Abstract
The Messolonghi lagoon complex in Western Greece receives agricultural and domestic effluents both from point and diffused sources. Surface sediments were analyzed for grain size, organic carbon, total nitrogen, total sulfur, major and minor elements, aiming at the identification of geochemical relationships between all variables. Enrichment factors and the modified degree of contamination methods were applied to assess potential heavy metal enrichment related to human activities. Sediment texture was highly variable, with muddy sediments prevailing. In the central sector of the Messolonghi lagoon, organic carbon contents were high. Principal factor analysis revealed the following main groups of variables with common geochemical behavior: (1) terrigenous aluminosilicates (2) organic matter, (3) biogenic carbonates, (4) mineral quartz-aluminosilicates, and (5) Mn-oxides. Enrichment factors estimated for V, Cr, Mn, Co, Ni, Cu, Zn, and Pb using local pre-industrial sediment showed that all metals exhibit almost natural background levels, except for Pb, which was found to be slightly elevated (legacy of leaded fuel). Estimation of contamination factors concluded in similar results, whereas the overall modified degree of contamination was at the lowest level, therefore suggesting that this transitional water body has not been affected by anthropogenic activities. The data set may be considered as a baseline for future monitoring projects according to EU policy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lagoons and river mouths, otherwise known as transitional waters between freshwater, marine and terrestrial ecosystems are sensitive water bodies, which are often influenced by human activities (Basset et al. 2006; Nicolaidou et al. 2005; Nriagu and Pacyna 1988). Several studies have demonstrated that heavy metal contamination is well recorded in sediments, and also pointed out that elevated heavy metal contents may be related to natural processes, such as weathering and erosion of adjacent rock formations, as well as anthropogenic inputs (Förstner and Wittmann 1979; Salomons and Förstner 1984). Although a great number of studies have focused on heavy metal contamination in rivers and lakes (e.g., Brügmann 1995; Mahler et al. 2006; Nikolaidis et al. 2004; Sanei et al. 2001; Scherer et al. 2003), geochemical surveys in lagoons (Bellucci et al. 2010; Beltrame et al. 2009; González et al. 2007; Huang et al. 1994; Siegel et al. 1994) are essential as lagoons have been recognized among the most productive ecosystems within the biosphere. Lagoons are included in the European Water Framework Directive (Directive 2000/60/EC) as ecosystems requiring continuous monitoring and conservation.
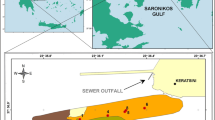
The Messolonghi–Aetoliko wetlands comprise lowlands, hills, and lagoons of 62,000 ha in the western part of the Greek mainland (Fig. 1). They have been formed by the deltaic deposits of the Acheloos River, which currently flows westwards into the Ionian Sea, as well as the Evvinos River, which flows eastwards into the Gulf of Patras (Anagnostou et al. 2009). The Messolonghi–Aetoliko lagoon complex that comprises the Aetoliko, Messolonghi, and Kleisova lagoons, as well as smaller ones, represents one of the most important Mediterranean lagoon systems and the largest in Greece, covering approximately 50% of the total Greek lagoon surface (Anonymous 2001).
This complex is the property of the Greek State, and it is a wetland of great ecological importance protected under the Ramsar Convention; some sectors are designated as specially protected areas that are included in the Natura 2000 network. The lagoons are very shallow, with depths ranging from 0.2 to 1.5 m, with the exception of Aetoliko lagoon, where water depth reaches 30 m. The geomorphological characteristics and tidal regime strongly influence water circulation in the lagoons (Basset et al. 2006). In the study area, the communication with the open sea is regulated by the seasonal wind regime (Katselis et al. 2007 and references therein). According to Tsimplis (1994), tides follow a semi-diurnal cycle, and the tidal range is in general less than 20 cm (Dimitriou 2007; Tsimplis and Blackman 1997). Fisheries, a traditional and very important activity for the local community decreased from 1,500 to 2,000 tons in the 1960s to 1,300–1,500 tons in recent years (Dimitriou et al. 1994). Salt-works are producing ~130,000 tons of salt annually, which amounts to 90% of Greece’s total production. The main pollution sources are agricultural and domestic effluents entering into the lagoon through temporary streams, drainage pumping stations and channels, and diffused sources. In addition, the municipal wastewater treatment plant of Messolonghi, and the dumping site of the city influence directly the artificially separated eastern sector of the Kleisova lagoon.
The aim of this study is to identify the geochemical relationships of organic carbon, nitrogen, sulfur, carbonate contents, total major and minor elements, determined in bulk surface sediment samples, with particular interest on the behavior and potential enrichment of heavy metals in the Messolonghi lagoon. Previous work regarding heavy metal levels for the Messolonghi lagoon has been reported by Voutsinou-Taliadouri et al. (1987), and for the Kleisova lagoon by Papatheodorou et al. (2002). In the former study, extractable levels of Cr, Mn, Fe, Co, Ni, Cu, Zn, and Pb, were determined, whereas in the latter, total and extractable levels of the same suite of metals were determined in sediments from the Kleisova lagoon only. Extractable and total heavy metal levels in sediments of Aetoliko lagoon are given by Dassenakis et al. (1994). The present work reports for the first time a multi-element data set obtained in a suite of surface lagoon sediments. According to the European Union (EU) Water Framework Directive (2000/60/EC), human pressures on transitional waters (e.g., lagoons) should be identified by member states, and by 2015 good chemical and ecological status has to be achieved. In this perspective, this study will provide valuable data for establishing a baseline on geochemical parameters.
Materials and methods
Sample collection
Thirteen surface sediment samples were recovered from the seabed of the study area during July 1999 by means of a Van Veen grab, operated from a fishing boat (Fig. 1). In some cases, due to water depths <1 m, sediments were sampled by sweeping gently the upper 1 cm of the seabed by hand, placing sediment directly into a plastic container. Because of the size of the fishing boat and the small height of the bridge separating the Messolonghi and Aetoliko lagoons, samples were not obtained from the latter lagoon.
Sample analysis
The samples were analyzed in the laboratory for their grain-size properties using Micromeritics® Sedigraph 5100, after separation of the sand fraction by wet sieving; sand (Ø > 63 μm), silt (2 μm < Ø < 63 μm), and clay (Ø < 2 μm) weight percentages were determined, and classification followed Folk’s nomenclature (Folk 1974).
Organic and inorganic carbon, total nitrogen, and total sulfur were determined in a Fisons Instruments EA-1108 CHNS analyzer. The operating parameters were very similar to those reported by Cutter and Radford-Knoery (1991), Nieuwenhuize et al. (1994) and Verardo et al. (1990). The precision of the method is within 5%. A detailed description of the analytical procedure is given by Karageorgis et al. (2009).
Major and minor elements were determined in fused beads, and powder pellets, respectively, by X-ray fluorescence, in a Philips PW-2400 wavelength analyzer equipped with a 3-kW Rh tube. Analytical accuracy was better than 4% for major elements, and better than 9% for minor elements; precision was better than 0.5%. The method is described in detail by Karageorgis et al. (2005). Loss on ignition (LOI) was determined after burning 1 g of sample for 1 h at 1,000°C.
Principal factor analysis
The data set was inspected for outliers using box plots (cases with values between 1.5 and 3 box lengths from the upper or lower edge of the box, where the box length is the interquartile range). Sample MS-11 was identified as an outlier in 6 out of 30 variables, thus it was excluded from further statistical analyses. All variables showed normal distribution, but after a Box–Cox transformation linearity and symmetry was improved. Distribution fitting was tested by the Kolmogorov–Smirnov normality tests, estimated by the moments method at 5% significance level. Variables were subject to principal factor analysis (PFA) with Varimax rotation and Kaiser normalization to study and visualize correlations between variables.
Enrichment factors
The geochemical behavior and spatial variability of both major and minor elements is greatly affected by the sediments’ grain-size distribution. In the case of this study, grain-size effects are more pronounced, since sediments’ texture is highly variable. In order to study element interrelationships, and to assess potential contamination issues (e.g., using Enrichment Factors; Salomons and Förstner 1984), a careful normalization is required. As demonstrated by Sanei et al. (2001) and Karageorgis et al. (2009), LOI represents a suitable normalizer to eliminate the variability of the carbonate and organic carbon contents. In the present paper, the estimation of EF is given by the following formula:
where reference sediment is a pre-industrial core sample. The core TEL was recovered from the deltaic plain of Acheloos River in 2000, and has a total length of 20 m (Mariolakos et al. 2004). The sample No. 42 (6.60 m, silty sand; Tziovara and Champilomati 2002) selected as background was analyzed exactly by the same procedure as the surface sediment, and, in addition, it was radiocarbon dated at 990 ± 40 year BP. If the EF is greater than 1.0, an enrichment due to anthropogenic activities with respect to a natural background could be hypothesized (Acquavita et al. 2010).
Degree of contamination
Håkanson (1980) proposed a sedimentological approach to facilitate pollution control, using a diagnostic tool named ‘degree of contamination’. More recently, Abrahim (2005) introduced the ‘modified degree of contamination; mC d’ in order to estimate the overall degree of contamination at a given site according to the formula:
where n = number of analyzed elements and i = ith element (or pollutant) and C f = Contamination factor. For each pollutant, C f is estimated, based on the average of at least five surface sediment contents. The latter need to be compared to background pristine sediment, according to the equation:
where M x and M b, respectively, refer to the mean concentration of a pollutant in the contaminated sediments and the pre-industrial sediments. Using this generalized formula to calculate the mC d allows the incorporation of as many metals as the study may analyzed with no upper limit (Abrahim and Parker 2008). For the classification and description of the modified degree of contamination (mC d) in estuarine sediments, the following levels are proposed:
-
mC d < 1.5 nil to very low degree of contamination
-
1.5 ≤ mC d < 2 low degree of contamination
-
2 ≤ mC d < 4 moderate degree of contamination
-
4 ≤ mC d < 8 high degree of contamination
-
8 ≤ mC d < 16 very high degree of contamination
-
16 ≤ mC d < 32 extremely high degree of contamination
-
mC d ≥ 32 ultra high degree of contamination.
Results and discussion
Sediment texture
Surface sediment grain size varies substantially from muddy sands to clays (Table 1). Sand prevails in the western sector of the lagoon (Fig. 2a), silt content increases gradually from west to east towards Kleisova (Fig. 2b), whereas clay content exhibits highest values (95%) in the northern sector of the Messolonghi lagoon (Fig. 2c). The western sector of the Messolonghi lagoon, which comprises many small islands, represents older and currently inactive channels of the Acheloos River (Anagnostou et al. 2009). The coarser texture of the sediments possibly portrays bed load deposits of the Acheloos River.
Organic carbon, total nitrogen, total sulfur, and carbonate content
Organic carbon content varies from 1.32 to 5.95% (Table 1); the highest values are observed in the central-eastern sector of the lagoon, nearby the salt-works, and with decreasing trends from the west to the east (Figs. 1, 3a). Given that organic carbon content is usually enriched in the clayey sediments (e.g., Horowitz 1991), its behavior here deviates from typical trends. Sediments from stations MS-11 and MS-12 are composed of clay, but only MS-12 exhibits high organic carbon content (5.18%). In contrast, sediment from station MS-10 (sandy mud) shows organic carbon maximum content (5.95%). Voutsinou-Taliadouri et al. (1987) have also reported high Corg content in the same area (max. 7.5%). In the Kleisova lagoon, which receives poorly treated wastes from the municipal sewage treatment plant of the Messolonghi city (Hotos and Avramidou 1997), average organic carbon content of 3.32% is observed. The latter value is slightly lower than the estimates of Papatheodorou et al. (2002) (average Corg 4.22%).
Organic carbon is significantly correlated to total nitrogen (R = 0.977; p < 0.001) and to total sulfur (R = 0.868; p < 0.001). The strong correlations are also reflected in great similarities of the spatial distribution patterns of total N, and total S (Fig. 3b, c). In both cases, highest contents appear in the central-eastern sector of the Messolonghi lagoon. Since the central sector of the lagoon is not associated with anthropogenic activities, unlike the Kleisova lagoon, this suggests that elevated values of Corg, N, and S can be attributed to natural causes, i.e., the decomposition of benthic vegetation. Shallow water depths, limited water renewal, and possibly elevated productivity may also explain the elevated values.
Carbonate content exhibits values from 12.0 to 78.4% (Table 1). The highest values are observed in the western sector of the study area (Fig. 3d), whereas in the central sector and in Kleisova lagoon, values are generally <30%. Carbonate content is negatively correlated to Corg, total N, and total S, illustrating its different origin. To some extent, the spatial distribution of carbonates resembles the distribution patterns of sand, but the two variables are not correlated (R = 0.132). Most probably, high carbonate contents are associated with the presence of benthic organisms with calcareous skeletons, and their fragments, which were recorded during sampling (mainly molluscs; Nicolaidou et al. 1988).
The pool of organic matter in lagoons, estuaries and other coastal regions comprises a spectrum of dissolved, colloidal and particulate material introduced into the system from various sources, including terrestrial river borne and marine allochthonous components. These sources are mixed with autochthonous biomass derived from planktonic and benthic primary production. Additional inputs are supplied by marginal vegetation and from anthropogenic sources. The variation in the importance of these sources determines the C/N ratio within the sediments that is often used as an indicator of the origin and quality of organic matter in aquatic sediments. Marine phytoplankton has a mean molar organic C/N ratio of 6.6 (106:16; Redfield et al. 1963), seagrass tissues have a C/N ratio close to 20 (550:30; Atkinson and Smith 1983; 474:24, Duarte 1990; 23:1, Pirc and Wollenweber 1988), while terrestrial plants are relatively impoverished in N, with characteristic C/N ratios ranging from 10 to 100 for soft tissues, and from 100 to 1,000 for woody tissues (Ruttenberg and Goni 1997 and references therein).
In the study area, C/N ratios ranged between 8.6 and 13.4 (Table 1) and were closer to the ratio of marine phytoplankton than of terrestrial plants, suggesting that the contribution of terrestrial organic matter was minor in the lagoons. This is not surprising since the Messolonghi and Kleisova lagoons are not regularly flushed by fresh water, and therefore marine and autochthonous organic matter (in situ plankton, macrophytes) dominate the organic matter inputs to the sediments. However, the C/N ratios were rather higher than the theoretical marine plankton ratio, suggesting either a small relative contribution of marine and/or terrestrial plants, either the increased presence of detrital organic matter. In particular, sediments from stations MS-2, MS-11, and TL, although different in terms of texture and geochemistry, exhibit the highest C/N ratios. The low organic carbon and nitrogen content of the surficial sediments of stations MS-2 and MS-11 imply that there is not any significant recent supply of organic matter, whereas the enriched in organic matter sediments of the TL station reflect the more eutrophic conditions of the Kleisova lagoon (Hotos and Avramidou 1997). Furthermore, the elevated C/N ratios of the three aforementioned sites suggest that the decomposed organic matter has lost more nitrogen in relation to carbon. Proteins, and their constituent amino acids, account for the majority of N in primary producers. In most sedimentary environments, proteins are considered more labile (e.g., Cowie and Hedges 1992) than other forms of organic carbon. This diagenetic liability generally results in higher C/N ratios in sediments relative to the source organisms (e.g., Cowie and Hedges 1992).
Geochemistry of major and minor elements
The contents of major, minor elements and LOI in surface sediments from the Messolonghi lagoon are presented in Table 2. In order to study major and minor element interrelationships and their association with grain-size variations, organic carbon, total nitrogen, carbonate content, and total sulfur, principal factor analysis was used to identify factors with common physicochemical characteristics. A model of four factors accounts for 89.5% of the total variance and the final communalities of all variables are >0.826 (Table 3). Factor 1 (54.6%) can be clearly identified as the ‘terrigenous aluminosilicates’ factor, showing high loadings for Al, Ti, Fe, K, V, Cr, Co, Ni, Cu, Zn, Rb, and Ba (Table 3; Fig. 4). It is notable that most of the heavy metals are mainly represented in this factor, identifying their primarily detrital origin. Aluminosilicates are mostly in the silt fraction, which shows positive loading in Factor 1. Silicon is also positively loaded in Factor 1, and to a lesser extent Mn, and other elements. Factor 1 is negatively loaded in Ca, and the sand fraction, implying its antipathetic relation to autochthonous biogenic carbonates, which are coarse-grained (Fig. 4). Organic carbon is markedly loaded in Factor 2 (20.6%), followed by total N and S, Na, Mg, Mo, Pb, and the clay fraction. The ‘organic matter’ factor represents the organic rich (C and N) clayey sediments, which scavenge effectively some metals, eventually in the form of sulfides (Calvert and Pedersen 1993; Horowitz 1991). It is possible that during organic matter degradation and the microbial utilization of oxygen, episodic reducing conditions may appear (Calvert and Pedersen 1993; Hotos and Avramidou 1997; Papatheodorou et al. 2002), thus explaining the presence of Stot, Mo, and Pb in Factor 2. Positively loaded Na and Mg could be related to sea salts, since samples were unwashed prior to analysis. Alternatively, magnesium being the metallic part of chlorophyll can be found in association with phytoplankton and macrophytes debris, which contribute to the organic matter content of the sediments. The Mg released through the chlorophyll a degradation during cellular senescence and death (Louda et al. 1998) could replenish some of the Mg content of the sediment. Factor 2 is characterized by high negative loadings for sand, the carbonate content, Ca, and Sr, which constitute the ‘biogenic’ group (Table 3; Fig. 4), representing shells of calcareous organisms and their fragments. The latter elements are typically associated, as Sr often substitutes for Ca due to similar ionic radii (215 and 197 pm, respectively). Factor 3 (8.8%) is another bipolar factor showing similarities with Factor 1 (positive loadings for Si, Al, Ti, Fe, K, Na, P, Cr, Co, Zn, Pb, and silt), indicating again the influence of terrigenous aluminosilicates. However, high positive loading for Si, probably represents here the presence of terrigenous quartz (Table 3). Negative loadings are recorded for the biogenic group of elements (Ca, Sr, carbonate content), apparently due to the allochthonous versus autochthonous nature of quartz–aluminosilicates and biogenic carbonates. Factor 4 (5.5%) is positively loaded in Mn, P, and to a lesser extent in the metals Pb, Zn, Ni, Co, and Fe (Table 3). This is the ‘Mn-oxides’ group representing another common geochemical process of metal scavenging (Calvert and Pedersen 1993; Papatheodorou et al. 2002). The presence of P in Factor 1 and Factor 4 shows that phosphorous is partly of terrigenous origin, whereas another part is related to Mn oxyhydroxides (Daesslé et al. 2004).
Enrichment factors
Enrichment factor values of seven selected heavy metals are illustrated in Fig. 5. According to the EFs, Cr and Cu exhibit negative values, thus they are not enriched in the surface sediments of the Messolonghi lagoon. EFs for elements Co, Fe, Mn, Ni, and Zn, exhibit values slightly over unity, and are not considered as potential contaminants. Only V (1.6) and Pb (2.0) show EF values that could be related to contamination. The highest EF for vanadium appears at station MS-4 and secondly at Kleisova lagoon. The absence of any continuity in space of those values, which by ever means are merely above 1, suggest that vanadium is not related to contamination. Lead exhibits maximum EF value (1.96) at TL station, in Kleisova lagoon. Papatheodorou et al. (2002) documented that greater weak acid-extractable proportions of Pb, and Cu were recorded near the city of Messolonghi, and should be attributed to anthropogenic activities. Nevertheless, the latter samples were collected very near the northern coast of Kleisova lagoon, close to the city and the sewage treatment plant. In conclusion, relatively higher EFs for Pb in the Messolonghi lagoon, and particularly in the Kleisova lagoon, may be attributed to human activities, whereas for Cu, present data do not justify similar sources. Lead from gasoline, municipal waste incineration and exterior paints could be associated with lead emissions (Mahler et al. 2006).
Degree of contamination
In the present study, the modified Håkanson formula was used to calculate the contamination factors (C f) and the modified degree of contamination (mC d) for eight selected heavy metals (V, Cr, Mn, Co, Ni, Cu, Zn, and Pb). The results of for the Messolonghi lagoon are presented for comparison together with C f’s and mC d’s from the lagoons Rodia, Tsoukalio, Logarou, and Tsopeli, in the Amvrakikos Gulf, NW Greece (Karageorgis 2007), as well as the Koumoundourou Lake near Athens, the latter communicating with the sea (Karageorgis et al. 2009) (Table 4).
For the Messolonghi lagoon, the contamination factors are lower than 1.5, thus classifying the area to the level ‘Nil to very low degree of contamination’. Only Pb reaches the upper limit of this class (1.5), therefore in agreement with the results obtained by the enrichment factors contamination assessment method, which indicated a slight enrichment of the surface sediments in Pb. The overall assessment described by the modified degree of contamination mC d, indicates that the study area is not contaminated by the eight heavy metals considered here. Likewise, the four lagoons of the Amvrakikos Gulf complex, exhibit low mC d’s (Table 4); and they are classified at the ‘Nil to very low degree of contamination’ level. Karageorgis (2007) proposed that elevated contents of the selected heavy metal levels (e.g., Cr, Ni, Zn) are attributed to natural weathering of metal-bearing ultra-basic rocks. The factors for the Koumoundourou Lake are elevated, and this study site is classified at the ‘Moderate degree of contamination’ level. According to Karageorgis et al. (2009) the lakes’ sediments are contaminated by heavy metals derived from various human activities.
In order to perform a comparison with another European lagoon greatly influenced by human activities, we used the data of Bernardello et al. (2006) for heavy metals and metalloids (Cr, Co, Ni, Cu, Zn, As, Cd, Hg, and Pb, determined in 25 surface sediments) and background data for the same elements obtained by Pavoni et al. (1987), to calculate the modified degree of contamination for the years 1987, 1993, and 1997. The results have shown mC d’s 2.2, 2.0, and 2.1, for the 3 years, respectively, thus classifying Venice lagoon in the ‘Moderate degree of contamination’ level. In Venice lagoon, it appears that the major contaminant is mercury (contamination factors 10, 9, 8 for the years 1987, 1993, and 1997, respectively) originating from a chloralkali plant in Porto Marghera using Hg cathodes since the 1950s (Bernardello et al. 2006). However, the overall mC d’s are relatively low, because the stations’ network spans the entire lagoon, whereas severe contamination is reported mainly in the industrial zone and the city of Venice (Basu and Molinaroli 1994; Bellucci et al. 2002; Donazzolo et al. 1984; Pavoni et al. 1987).
The comparisons demonstrate that the method introduced by Håkanson (1980) and modified by Abrahim and Parker (2008), is a reliable method for heavy metal contamination assessment, if local pre-industrial background (baseline) is available for the heavy metals under investigation. However, it may be noted that baseline values should be selected carefully, taking into consideration the texture of the analyzed sediments. Since no normalization step is used (e.g., element ratios to Al or other conservative element), analyzed surface sediments and baseline sediment should have similar grain-size characteristics. For example, in the case of the Amvrakikos Gulf lagoons, contamination factors appeared much higher, when pre-industrial sediment of different texture was used as baseline.
Conclusions
New data on the geochemistry of organic carbon, total nitrogen, and total sulfur, major and minor elements are reported for the Messolonghi lagoon complex. Measured variables are associated mainly with terrigenous aluminosilicates, organic matter, and calcareous organisms. Heavy metal contamination was assessed by two methods: (1) the enrichment factors, and (2) the modified degree of contamination. Both methods show that the Messolonghi lagoon is largely unaffected by human activities, with a reservation regarding Pb, which appears to be slightly elevated, probably due to the legacy of leaded gasoline, which was banned for European Union countries in 1989. In agreement with Papatheodorou et al. (2002), the Kleisova lagoon appears to be more vulnerable to anthropogenic pressure, when compared to the central Messolonghi lagoon. However, the overall degree of contamination is the lowest in the Håkanson’s scale, suggesting that the lagoon is practically in almost pre-industrial conditions. Given that the samples analyzed were recovered in 1999, geochemical results may be considered as baseline data, which may be used for comparison in future monitoring of the Messolonghi lagoon complex, in line with the Water Framework Directive of the European Union.
References
Abrahim GMS (2005) Holocene sediments of Tamaki Estuary: characterisation and impact of recent human activity on an urban estuary in Auckland, New Zealand. PhD thesis, University of Auckland, Auckland, New Zealand, p 361
Abrahim GMS, Parker RJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediments from Tamaki Estuary, Auckland, New Zealand. Environmental Monitoring Assessment. doi:10.1007/s10661-007-9678-2
Acquavita A, Predonzani S, Mattassi G, Rossin P, Tamberlich F, Falomo J, Valic I. (2010) Heavy metal contents and distribution in coastal sediments of the Gulf of Trieste (Northern Adriatic Sea, Italy). Water Air Soil Pollut. doi:10.1007/s11270-009-0284-5
Anagnostou Ch, Karageorgis A, Hatiris G, Drakopoulou P (2009) The deltaic-lagoonal systems of Acheloos and Evinos Rivers (Western Greece): evolution and changes related to anthropogenic activities and sea-level rise. Abstracts, Fourth European Conference on Coastal Lagoon Research, December 14–18, 2009, Montpellier, France, p 70
Anonymous (2001) Study of management of fishery exploitation of Greek lagoons. Project PESCA. Ministry of Agriculture of Greece, Direction of Aquaculture (in Greek)
Atkinson MJ, Smith SV (1983) C:N:P ratios of benthic marine plants. Limnol Oceanogr 28:568–574
Basset A, Sabetta L, Fonnesu A, Mouillot D, Do Chi T, Viaroli P, Giordani G, Reizopoulou S, Abbiati M, Carrada GC (2006) Typology in Mediterranean transitional waters: new challenges and perspectives. Aquat Conserv Mar Freshw Ecosyst 16:441–455
Basu A, Molinaroli E (1994) Toxic metals in Venice lagoon sediments: model, observations, and possible removal. Environ Geol 24:203–216
Bellucci LG, Frignani M, Paolucci D, Ravanelli M (2002) Distribution of heavy metals in sediments of the Venice Lagoon: the role of the industrial area. Sci Total Environ 295:35–49
Bellucci LG, Giuliani S, Mugnai C, Frignani M, Paolucci D, Albertazzi S, Ruiz Fernandez AC (2010) Anthropogenic metal delivery in sediments of Porto Marghera and Venice Lagoon (Italy). Soil Sediment Contam Int J. doi:10.1080/15320380903390562
Beltrame MO, De Marco SG, Marcovecchio JE (2009) Dissolved and particulate heavy metals distribution in coastal lagoons. A case study from Mar Chiquita Lagoon, Argentina. Estuar Coast Shelf Sci 85:45–56
Bernardello M, Secco T, Pellizzato F, Chinellato F, Sfriso A, Pavoni B (2006) The changing state of contamination in the Lagoon of Venice. Part 2: Heavy metals. Chemosphere 64(8):1334–1345
Brügmann L (1995) Metals in sediments and suspended matter of the river Elbe. Sci Total Environ 159:53–65
Calvert SE, Pedersen TF (1993) Geochemistry of recent oxic and anoxic marine sediments: implications for the geological record. Mar Geol 113:67–88
Cowie GL, Hedges JI (1992) Sources and reactivities of amino acids in a coastal marine environment. Limnol Oceanogr 37:703–729
Cutter GA, Radford-Knoery J (1991) Determination of carbon, nitrogen, sulfur and inorganic sulfur species in marine particles. In: Hurd DC, Spencer DW (eds) Marine particles: analysis and characterization American Geophysical Union, Geophysical Monograph Series 63, pp 57–63
Daesslé LW, Camacho-Ibar VF, Carriquiry JD, Ortiz-Hernández MC (2004) The geochemistry and sources of metals and phosphorus in the recent sediments from the Northern Gulf of California. Cont Shelf Res 24:2093–2106
Dassenakis M, Krasakopoulou E, Matzara B (1994) Chemical characteristics of Aetoliko Lagoon, Greece, after an ecological shock. Mar Pollut Bull 28:427–433
Dimitriou E (2007) Contribution to the study of growth and behaviour of the sea bream (Sparus aurata L.) in the Messolonghi–Etoliko lagoons. PhD thesis, University of Patras, 207 p (in Greek with English abstract)
Dimitriou E, Rogdakis Y, Leonardos J, Athanasopoulos A (1994) The quantitative and qualitative composition of fishes catch of Messolonghi–Aetoliko lagoon as index to fishing management. Fish News Int 155:82–91 (in Greek)
Donazzolo R, Orio AA, Pavoni B, Perin G (1984) Heavy metals in sediments of the Venice lagoon. Oceanol Acta 7:25–32
Duarte CM (1990) Seagrass nutrient content. Mar Ecol Prog Ser 67:201–207
Fang TH, Li JY, Feng HM, Chen HY (2009) Distribution and contamination of trace metals in surface sediments of the East China Sea. Mar Environ Res 68:178–187
Folk RL (1974) Petrology of sedimentary rocks. Hemphil, Austin
Förstner U, Wittmann GTW (1979) Metal pollution in the aquatic environment. Springer, New York
González I, Águila E, Galán E (2007) Partitioning, bioavailability and origin of heavy metals from the Nador lagoon sediments (Morocco) as a basis for their management. Environ Geol 52:1581–1593
Håkanson L (1980) Ecological risk index for aquatic pollution control, a sedimentological approach. Water Res 14:975–1001
Horowitz AJ (1991) A primer on sediment—trace element chemistry. Lewis Publishers Ltd., Chelsea
Hotos GN, Avramidou DN (1997) A one year water monitoring study of Klisova lagoon (Mesolonghi, W. Greece). GeoJournal 41:15–23
Huang W, Campredon R, Abrao JJ, Bernat M, Latouche C (1994) Variation of heavy metals in recent sediments from Piratininga lagoon (Brazil): interpretation of geochemical data with the aid of multivariate analysis. Environ Geol 23:241–247
Karageorgis AP (2007) Geochemical study of sediments from the Amvrakikos Gulf lagoon complex, Greece. Trans Waters Bull. doi:10.1285/i1825229Xv1n3p3
Karageorgis AP, Anagnostou CL, Kaberi H (2005) Geochemistry and mineralogy of the NW Aegean Sea surface sediments: implications for river runoff and anthropogenic impact. Appl Geochem. doi:10.1016/j.apgeochem.2004.07.008
Karageorgis AP, Katsanevakis S, Kaberi H (2009) Use of enrichment factors for the assessment of heavy metal contamination in the sediments of Koumoundourou Lake, Greece. Water Air Soil Pollut. doi:10.1007/s11270-009-0041-9
Katselis G, Koukou K, Dimitriou E, Koutsikopoulos C (2007) Short-term seaward fish migration in the Messolonghi–Etoliko lagoons (Western Greek coast) in relation to climatic variables and the lunar cycle. Estuar Coastal Shelf Sci. doi:10.1016/j.ecss.2007.02.010
Louda JW, Li J, Liu L, Winfree MN, Baker EW (1998) Chlorophyll-a degradation during cellular senescence and death. Org Geochem 29:1233–1251
Mahler BJ, Van Metre PC, Callender E (2006) Trends in metals in urban and reference lake sediments across the United States, 1970 to 2001. Environ Toxicol Chem. doi:10.1897/05-459R.1
Mariolakos I, Mariolakos D, Fountoulis I, Tziovara A, Champilomati A, Anagnostou Ch, Sakellariou D (2004) Shallow sampling drillings in the Acheloos delta area: preliminary results and radiocarbon dating. Abstracts, 10th International Congress of the Geological Society of Greece, 15–17 April, Thessaloniki, Greece, 496–497
Nicolaidou A, Bourgoutzani F, Zenetos A, Guelorget O, Perthuisot JP (1988) Distribution of molluscs and polychaetes in coastal lagoons in Greece. Estuar Coast Shelf Sci 26:337–350
Nicolaidou A, Reizopoulou S, Koutsoubas D, Orfanidis S, Kevrekidis T (2005) Biological components of Greek lagoonal ecosystems: an overview. Mediterranean Mar Sci 6:31–50
Nieuwenhuize J, Maas YEM, Middelburg JJ (1994) Rapid analysis of organic carbon and nitrogen in particulate materials. Mar Chem 45:217–224
Nikolaidis NP, Dobbs GM, Chen J, Lackovic JA (2004) Arsenic mobility in contaminated lake sediments. Environ Pollut 129:479–487
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water, and soils by trace metals. Nature 33:134–139
Papatheodorou G, Hotos G, Geraga M, Avramidou D, Vorinakis T (2002) Heavy metal concentrations in sediments of Klisova lagoon (southeast Mesolonghi–Aetolikon lagoon complex), W. Greece. Fresenius Environ Bull 11:951–956
Pavoni B, Donazzolo R, Marcomini A, Degobbis D, Orio AA (1987) Historical development of the Venice lagoon contamination as recorded in radiodated sediment cores. Mar Pollut Bull 18:18–24
Pirc H, Wollenweber B (1988) Seasonal changes in nitrogen, free amino acids, and C/N ratio in Mediterranean seagrasses. PSZNI Mar Ecol 9:167–179
Redfield AC, Ketchum BH, Richards FA (1963) The influence of organisms on the composition of sea water. In: Hill MN (ed) The sea, ideas and observations on progress in the study of the seas. Interscience, New York, pp 26–77
Ruttenberg KC, Goni MA (1997) Phosphorus distribution, C:N:P ratios, and δ13Coc in arctic, temperate, and tropical coastal sediments: tools for characterizing bulk sedimentary organic matter. Mar Geol 139:123–145
Salomons W, Förstner U (1984) Metals in the hydrocycle. Springer, New York
Sanei H, Goodarzi F, Van Der Flier-Keller E (2001) Historical variation of elements with respect to different geochemical fractions in recent sediments from Pigeon Lake, Alberta, Canada. J Environ Monitor. doi:10.1039/b006819p
Scherer U, Fuchs S, Behrendt H, Hillenbrand T (2003) Emissions of heavy metals into river basins of Germany. Water Sci Technol 47:251–257
Siegel FR, Slaboda ML, Stanley DJ (1994) Metal pollution loading, Manzalah lagoon, Nile Delta, Egypt: implications for aquaculture. Environ Geol 23:89–98
Tsimplis MN (1994) Tidal oscillations in the Aegean and Ionian seas. Estuar Coast Shelf Sci 39:201–208
Tsimplis MN, Blackman D (1997) Extreme sea-level distribution and return periods in the Aegean and Ionian Seas. Estuar Coast Shelf Sci 44:79–89
Tziovara A, Champilomati A (2002) Sedimentology of deltaic deposits of Acheloos and its contribution to the understanding of the recent evolution of the deltaic system. BSc thesis, University of Athens, 90 p + Annexes (unpublished) (in Greek)
Verardo DJ, Froelich PN, McIntyre A (1990) Determinations of organic carbon and nitrogen in marine sediments using the Carlo Erba NA-1500 Analyzer. Deep Sea Res 37:157–165
Voutsinou-Taliadouri F, Satsmadjis J, Iatridis B (1987) Granulometric and metal composition in sediments from a group of Ionian lagoons. Mar Pollut Bull 18:49–52
Acknowledgments
The help of M. Taxiarchi and A. Papageorgiou in laboratory analysis is appreciated. Constructive suggestions of an anonymous reviewer are gratefully acknowledged. Also, we wish to thank J. Nystuen for improving language flow.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karageorgis, A.P., Sioulas, A., Krasakopoulou, E. et al. Geochemistry of surface sediments and heavy metal contamination assessment: Messolonghi lagoon complex, Greece. Environ Earth Sci 65, 1619–1629 (2012). https://doi.org/10.1007/s12665-011-1136-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-011-1136-3