Abstract
Measurement of elevated trace elements is an important component of environmental assessment and management of estuarine marine sediments in systems adjacent to concentrated human activity. The present study surveys the estuarine sediments in selected tributary bays, creeks, and the upper segments of the Georges River system, NSW, Australia, which flows into the Tasman Sea through Botany Bay. A total of 146 surface sediment samples were analysed by X-ray fluorescence. Potential pollution of sediments was evaluated using potential load index, modified degree of contamination, and potential ecological risk index. The spatial distribution of trace elements varies between sites. Variable sources of contamination, including runoff from catchment areas, and emissions from watercraft and boatyards are contributing sources. Bay morphologies and their interactions with catchment and tidal flows play significant roles in the distribution of trace elements. The greatest concentration of trace elements occurs around discharge points and in the inner parts of bays that have high percentages of mud particles and organic matter. The lowest contamination by trace elements was found in sandy sediments along the shoreline and edges of the bays. Trace element distributions decline in concentration in residential-free areas and reach background levels in deeper sediment cores. The concentrations of trace elements were controlled by discharge points from the catchment area, marine boat activities, bay morphology, and sediment types (sand, silt, and clay). The highest pollutant concentrations are the result of past legal, but uncontrolled, discharge of waste from manufacturing into Salt Pan Creek.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Coastal and estuarine ecosystems can play a significant role as reservoirs for many persistent organic and inorganic contaminants, such as nutrients and trace elements, through adsorption onto suspended matter and subsequent sedimentation (Zhan et al. 2010; Junakova et al. 2015). The main sources of contamination in estuaries are derived from catchment area runoff and include mining, industries, commercial waste, urban development, sewage discharge, agricultural and irrigation runoff, and atmospheric deposition (Karageorgis et al. 2012; Cheng et al. 2015; Singovszka et al. 2015; Kim et al. 2016). In addition, contamination may come from boat moorings and boatyards within bays, dredging, localized oil pollution, and the use of antifouling products, anticorrosive paints and metallurgical processing (Attia et al. 2012; Tarique et al. 2012; Zhang et al. 2012). Areas surrounding many industrial and commercial centres contain particularly high concentrations of trace elements. These concentrations result from the inflow of untreated or poorly treated runoff onto the land or waterways (Martley et al. 2004). As a result, the global distribution of toxic trace elements, such as Hg, As, Pb, Cu, Zn, Cd, Ni, and Se, are considered to represent one of the environmental crises associated with rapid economic development around the entire world. Marine sediments in estuaries, lagoons, and bays effectively act as sinks and/or a secondary source for anthropogenic pollutants comprising trace elements and organic matter (Qiu et al. 2011). Trace elements are released again into the water column when there are changes in chemical, physical, and biological process, such as pH, Eh, and bioturbation. Consequently, premeditated and/or unintentional human activities, such as waste disposal and discharge of waste into the marine system (estuaries, lagoons, and bays), causes contamination and often disperses into the vicinity of the marginal marine setting.
The distribution of trace elements in surface sediments from Izmit Bay, Turkey, was evaluated by Pekey (2006a) and found to be influenced by contamination in a local stream. The results demonstrated that the most concentrated element was Sn. Moreover, trace elements, such as Cd, As, Pb, and Zn, were considered to be moderately concentrated within the bay. In contrast, only background concentrations of elements, such as Co, Cr, Cu, Fe, Mg, Mn, and Ni, were found. The study suggested that the increased concentration of some trace elements coincides with an increased source of anthropogenic pollution, which arises from increased urbanization and industrialization, and organic matter from industrial plant developments and shipping traffic.
Assessment of contamination by trace metals, such as Cr, Pb, Zn, and V, in soils at the Jahmau and Unnao areas of the Ganga Plain, India, was completed by Gowd et al. (2010). The results showed that the impact of anthropogenic activities increased the amount of heavy metals in the soils of the study areas. They were found to be extremely contaminated because of many years of random dumping of hazardous waste and the free discharge of drains by a number of industries, such as cotton and wool textile mills, tanning and leather manufacturing, fertilizer factories, and several arms factories.
Estimation of enrichment factors for contamination by trace metals, such as Pb, Cu, Ni, and V, in the surface and subsurface sediments of Koumounfourou Lake, Greece, was investigated by Karageorgis et al. (2009). The sources of these heavy metals were various industries and oil refineries. Furthermore, the lake receives freshwater from underwater springs and water outlets from an industrialised catchment and the Athens landfill. Another study performed by Amin et al. (2009) also evaluated the concentration and distribution of trace elements to determine the pollution status of the coastal surface sediments near Dumai, Indonesia. The results showed that the sediments in Dumai City and its vicinity had higher trace element concentrations compared with other sites in the area. The concentration of trace elements was compared to other regions of the world by using the standard sediment quality guidelines; trace elements were not deemed to be significant pollutants in this moderately polluted coastal environmental. Research has suggested that the main source of contamination from trace elements in coastal sediments emanates from human activities and industrial development (Romano et al. 2004; Amin et al. 2009). Moreover, coastal topography and transfer by water currents have an important role in the concentration of trace element contaminants in sediment, especially in the eastern Dumai coastal lagoon.
These contaminants are rapidly transported and adsorbed onto sediments, especially fine particles, such as clay minerals and organic matter, via chemical, physical, and biological processes. When changes in environmental conditions occur, the resultant chemical (pH and Eh), physical (temperature and turbidity), and biological processes can release contaminants back into the water column. This secondary contamination can have a significant effect on marine ecosystems (Karageorgis et al. 2012; Naser 2013). Accumulation of trace element pollution occurs in the upper layer of sediments in aquatic environments through geochemical and biological processes and may become toxic to sediment-dwelling organisms and fish (Cosentino et al. 2013; Li et al. 2013). As a consequence, trace element pollution is one of the largest problems in coastal marine ecosystems causing potential bio-accumulation and bio-magnification, resulting in long-term implications, because it can enter the human body via the food chain. Coastal environments (estuaries and embayments) are important, because they act as a sink for sediments and trap large quantities of fine particles. These sediments are related to pollution, because the fine particles have charged surfaces and occur in a protected environment, sheltered from fast currents, waves, tides, and wind activities (Duan et al. 2011). Both the sediment particles and pollutants may be derived from the adjacent land and transported by runoff, stormwater outlets, tidal activity, and waves. The entrapment is due to estuarine environments having a circulatory current activity, which can inhibit the sediment particles from escaping the estuary. Depositional processes for sediments within the estuaries are controlled by flocculation and transportation by tidal current activity and nontidal waves. Sediment size has an important role in the depositional processes, because fine particles can be moved in suspension (McLusky 1989; Palinkas et al. 2014).
The deposition of fine sediment particles can be inhibited by strong tidal activities and large river flow discharges, which occur at the mouth and head of the bay, respectively. Away from the mouth of the bay, the strength of tidal currents and waves declines into the side embayments. As a consequence, the deposition of sediment particles occurs gradually in the inner parts of bays mainly as a result of flocculation. Originally, the coarse sediment fractions comprising pebbles and coarse sands are deposited close to river and stormwater discharge points. The deposition of coarse to fine sand also can occur near the shoreline where the sediment is reworked by wave action. Fine to very fine sediment particles (<63 µm) are concentrated in the inner and upper reaches of the estuary and bays, which contain organic matter (McLusky 1989; Hein et al. 2013). The objectives of this study were to determine the spatial distribution of trace elements, identify pollution sources, and assess potential risks from sediments in the Georges River system, NSW, Australia.
Study Area
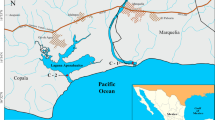
The Georges River system is located approximately 15 km south of the central business district of Sydney (33°55′S, 151°11′ E; Fig. 1) and is classified as a drowned river valley estuary, discharging into a broad bay (Botany Bay) with an unrestricted entrance to the ocean (Roy 1984). The smaller Cooks River also drains into Botany Bay to the north of the Georges River. The whole system of the two river estuaries and Botany Bay form an area of approximately 49 km2 with a mouth, approximately 1.1-km wide (Fraser et al. 2006). The system is partly protected from the oceanic waves by headlands. Both main rivers entering the bay have catchment areas that extend into Sydney; thus they have large discharges of urban and industrial stormwater, feeding Botany Bay with an annual water runoff of approximately 520 × 106 m3 (Fraser et al. 2006). This study investigates selected sub-environments in the Georges River estuary comprising Woolooware Bay, Kogarah Bay, Oyster Bay, Oatley Bay, Woronora River, Salt Pan Creek, and the main arm of the upper Georges River estuary (Fig. 1).
Materials and Methods
Sample Collection and Analyses
A total of 146 surface sediment samples from different water depths were collected using a Van Veen grab sampler during summer 2013, and they were combined with 179 samples from previous work (Fig. 1; Pease 2007; Alyazichi et al. 2015a; Alyazichi et al. 2015b). The surface 5 cm of sediment was reserved for analysis. Water depth and sample locations were recorded at each site using echo-sounding (sonar) and a Geographical Position System (GPS). Grain size analyses were conducted using a Malvern Mastersizer 2000, and classified according to Folk (1974). Trace elements were measured using an XRF Spectro-Xepos energy dispersive spectrometer fitted with a Si-docile detector, following an established standard procedure (Norrish and Chappell 1977). While a full spectrum of trace elements was determined by the XRF analyses, only chromium (Cr), nickel (Ni), arsenic (As), copper (Cu), zinc (Zn), lead (Pb), rubidium (Rb), and bromine (Br) are presented because of limited space. The other elements exhibited similar spatial distributions. International reference standards (G-2, BIR-1 and AC-G) were analysed to calibrate the precision and accuracy of the analytical procedure. Detection limits for lead and bromine were 1 mg/kg and 0.5 mg/kg, respectively.
Subsurface sediment samples from deeper cores (75 mm diameter and 2.5 m below the sediment surface) were used to assess the more stable pre-European background subsurface trace element concentrations (Pease 2007). These background concentrations were used to determine the amount of trace element contamination in the samples.
Sediment Risk Assessments
Assessment factors used to evaluate sediment quality in this study include the modified degree of contamination (mC d), potential load index (PLI), and potential ecological risk index (RI). Abrahim and Parker (2008) introduced a modified and generalised formula based on Hakanson’s (1980) original formula to calculate the modified degree of contamination for trace element pollution in estuarine sediments.
CF i is the contamination factor, C sample is the measured concentration of each trace element, C background is the concentration of the trace elements in a vibracore (75 mm diameter at 2.5 m depth), mC d is the modified degree of contamination, and n is number of trace elements analysed.
The potential load index (PLI) was proposed by Sheppard (1998) to calculate contamination, because it allows comparison of contaminant concentrations between locations at different times. The PLI was calculated by using the following formula, and the PLI values were classified.
The potential ecological risk index (RI) was used to assess the effects of the trace element pollution in the study area. The RI was originally defined by Hakanson (1980) and was calculated using Reboredo’s (1993) modification:
CF i is the contamination factor; \( E_{\text{r}}^{i} \) is the monomial potential ecological risk factor; \( T_{\text{r}}^{i} \) is the response coefficient for the toxicity of a single trace element, which was adopted as the evaluation criterion, i.e., Cr = 2, Ni = Cu = Pb = 5, Zn = 1 and As = 10 (Hakanson 1980; Mei et al. 2011); and RI is the sum of all risk factors for trace elements in the sediments. According to Hakanson (1980), the RI values are rated in four classes from low to very high risk.
Geostatistical Analyses
ArcGIS desktop software, version 10.2, was used to create contour maps for the variables measured for the sediment samples from each bay. For each of the variables analysed (e.g., water depth, mud particles, and trace elements), values between the sampled sites were interpolated using the “Spline with Barriers” method from the ArcGIS Spatial Analyst Toolbox. Raster layers were created for each variable for analysis and mapping purposes. The final maps are presented as contour plots of relevant variables (Duan et al. 2015).
Results and Discussion
Grain size Analysis
The results of the grain size analyses provide information to classify the sedimentary environments and to assist in the evaluation of depositional processes (Folk 1974). The grain size ternary diagram for the Georges River system is based on Folk’s 1974 classification and shows a continuum of sizes from sandy silt to clean sand with a few samples in the clay and silty clay zone (Fig. 2). The textural distributions in the estuary samples are related to the source rocks in the catchment, which are predominantly sedimentary.
Classification of surface sediments in the Georges River system after Folk (1974). Where S sand, s sandy, Z silt, z silty, M mud, m muddy, C clay and c clayey
The spatial distribution of the textural classifications is made using a classification presented by Blott and Pye (2012) and is illustrated in Fig. 3a. Muddy (silt+clay) particles dominate the central parts of the tributary bays where grain size ranges between mud and very fine sand. Fine and medium sands become dominant close to the shallow margins. The sand content ranges from 0 to 100% (Fig. 3b). The highest percentages of sand have accumulated in shallow water, especially at the mouth and along the edges and shoreline of each bay, where wave activity is highest, causing transport of fine particles and leaving medium and coarse particles close to the shoreline. High percentages of sand also were found in the Woronora River and the previously dredged main channel of Georges River below Salt Pan Creek (Fig. 3b).
In general, the deepest water areas have the lowest percentages of sand. This is related to low current activity at depth within the bays where waves do not disturb the bottom sediments. Fine particles such as silt and clay can be transported by the currents and tides into the deeper water and then gradually settle and concentrate in these deeper areas (Fig. 3c, d).
The percentages of mud particles in the Georges River system also range between 0 and 100% (Fig. 3c). The clay particle percentages are low compared with those of silt and sand (Fig. 2). The highest percentages of mud accumulate in the inner and middle sites of the bays, such as Kogarah Bay, Oyster Bay, Oatley Bay, and Woolooware Bay, because the wave and current activity is less compared with the main channel. Mud content is low in the main channel of the Woronora River, as well as around the edges and mouths of the bays, where local wind waves prevent deposition of fine particles or resuspended them; these sites are dominated by sand (Alyazichi et al. 2015b). The percentage of mud declines as depth becomes shallower along the shoreline (Fig. 3c). Fine organic matter also can stay undisturbed in deeper water areas, because current velocities and waves are less effective (Fig. 4h). Exceptions to this pattern included Salt Pan Creek and Oyster Bay, which had shallow water depths (<1.5 m; Fig. 3d) but also low percentages of sand. Their sediment consisted of high percentages (approximately 80%) of fine to very fine particles, which may have been because of the low wave and current activity in their sheltered environments; consequently, the fine particles do not escape from Salt Pan Creek and Oyster Bay.
Spatial Distribution of Trace Elements
The trace element concentrations Cr, Ni, As, Cu, Zn, Pb, Rb, and Br in the surface sediments within the inner parts of the Georges River system are illustrated in (Table 1). Generally, it was found that concentrations of trace elements had similar patterns in most embayments in the Georges River system with the highest concentrations at the head and in the middle of the bays (Figs. 4a–h). Trace elements, such as Cu, Zn, and Pb, are concentrated within the deeper inner portions of the bays. In addition, trace elements were concentrated around harbours and marinas where many boats are moored.
The concentrations of trace elements declined markedly towards the edges and mouths of the bays as well as in the main Georges River channel and Woronora River where the sandy sediments had the lowest concentrations of trace elements. These oxidising environments had a high sand content and low percentages of mud particles and organic matter (Fig. 3b). Most of the bays had their highest concentrations of trace elements located around drains and discharge points and within the inner parts of the bays. There are several reasons for this pattern. First, surface sample sites close to discharge points and stormwater drains from roadways and residential areas have sediment particles combining with trace elements, flocculating and settling close to the discharge points due to the mixing of fresh and salt water. Second, samples that are close to boatyards where boats are painted to prevent fouling have high contaminant concentrations. There also are large numbers of moored watercraft with potential contaminant spills. Third, the high percentages of mud (Fig. 3c), including clay minerals, such as illite, kaolinite, and chlorite, at the surface sample sites can accumulate and trap trace elements through absorption, ion exchange, and metal substitution with muddy particles (Karageorgis et al. 2012; Gunawardana et al. 2014; Xu et al. 2014; Alyazichi et al. 2015a). The high clay content is shown by the high concentrations of Rb between 50 mg/kg and 90 mg/kg in the inner parts of the bays (Fig. 4g) that are typically associated with the aluminosilicate mud particles. Finally, increased percentages of organic matter (total carbon) also were higher at these deeper muddy sites (Sansalone and Kim 2008). The concentration of Br, which has a positive relationship with organic matter, ranged from 100 to 500 mg/kg in the inner parts of the bays, and it exceeded 300 mg/kg in some bays, such as Kogarah Bay and Woolooware Bay (Fig. 4h). These sites represent anoxic conditions, allowing trace elements to be captured by the minerals and organic matter (Zhao et al. 2014). According to previous studies (Sansalone and Kim 2008; Zhao et al. 2014), organic matter has an affect on chemical speciation transformation and the absorption of trace elements in sediments and can affect the toxicity and bioavailability of trace elements. Finally, increased percentages of organic matter (total carbon) may be correlated with anoxic environments in which trace elements can be captured (Sansalone and Kim 2008; Nath et al. 2013). Salt Pan Creek is considered to be a very highly polluted creek and has the highest concentrations of trace elements in the analysed samples from the Georges River. Concentrations ranged between 24 and 86 mg/kg for Cr; 17 and 30 mg/kg for Ni; 74 and 138 mg/kg for Cu; 349 and 788 mg/kg for Zn; 11 and 26 mg/kg for As; and 138 and 268 mg/kg for Pb.
Historical location of industries using or generating various chemicals in the catchment of Salt Pan Creek had previously produced and discharged large amounts of trace elements into both the creek and the Georges River, as well as discharges from stormwater and wastewater that are thought to be an important ongoing contributor to the general pollution of these waterways. Second, Salt Pan Creek contains high percentages of mud particles, ranging from 70 to 96%. Finally, the high percentages of pyrite (1.6–3.6%) and the high Br contents (organic matter) represent anoxic conditions in which trace elements tend to be concentrated (MacDonald et al. 2004; Nath et al. 2013).
Correlation Between Sediment Particle Size and Trace Elements
A correlation matrix was constructed to search the relationship between sediment particle size and trace elements. Table 2 summarizes the correlation matrix results for the Georges River samples. There is a strongly significant correlation between Cr, Ni, Cu, Zn, As, and Pb, muddy particles (clay and silt), and organic matter, as shown by the regression values. The correlation coefficients also support the previously described relationship between trace elements and mud particles. Muddy particles can be indicated by Rb while organic matter can be indicated by Br. It is apparent that the concentrations of trace elements increase with increasing percentages of mud particles and organic matter (Table 2). This represents an anoxic environment, which can absorb and accumulate trace elements. Trace elements have a negative relationship with increased sand fraction (Table 2). This is because sand is left as a residual deposit where local waves become more active in shallow marginal areas, reworking finer sediments and trace elements, which will then deposit in calmer and/or deeper sections of the creeks and bays.
Assessment of Sediment Contamination
As outlined previously, three parameters, the potential load index (PLI), modified degree of contamination (mC d), and potential ecological risk index (RI) were applied to evaluate sediment contamination by trace elements (Hakanson 1980; Mmolawa et al. 2011). The potential ecological risk index (RI) is the most popular method used in order to evaluate the hazards of trace elements for both human and environmental ecosystems. It indicates the concentration of trace elements that may cause adverse effects in biological toxicology, environmental chemistry, and ecology (Fig. 5c; Guo et al. 2010; Jiang et al. 2014).
The values of the three contamination factors were found to vary between samples in the same bay and from one bay to another in the surface sediments. The values vary from very low risk and/or unpolluted to a high degree of contamination or pollution (Fig. 5a–c) and are controlled by variables, such as distance from source of pollution, sediment grain size, and the type and amount of various minerals. Higher degrees of contamination indicated by highly polluted and very high risk values (exceeding and/or slightly less than 120 RI) indicate that some sites in Kogarah Bay, Oyster Bay, and Salt Pan Creek are highly polluted. This is because these sites are close to discharge points, stormwater outlets, watercraft, or boatyards, as well as having sediment types that are dominated by muddy particles in the inner parts of the bays where they are unaffected by active waves and currents. In contrast, other nearby sites in the study areas have low degrees of contamination, are not polluted, and have low risk values. These were found along the shorelines, edges and in the mouths of bays, such as in Oyster Bay, Georges River, and Woronora River that are dominated by coarse particles (sand and/or coarse silt). At these sites, currents and waves are more active, leading to transportation of fine particles and trace elements toward deeper areas. The highest values PLI, mC d and RI for Cu, Zn, and Pb, respectively, all occur in the very highly contaminated Salt Pan Creek, which includes contamination from previous manufacturing and a waste dump (Fraser et al. 2006).
Comparison with Australian Estuaries and Sediment Quality Guidelines
In this study, the range and mean values of the trace element concentrations in marine sediment samples were compared with the Australian New Zealand Environment and Conservation Council (ANZECC) and Natural Health and Medical Research Council (NHMRC) (ANZECC and NHMRC 2000) sediment and water quality guidelines (Table 3). Some additional elements are included in this table for illustration. This comparison determined whether the trace elements were within acceptable ranges (low risk), between low and high triggers values, or exceeded the high trigger values that require further investigation according to the national guidelines. Adverse biological effects of trace elements are occasionally detected between the low and high values in the ANZECC and NHMRC (2000) guidelines.
According to ANZECC and NHMRC (2000) protocols, the anthropogenic pollution of trace elements, such as Cr, Ni, Cu, Zn, As, and Pb, within the sediments of the study sites were generally below the interim sediment quality guideline values (ISQG-low) except for elements, such as Pb in Kogarah Bay, Oyster Bay, and Salt Pan Creek; Cu and Ni in Salt Pan Creek; and Zn in Oyster Bay and Salt Pan Creek (Table 3). Table 3 is divided into sections: the study sites in the Georges River system and, for comparison, other estuaries where comprehensive trace element studies have been completed.
For the Georges River system, the trace element concentrations were noted to be between the ISQG-low ANZECC and NHMRC (2000) and high values at some sites in all bays. These concentrations depend on the sources and discharge points as well as boatyards and watercraft. Salt Pan Creek is the most highly polluted by trace elements in the study area. The levels can be correlated with both water depth and muddy particles but show the highest concentrations in the study areas. This can be attributed to historical dumping of waste into the creek and chemical industry (e.g., oil refinery) discharged waste within it. The mean values of trace element pollution for Cu, Zn, and Pb in the surface sediments of the study areas generally had higher concentrations compared with the more pristine environments in both Burrill Lake and the Huon estuary (Killian 1999; Jones et al. 2003b). The surface sediments in both the Huon estuary and Burrill Lake are dominated by clean sand fractions and low percentages of organic matter, and their catchment areas are non industrialised and lightly developed (Killian 1999; Jones et al. 2003a). The results for the current study are slightly higher than for a previous study in Botany Bay (Birch et al. 1996). Trace element concentrations for Cr, Ni, and As in surface sediments from the Huon River were similar to all the present study areas, except for Ni in Salt Pan Creek and As in Oatley Bay (Table 3). This is because of the large amount of dolerite in the source rock in the Huon River releasing trace metals as it weathers. Thus, it is important to understand the natural contributions from the catchment geology when assessing the amount of pollution in any area. This factor has been overcome for the Georges River sites by using local background trace element concentrations from a time prior to significant anthropogenic pollution.
Surface sediments in Salt Pan Creek were more concentrated in trace elements compared with the Huon estuary and Burrill Lake (e.g., 3× times for Cu, 14× times for Zn, and 7× times for Pb). This was caused by dumping waste from an oil refinery and other waste disposal sites; for this reason, Salt Pan Creek is markedly more polluted compared with other parts of the study areas. However, the values of trace elements in surface sediments from the current study area had low concentrations in comparison to previous studies in Port Hunter, Sydney Harbour, Port Kembla, Griffins Bay, and the Derwent River, except for Zn and Pb in Salt Pan Creek. Surface sediments in Sydney Harbour (Irvine and Birch 1998) and the Derwent River (Jones et al. 2003a) have been subject to large amounts of contamination from urban, industrial and commercial activities, such as a zinc refinery, stormwater runoff, mine discharges, vehicles exhausts, sewage effluent, and watercraft, as well as leaching from reclamation areas.
Comparison with Estuaries Worldwide
Most estuaries and embayments located near population concentrations around the world are typically polluted by trace elements derived largely from human activity. Selected examples of trace element concentrations in other locations are summarized in Table 4. There is considerable variation between trace element concentrations in the study areas and trace elements in the estuaries around the world for several reasons: the sediment types; chemical, physical and hydrological characteristics of the water; the sources and amounts of pollution discharged into the bays (e.g., industrial activities, farming, fishing, and transportation as well as residential, commercial, and agricultural runoff); background concentrations of trace elements; and possibly the use of different analytical methods to estimate the concentrations of trace elements. As a result, the behaviour of trace elements is varied in many estuaries and bays around the world. The different concentrations of trace elements in the various countries in Table 4 reflect differing industrial and other use concentrations in source areas with varying concentrations of development, as well as pollutant control measures. These average figures do not provide information on the distribution of trace elements with these systems. Detailed mapping of pollutant distribution within bays and estuaries is required before any management or remediation action can be considered. Limited comparison could be made if distributions are linked to morphological or hydrodynamic properties as a system example but detailed spatial mapping is far more effective.
Summary of the Differences Between Bays
The distribution pattern of sediment particles and trace elements in the different bays in this study appears to be related to different bay morphologies and their interactions with catchment and tidal flows. In wide bays with wide-open mouths (Kogarah Bay and Oyster Bay) deposition of trace elements generally follows the current and tide trajectories, with secondary concentrations in boatyards and under moored boats where leaching, anti-fouling and fuel spillages add to the pollutant sources. Linear and elongated bays usually have a single major point source for water and thus pollutant flows show trace element distributions that are constrained by the narrow bay form. Trace element pollution is concentrated in Salt Pan Creek, which has the highest concentrations of trace elements around the Georges River, with the concentrations significantly declining when the creek joins with the main Georges River channel. The current velocities are faster in this creek compared with other bays, largely because the creek has a narrow, elongated channel form, aiding distribution of trace elements well away from initial discharge points.
Conclusions
Chemical composition of sediments can be used as a sensitive indicator for both spatial and temporal trend monitoring of contaminants in the coastal marine environment. Marginal marine sediment contamination is considered to be one of the main environmental concerns for marine ecosystems. A combined total of 325 surface sediments were analyzed from the Georges River system, NSW, Australia. Highly polluted and high risk sites were in Salt Pan Creek and some locations within other bays. However, low polluted and low-risk sites were found in Woronora River and close to shoreline areas in bays. Toxic pollutants, such as trace elements, and organic matter originating from human activities like transport, industry, stormwater runoff drains, boatyards, and watercraft, are continuing to be introduced to aquatic environments through rivers, waste dumping, and emission processes. They are then deposited in marine sediments, which act as both a sink and source of pollution in the aquatic environment. This study provides useful baseline information to contribute to future management and water quality planning. It is necessary to investigate the distribution and degree of trace element pollution in order to protect environmental ecosystems from accumulating pollution and to provide basic information for coast utilization and management.
References
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monitor Assess 136:227–238
Alyazichi YM, Jones BG, McLean E (2015a) Source identification and assessment of sediment contamination of trace metals in Kogarah Bay, NSW, Australia. Environ Monitor Assess 187:1–10
Alyazichi YM, Jones BG, McLean E (2015b) Spatial and temporal distribution and pollution assessment of trace metals in marine sediments in Oyster Bay, NSW, Australia. Bull Environ Contam Toxicol 94:52–57
Amin B, Smail A, Arshad A, Yap CK, Kamarudin MS (2009) Anthropogenic impacts on heavy metal concentrations in the coastal sediments of Dumai, Indonesia. Environ Monit Assess 148:291–305
ANZECC and NHMRC (2000) Australian and New Zealand guidelines for the assessment and management of contaminated sites. Australian and New Zealand Environment Conservation Council and National Health and Medical Research Council, Canberra
Attia OEA, Abu Khadra AM, Nawwar AH, Radwan GE (2012) Impacts of human activities on the sedimentological and geochemical characteristics of Mabahiss Bay, north Hurghada, Red Sea. Egypt Arab J Geosci 5:481–499
Birch GF, Evenden D, Teutsch ME (1996) Dominance of point source in heavy metal distributions in sediments of a major Sydney estuary (Australia). Environ Geol 28:169–174
Birch GF, Ingleton T, Taylor SE (1997) Environmental status of the world’s largest coal exporting harbour, Port Hunter, Neweastle, Austraila. J Mar Environ Eng 4:133–145
Blott SJ, Pye K (2012) Particle size scales and classification of sediment types based on particle size distributions: review and recommended procedures. Sedimentology 59:2071–2096
Cheng H, Li M, Zhao C, Yang K, Li K, Peng M, Yang Z, Liu F, Liu Y, Bai R, Cui Y, Huang Z, Li L, Liao Q, Luo J, Jia S, Pang X, Yang J, Yin G (2015) Concentrations of toxic metals and ecological risk assessment for sediments of major freshwater lakes in China. J Geochem Explor 157:15–26
Cosentino C, Peoe F, Scopelliti G, Calabro M, Caruso A (2013) Benthic foraminiferal response to trace element pollution—the case study of the Gulf of Milazzo, NE Sicily (Central Mediterranean Sea). Environ Monit Assess 185:8777–8802
Duan L, Song J, Xu Y, Li X (2011) The concentrations, fluxes, enrichments and chronologies of potential harmful elements in sediment cores from Bohai Bay, North China. Environ Earth Sci 64:2259–2269
Duan X, Zhang G, Rong L, Fang H, He D, Feng D (2015) Spatial distribution and environmental factors of catchment-scale soil heavy metal contamination in the dry-hot valley of Upper Red River in southwestern China. Catena 135:59–69
Fernandez-Cadena JC, Andrade S, Silva-Coello CL, De La Iglesia R (2014) Heavy metal concentration in mangrove surface sediments from the north-west coast of South America. Mar Pollut Bull 82:221–226
Folk RL (1974) Petrology of sedimentary rocks. The University of Texas, Austin
Fraser C, Hutchings P, Williamson J (2006) Long-term changes in polychaete assemblages of Botany Bay (NSW, Australia) following a dredging event. Mar Pollut Bull 52:997–1010
Gowd S, Ramakrishan Reddy M, Govil PK (2010) Assessment of heavy metal contamination in soils at Jajmau (Kanpur) and Unnao industrial areas of the Ganga Plain, Uttar Pradesh, India. J Hazard Mater 174:113–121
Gunawardana C, Egodawatta P, Goonetilleke A (2014) Role of particle size and composition in metal adsorption by solids deposited on urban road surfaces. Environ Pollut 184:44–53
Guo W, Liu X, Liu Z, Li G (2010) Pollution and potential ecological risk evaluation of heavy metals in the sediments around Dongjiang Harbor, Tianjin. Procedia Environ Sci 2:729–736
Hakanson L (1980) An ecological risk index for aquatic pollution control: a sedimentological approach. Water Res 14:975–1001
Hamer K, Karius V (2002) Brick production with dredged harbour sediments. An industrial-scale experiment. Waste Manag 22:521–530
Hein H, Hein B, Pohlmann T (2013) Recent sediment dynamics in the region of Mekong water influence. Glob Planet Chang 110:183–194
Hyun S, Lee C-H, Lee T, Choi J-W (2007) Anthropogenic contributions to heavy metal distributions in the surface sediments of Masan Bay, Korea. Mar Pollut Bull 54:1059–1068
Irvine I, Birch GF (1998) Distribution of heavy metals in surficial sediments of Port Jackson, Sydney, New South Wales. Aust J Earth Sci 45:297–304
Jiang X, Teng A, Xu W, Liu X (2014) Distribution and pollution assessment of heavy metals in surface sediments in the Yellow Sea. Mar Pollut Bull 83:366–375
Jones BG, Chenhall BE, Debretsion F, Hutton AC (2003a) Geochemical comparisons between estuaries with non-industrialised and industrialised catchments: the Huon and Derwent River estuaries, Tasmania. Aust J Earth Sci 50:653–667
Jones BG, Killian HE, Chenhall BE, Sloss CR (2003b) Anthropogenic effects in a coastal lagoon: geochemical characterization of Burrill Lake, NSW, Australia. J Coast Res 19:621–632
Junakova N, Junak J, Balintova M (2015) Reservoir sediment as a secondary raw material in concrete production. Clean Technol Environ Policy 17:1161–1169
Karageorgis AP, Katsanevakis S, Kaberi H (2009) Use of enrichment factors for the assessment of heavy metal contamination in the sediments of Koumoundourou Lake, Greece. Water Air Soil Pollut 204:243–258
Karageorgis A, Sioulas A, Krasakopoulou E, Anagnostou C, Hatiris G, Kyriakidou H, Vasilopoulos K (2012) Geochemistry of surface sediments and heavy metal contamination assessment: Messolonghi lagoon complex, Greece. Environ Earth Sci 65:1619–1629
Killian HE (1999) The spatial distribution of sediment-bound trace metals within the surface sediments of Burrill Lake, New South Wales, Honours thesis, University of Wollongong, Wollongong
Kim BSM, Salaroli AB, Ferreira PADL, Sartoretto JR, Mahiques MMD, Figueira RCL (2016) Spatial distribution and enrichment assessment of heavy metals in surface sediments from Baixada Santista, Southeastern Brazil. Mar Pollut Bull 103:333–338
Li T, Xiang R, Li T (2013) Benthic foraminiferal assemblages and trace metals reveal the environment outside the Pearl River estuary. Mar Pollut Bull 75:114–125
Macdonald BCT, Smith J, Keene AF, Tunks M, Kinsela A, White I (2004) Impacts of runoff from sulfuric soils on sediment chemistry in an estuarine lake. Sci Total Environ 329:115–130
Martley E, Gulson BL, Pfeifer HR (2004) Metal concentrations in soils around the copper smelter and surrounding industrial complex of Port Kembla, NSW, Australia. Sci Total Environ 325:113–127
Mclusky DS (1989) The estuarine ecosystem, 2nd edn. Blackie Academic Professional, Glasgow
Mei J, Li Z, Sun L, Gui H, Wang X (2011) Assessment of heavy metals in the urban river sediments in Suzhou City, northern Anhui Province, China. Procedia Environ Sci 10:2547–2553
Mmolawa K, Likuku A, Gaboutloeloe G (2011) Assessment of heavy metal pollution in soil along roadside areas in Botswana. Afr J Environ Sci Technol 5:186–196
Naser HA (2013) Assessment and management of heavy metal pollution in the marine environment of the Arabian Gulf: a review. Mar Pollut Bull 72:6–13
Nath B, Birch G, Chaudhuri P (2013) Trace metal biogeochemistry in mangrove ecosystems: a comparative assessment of acidified (by acid sulfate soils) and non-acidified sites. Sci Total Environ 463–464:667–674
Norrish K, Chappell B (1977) X-ray fluorescence spectrometry. In: Zussman J (ed) Physical methods in determinative mineralogy. Academic Press, London
Palinkas CM, Halka JP, Li M, Sanford LP, Cheng P (2014) Sediment deposition from tropical storms in the upper Chesapeake Bay: field observations and model simulations. Cont Shelf Res 86:6–16
Pease J (2007) Sedimentation and geochemistry in Oatley Bay, Georges River, Sydney, New South Wales. B.Sc. (Honours) thesis, University of Wollongong
Pekey H (2006a) The distribution and sources of heavy metals in Izmit Bay surface sediments affected by a polluted stream. Mar Pollut Bull 52:1197–1208
Pekey H (2006b) Heavy metal pollution assessment in sediments of the Izmit Bay, Turkey. Environ Monit Assess 123:219–231
Qiu Y-W, Lin D, Liu J-Q, Zeng EY (2011) Bioaccumulation of trace metals in farmed fish from South China and potential risk assessment. Ecotoxicol Environ Saf 74:284–293
Raj SM, Jayaprakash M (2008) Distribution and enrichment of trace metals in marine sediments of Bay of Bengal, off Ennore, south-east coast of India. Environ Geol 56:207–217
Reboredo F (1993) How differences inthe field influence Cu, Fe and Zn uptake by Halimione-portulacoides and Spartina-maritima. Sci Total Environ 133:111–132
Romano E, Ausili A, Zharova N, Celiamagno M, Pavoni B, Gabellini M (2004) Marine sediment contamination of an industrial site at Port of Bagnoli, Gulf of Naples, southern Italy. Mar Pollut Bull 49:487–495
Roy PS (1984) Holocene sedimentation histories of estuaries in southeastern Australia. Estuarine environments of the southern hemisphere. Department of Conservation and Environment, Western Australia, Bulletin No. 161: 23–59
Sansalone JJ, Kim J-Y (2008) Suspended particle destabilization in retained urban stormwater as a function of coagulant dosage and redox conditions. Water Res 42:909–922
Sheppard SC (1998) Geophagy: who eats soil and where do possible contaminants go? Environ Geol 33:109–114
Singovszka E, Balintova M, Holub M (2015) Heavy metal contamination and its indexing approach for sediment in Smolnik creek (Slovakia). Clean Technologies and Environmental Policy 18(1):305–313
Tarique Q, Burger J, Reinfelder JR (2012) Metal concentrations in organs of the clam Amiantis umbonella and their use in monitoring metal contamination of coastal sediments. Water Air Soil Pollut 223:2125–2136
Xu G, Liu J, Pei S, Kong X, Hu G (2014) Distribution and source of heavy metals in the surface sediments from the near-shore area, north Jiangsu Province, China. Mar Pollut Bull 83:275–281
Zhan S, Peng S, Liu C, Chang Q, Xu J (2010) Spatial and temporal variations of heavy metals in surface sediments in Bohai Bay, North China. Bull Environ Contam Toxicol 84:482–487
Zhang W, Liu X, Cheng H, Zeng EY, Hu Y (2012) Heavy metal pollution in sediments of a typical mariculture zone in South China. Mar Pollut Bull 64:712–720
Zhao L, Xu Y, Hou H, Shangguan Y, Li F (2014) Source identification and health risk assessment of metals in urban soils around the Tanggu chemical industrial district, Tianjin, China. Sci Total Environ 468–469:654–662
Acknowledgements
This paper is a part of the first author’s PhD. thesis undertaken at School of Earth and Environmental Sciences, University of Wollongong. It was financially supported by the Ministry of Higher Education and Scientific Research in the Iraqi Government, and by the GeoQuEST Research Centre, University of Wollongong, Australia. We would like to acknowledge the anonymous reviewers for their useful comments to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alyazichi, Y.M., Jones, B.G., McLean, E. et al. Geochemical Assessment of Trace Element Pollution in Surface Sediments from the Georges River, Southern Sydney, Australia. Arch Environ Contam Toxicol 72, 247–259 (2017). https://doi.org/10.1007/s00244-016-0343-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-016-0343-z