-term follow-up. Pediatr Cardiol 41(4):703–708
Itkin M et al (2017) Protein-losing enteropathy in patients with congenital heart disease. J Am Coll Cardiol 69(24):2929–2937
Healy F, Hanna BD, Zinman R (2012) Pulmonary complications of congenital heart disease. Paediatr Respir Rev 13(1):10–15
Avitabile CM et al (2014) A multifaceted approach to the management of plastic bronchitis after cavopulmonary palliation. Ann Thorac Surg 98(2):634–640
Daniels CJ et al (2017) Fontan-associated liver disease: proceedings from the American College of Cardiology stakeholders meeting, October 1 to 2, 2015, Washington DC. J Am Coll Cardiol 70(25):3173–3194
Bae EJ et al (2003) De novo creation of fenestration and stent implantation for failed extracardiac conduit Fontan operation. Int J Cardiol 88(2–3):321–322
Chaudhari M, Stumper O (2004) Plastic bronchitis after Fontan operation: treatment with stent fenestration of the Fontan circuit. Heart 90(7):801
Vyas H et al (2007) Results of transcatheter Fontan fenestration to treat protein losing enteropathy. Catheter Cardiovasc Interv 69(4):584–589
Funding
The authors did not receive funding from any organization for the submitted work.
Ethics declarations
Competing Interests
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devanagondi, R., Leonard, G. Transcatheter Fontan Fenestration Closure: Sustained Improvements in Oxygen Saturation with Minimal Morbidity and Mortality. Pediatr Cardiol 44, 922–926 (2023). https://doi.org/10.1007/s00246-022-03077-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-03077-7