Abstract
The aim of this study was to evaluate the use of metal concentrations in clam organs to monitor metal contamination in coastal sediments. The concentrations of Cd, Cr, Cu, Hg, Ni, Pb, V, and Zn were measured in the kidneys, gonads, mantles, gills, digestive gland, and hearts of the infaunal clam Amiantis umbonella collected from a contaminated site near desalination and power plant discharges, and a reference site in Kuwait Bay. Metal concentrations in sediment and sediment pore water were also measured at the collection sites of individual clams at the contaminated site. The concentrations of all metals in all organs (except Zn in the digestive gland) were significantly higher in clams from the contaminated site than from the reference site. Metal concentrations in several organs in A. umbonella from the contaminated site were correlated with those in the sediments and pore waters to which they were exposed. However, fresh weights of gonads, gills, and mantles were significantly lower in clams from the contaminated site compared to the reference site, indicating that the observed elevated concentrations of metals in the organs of clams from the contaminated site largely reflect lower organ weights, rather than higher metal loads, and that these organs in A. umbonella and perhaps other clams are not appropriate for use as biomonitors of metal contamination. Metal concentrations in clam kidneys showed a wide dynamic range with respect to environmental contamination and kidney weight was not variable. Therefore, metal concentrations in clam kidneys provide a reliable biomonitor of contaminant metals in coastal marine sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
As part of a comprehensive strategy to assess the effects and trends of marine pollution, biomonitor organisms appropriate for a wide range of geographic regions are needed (Rainbow and Phillips 1993). Marine bivalves have been recognized as useful sentinel organisms of contamination in aquatic ecosystems because of their abundance, wide geographical distribution, sedentary habit, modes of feeding, their relative sensitivity to contaminated sediments and water (Chung et al. 2007), and their capacity to accumulate contaminants in proportion to environmental levels (Goldberg et al. 1983; Phillips and Rainbow 1995; O’Connor 2004).
The accumulation of metals in specific organs of marine bivalves has been proposed as a more sensitive and specific indicator of environmental contamination than whole body burdens (Ahn et al. 2001; Yap et al. 2006; Kavun and Podgurskaya 2009). The concentrations of metals in specific organs may be more responsive to environmental concentrations than whole body burdens (Ahn et al. 1996; Nigro et al. 1997) and are linked to specific pathways of accumulation and elimination (Baudrimont et al. 1997; Podgurskaya et al. 2004). However, the utility of metal concentrations in specific organs as biomarkers of metal pollution is confounded by several biological factors including variable weights of specific organs in contaminated and uncontaminated areas (Dragun et al. 2010) and reproductive status (Nørum et al. 2005). The response of metal concentrations in specific organs of marine bivalves to environmental exposures and the effects of organ size on the accumulation of metals in bivalve organs have only been examined in a few cases and rarely in native populations.
The goal of this study was to evaluate the sensitivity of metal accumulation in different organs of the marine clam Amiantis umbonella from populations living in point source contaminated and uncontaminated environments in Kuwait Bay and their utility as biomonitors of environmental contamination. The objectives were to (1) compare metal concentrations in different organs of clam A. umbonella from a point source impacted site and a reference site and (2) examine the relationships between metal concentrations in specific organs in clams from the contaminated site and metal concentrations in the sediment and pore water to which the clams were exposed. To address these objectives, the concentrations of eight metals (Hg, Cd, Cu, Ni, Pb, Cr, V, and Zn) were measured in seven organs of A. umbonella collected from a contaminated site near desalination and power plant discharges and from a reference site 5 km away. It was hypothesized that the concentrations of metals in specific clam organs reflect environmental contamination, but that the decrease in the mass of certain organs associated with growth in a contaminated environment confounds the interpretation of metal bioaccumulation in those organs.
Most of the urban, commercial, industrial, and recreational activities in Kuwait are concentrated within 15 km of its 400-km shoreline along the Arabian Gulf. Furthermore, Kuwait’s coast is virtually the only source of fresh water and energy in the country; several desalination/power plants were established along the shoreline to meet the country’s need for drinking water and electricity (Bu-Olayan and Thomas 2006). The seawater used for cooling the power plants is also discharged to the sea, which may increase temperature and salinity of the coastal water (Al-Bakri and Kittaneh 1998). The coastal area is considered a valuable natural resource containing an important ecosystem and supporting many organisms vital to the health of the Arabian Gulf. For example, the coastal zone is a very important nesting and feeding ground for many resident and migratory birds (Al-Bakri and Kittaneh 1998). The urbanization and industrialization of Kuwait’s coast raise concerns about increased pollution of coastal waters (Bu-Olayan et al. 2008) and human exposure to metals through the ingestion of sea food (Bu-Olayan and Al-Yakoob 1998). As a result, there is an ongoing need to develop biomonitors of metal contamination in the Arabian Gulf to support ecological health and risk assessment (Alyahya et al. 2011).
2 Materials and Methods
2.1 Clam Collection and Dissection
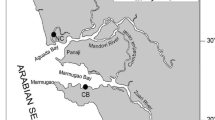
Individual clams of the facultative suspension feeder A. umbonella (2 to 4.5 cm shell length) were randomly collected at low tide from the sand/mud sediments of the intertidal zone of Kuwait Bay near Al Doha, Kuwait (Fig. 1). Clams were collected manually from a contaminated site near desalination and power plant discharges (29.378 N, 47.822 E) and a reference site 5 km away (29.404 N, 47.752 E) during December 1999 and January 2000. Clams from both sites were of similar shell length (see Fig. 3) to minimize effects of body weight (Marina and Enzo 1983). Most clams were partially exposed on the surface, while others were up to 10 cm below the surface. Clams were transported to the laboratory in aerated self-supporting tanks (150 L capacity) with recirculating seawater at 24–25°C. Clams were depurated in seawater for approximately 24 h. Seawater salinity and temperature were measured during each sampling.
All collected clams (33 from each site) were dissected. Clams were opened carefully using stainless steel scalpel blades, and soft organs including gonads, digestive gland, gills, mantles, foot, hearts, and kidneys were removed. The fresh weight of each organ was recorded prior to combining in composite samples. Organs from each group of 33 clams were combined to form composite samples of each organ from the contaminated and reference sites, transferred to separate glass vials, and stored at −20°C prior to trace metal analysis by ICP or AA (inductivity coupled plasma atomic emission spectrometer; result in Table 2). To investigate the variability of metal concentrations, a complete set of metal concentrations in all organs was produced for 17 clams individually from the contaminated site. All 17 clams were analyzed individually rather than as composites (Table 3). In addition, sediment and pore water samples associated with each of these individually analyzed clams were collected (see below). Organ samples were extracted by heating at 120°C in concentrated HNO3 until dry and were then redissolved in 0.1% HCl prior to analysis.
2.2 Water and Sediment Collection
Sediment pore water samples were collected in acid-cleaned plastic bottles except for mercury which was collected in glass bottles at low tide from standing pools on the surface of the sediment where each individual clam was collected. Sediment samples were collected in 75-ml plastic bottles by hand from the shoreline at low tide adjacent to the clam collection sites in Kuwait Bay. Sediments were air dried and stored frozen. Prior to analysis, sediment samples were freeze dried until the change in weight was less than 0.5%. Then, the sediment samples were homogenized by grinding and passed through a 2-mm sieve. Processed sediment samples were kept in acid-cleaned glass bottles prior to digestion. Seawater samples were filtered with a 0.45-μm membrane filter and acidified to a pH of between 5.0 and 5.5 by the addition of 2 N HNO3.
2.3 Metal Analysis
A weight of 0.5 g of the sediment powder was transferred to a Teflon beaker that was cleaned with 20% of HNO3. Five milliliters of concentrated HNO3/HClO4/HF in a ratio of 3:2:1 was added to the sample and evaporated to near dryness while covering the samples continuously for 3 h. The sample was passed through a filter paper and transferred to a 50-ml volumetric flask, and the volume was made up with 0.1% HCl. Three standard reference materials were digested to validate the analytical procedure, along with three blanks to set up the system baseline. The final sample was ready for elemental analysis by an inductively coupled plasma atomic emission spectrometer. Accuracy of the method was verified (ten replicates) by analyzing standard reference material oyster tissues (SRM-1566a) from the National Bureau of Standards. Recoveries were above 90% for all the trace metals measured (Table 1).
The acidified filtered seawater samples were passed through an ion exchange column at a rate of 2 ml min−1. The concentrations of dissolved trace metals in water samples were analyzed by the ion exchange column technique (Bruland et al. 1985). This technique employs Chelex-100 (sodium form, 100–200 mesh), pre-cleaned with 4 N HCl and 2.5 N HNO3, rinsed with distilled water, and converted to ammonia form prior to loading in a column. Samples were adjusted to pH 6 with ammonium acetate and were drawn through a column containing 7.5 ml of Chelex-100 resin (flow rate, 1–2 ml min−1). The column was then rinsed with 20 ml ultrapure ammonium acetate (pH 5.8) and 20 ml distilled water to rinse the bulk of the alkali and alkaline earth metals. The trace metals were eluted with 20 ml of Aristar 2 N HNO3, providing a concentration factor of 100:1. In spiked seawater, the percentage recovery averaged 92% for Hg, 108% for Cu and Cd, 88% for V, 95% for Pb, 93% for Cr, and 94% for Zn.
2.4 Statistical Analysis
T tests were used to evaluate differences in mean metal concentrations in clam organs from the contaminated and reference sites. Linear regression analysis and a coefficient of determination were used to assess correlations between clam length and weight and metal concentrations in specific organs and water or sediment. At the same time, ANOVA (F test) was used to determine the significance of the regression coefficient (R 2).
3 Results
3.1 Metal Concentrations in the Organs of A. umbonella from Kuwait Bay
In the composite samples, mean concentrations of all metals were significantly higher (p < 0.02) in the organs of A. umbonella clams collected from the contaminated site in Kuwait Bay compared with clams from the reference site with the exception of Zn in the digestive gland (Table 2). For example, in the kidneys of A. umbonella, Hg concentrations were about ten times higher, and Cd, Cu, Pb, Ni, and V concentrations were three to five times higher in clams from the contaminated site than in clams from the reference site. Similarly, the concentrations of Hg, Cd, and Cr in gills and mantles of A. umbonella were four to nine times higher in clams from the contaminated than reference site. In all tissues of A. umbonella except the digestive gland, Zn concentrations were significantly higher (p < 0.02) in clams from the contaminated site than from the reference (Table 2). The highest increase in Zn concentration was in the clam gills (1.3-fold higher), and the smallest increases were in the mantles, kidney, and heart (1.1-fold higher).
In clams from both sites, mean concentrations of Cd, Pb, V, and Zn were higher (p < 0.05) in the kidneys than in any other organs including digestive gland, mantles, or gonads, and within clam kidneys, concentrations of Cd, Pb, and V were higher than all other metals except Zn. In both the composite and individually analyzed clams, the accumulation of Cd and Pb almost exclusively in the kidneys of clams from the contaminated site contrasts with the fairly even distribution of Cu and Hg among the clam mantles, digestive gland, kidneys, and gonads (Tables 2 and 3).
3.2 Wet Weights of Clam Organs
For the clams that were used for the composite metal analysis, the mean wet weights of digestive gland, kidney, foot, and heart from A. umbonella from Kuwait Bay were not significantly different in clams from the contaminated site and the reference site (Fig. 2a). However, the mean wet weights of gonads, mantles, and gills were significantly lower in clams collected from the contaminated site compared to the reference site. In clams from the contaminated site, the mean wet weights of mantles and gills were 40% lower and wet weights of gonads were 80% lower than in clams from the reference site (Fig. 2b). Gonad wet weight for clams prior to composite analysis was positively correlated (R 2 = 0.70, p < 0.01) with length in clams from the reference site (Fig. 3a), whereas gonad wet weight was weakly, negatively correlated with length (R 2 = 0.36, p < 0.01) in clams from the contaminated site (Fig. 3b).
3.3 Metal Correlations Between Clam Organs and Their Environment
Within the set of individually analyzed A. umbonella clams collected from the contaminated site in Kuwait Bay, several correlations were observed between metal concentrations in clam organs and those in the sediment and sediment pore water to which individual clams were exposed (Table 4). For example, the concentration of Hg in clam gonads (Fig. 4a; R 2 = 0.53, p < 0.01) and the concentrations of Cd, Cu, and Pb in clam kidneys (Fig. 4b; R 2 = 0.49, p < 0.01, Fig. 4c; R 2 = 0.51, p < 0.01, Fig. 4d, R 2 = 0.52, p < 0.01, respectively) were correlated with the concentrations of these metals in the sediment in which the animals were living. Positive correlations were also found between metal concentrations in clam organs and sediment pore water for Hg in clam gonads (Fig. 5a; R 2 = 0.63, p < 0.01), Hg in gills (Fig. 5b; R 2 = 0.72, p < 0.05), Cd in clam mantles (Fig. 5c; R 2 = 0.65, p < 0.01), and Cu in clam gonads (Fig. 5d; R 2 = 0.53, p < 0.01).
Relationships between metal concentrations in water and specific organs of the clam A. umbonella from the contaminated site in Kuwait Bay. Data are for Hg in kidney (a), Hg in gills (b), Cd in mantles (c), and Cu in gonads (d). Hg concentrations in water are in parts per trillion (nanograms per liter)
4 Discussion
The distribution of metals among bivalve organs reflects the integrated bioavailability of metals in pristine or contaminated aquatic environments and their biological redistribution within the organisms. Metal distributions among bivalve organs have rarely been measured in native freshwater (Tessier et al. 1984) or marine (Okazaki and Panietz 1981; Ahn et al. 2001; Kavun and Podgurskaya 2009) populations and may differ from those in laboratory-exposed animals (Giguere et al. 2003). In the present study, A. umbonella clams from both the contaminated and reference sites in Kuwait Bay accumulated most of their body burdens of Cd, Pb, V, and Zn in the kidneys, while Hg, Cu, and Ni were evenly distributed among organs and Cr was concentrated in the foot and mantles. High absolute and relative levels of metal accumulation in bivalve kidneys were previously observed in the Antarctic clam Laternula elliptica (Ahn et al. 1996) and in Pacific mussels (Podgurskaya and Kavun 2005; 2006), but in the oysters Crassostrea gigas and Crassostrea virginica, metal concentrations in the kidneys were similar to those in other organs in oysters from a contaminated site or lower than those in other organs in oysters from an uncontaminated site (Okazaki and Panietz 1981).
All metals accumulated to higher concentrations in the gills and mantles of A. umbonella from the contaminated site in Kuwait Bay than in clams from the reference site, but most metals did not accumulate to the greatest extent in these organs overall. Indeed, in A. umbonella from the contaminated site, internal organs including digestive gland, kidneys, and gonads accumulated the highest concentrations of Hg, Cd, Cu, Pb, Ni, and V consistent with previous findings (Okazaki and Panietz 1981; Podgurskaya and Kavun 2005).
The accumulation of metals in specific organs of bivalves is a consequence of specific pathways of uptake, transport, storage, and excretion (Simkiss and Mason 1983; Phillips and Rainbow 1989; Fisher et al. 1996). For example, the accumulation of metals in gills and mantles indicates uptake of metals from the dissolved phase (Bebianno et al. 1993; Reinfelder et al. 1998, 1997; Cooper et al. 2010), especially for metals like Cd which are primarily present in marine waters in dissolved form (Balls 1985). Positive correlations between Cd and Cr concentrations in A. umbonella gills and mantles and in sediment pore water indicate primarily dissolved accumulation of these metals in these organs. In contrast, strong correlations between the concentrations of Cd, Pb, Cu, Cr, and V in A. umbonella kidneys and sediment indicate trophic accumulation. A. umbonella is an infaunal “interface” feeder that can change its mode of feeding from deposit to suspension feeding in response to stress, poor quantity of suspended particulate matter, or flow regime (Taghon et al. 1980; Dauer et al. 1981) and may therefore accumulate metals from either ingested sediments or suspended particles in the overlying water (King et al. 2010).
The observation of higher concentrations of most metals in the organs of A. umbonella clams collected from the contaminated site in Kuwait Bay than from the reference site is consistent with previous studies in which metal concentrations in bivalve organs were compared in native populations from contaminated and uncontaminated sites (Okazaki and Panietz 1981; Podgurskaya and Kavun 2005). The small range of Zn concentrations in the organs of A. umbonella from the contaminated and reference sites is consistent with measurements of Zn in whole soft tissues of other bivalves (Klumpp and Burdon-Jones 1982; Amiard-Triquet et al. 1986), indicating that Zn levels in bivalves do not vary considerably between polluted and unpolluted areas as a result of biological regulation.
Significant positive correlations between the concentrations of metals in the organs of A. umbonella from the contaminated site in Kuwait Bay and those in the environment (Table 4, Figs. 4 and 5) indicate that metal concentrations in A. umbonella organs are sensitive to changes in the concentrations of metals in the sediments and sediment pore waters to which these clams are exposed. Among all the organs, the gills, gonads, and kidneys had metal concentrations that were most frequently correlated with the concentrations of metals in sediments and sediment pore waters. These organs are therefore the best potential candidate biomonitors of metal contamination in coastal marine environments. Unlike in other studies (Tessier et al. 1984; Ahn et al. 2001), few correlations between metal concentrations in A. umbonella digestive glands and the environment were observed.
A. umbonella accumulated Hg, Cd, Pb, Cr, and V in its gills in proportion to their concentrations in the sediment pore water to which it was exposed, indicating that gills may be a suitable biomonitor for changes in dissolved metal levels. Similar correlations between metal concentrations in the gills and environmental compartments have been observed in other mollusks (Tessier et al. 1984; Langston and Zhou 1987; Roesijadi and Robinson 1994; Odzak et al. 1994; Inza et al. 1997). Correlations between metal concentrations in bivalve gonads and environmental compartments have also been reported (Yap et al. 2006). The kidneys of the clam A. umbonella might serve as a useful biomonitor of metals in sediments since they accumulated Cd, Pb, Cu, Cr, and V in proportion to concentrations in the sediments to which the clams were exposed. Few correlations of metal concentrations in bivalve kidneys and environmental concentrations have been reported. Concentrations of Cd, Cu, Pb, and Zn were elevated in the kidneys of mussels from a contaminated bay off Russia’s Pacific coast near Vladivostok compared with mussels from uncontaminated sites (Podgurskaya and Kavun 2005). Cd levels increased in kidneys in proportion to environmental exposure in the clam Megapitaria squalida in La Paz Bay, Baja California Sur, Mexico (Escobedo-Fregoso et al. 2010).
The use of bivalves as biomonitors of environmental metal contamination requires an understanding of how metal concentrations in bivalve soft tissues vary with environmental and biological factors. For example, the use of metal concentrations in bivalve organs to assess metal contamination is confounded by several biological factors including variable organ size (Dragun et al. 2010) and reproductive status (Nørum et al. 2005). Soft tissue weight often decreases as metal concentrations increase such that there may be little to no variation in total metal load to the animal (Mouneyrac et al. 1998). In this case, relationships between metal concentrations in the animal and the environment are unclear. A similar effect would complicate the use of bivalve organs as biomonitors if organ weight decreased as metal exposures increase. In A. umbonella, fivefold lower wet weights of gonads in contaminated clams compared to clams from the reference site in Kuwait Bay could, within analytical uncertainty, account for all of the apparent increases in the concentrations of all eight metals analyzed. Similarly, the 1.7-fold lower wet weights of gills and mantles in contaminated clams could account for apparent increases of Cu, Pb, Ni, and Zn in clam mantles and of Pb, Ni, and Zn in clam gills. Although in the present case the concentrations of Hg and Cd in gills and mantles and Cu in gills increased by more than the factor of 2 decrease in gill and mantle weights, in the environment, such decreases in gill and mantle weights would complicate the relationships between metal concentration increases in these organs and environmental contamination. With respect to metal accumulation in bivalve gonads, changes in gonad weight over the reproductive cycle would have a similarly complicating effect on the interpretation of gonad metal concentrations. Indeed, reproductive status affected the concentrations of the greatest number of metals in the gonads compared with other organs in spiny and Pacific scallops (Nørum et al. 2005). Metal accumulation in the gonads, gills, and mantle of A. umbonella and perhaps other bivalves is therefore not appropriate for use as biomonitors of sediment contamination in marine environments.
Decreased organ weights in clams from contaminated environments compared with uncontaminated clams may indicate organ-specific stress on tissue development, a general toxic effect on animal growth, or stress related to poor quality or low quantity of food. In the present study, environmental contamination only affected the weights of three out of seven clam organs and did not affect clam size (shell length). Therefore, a general toxic effect on animal growth is unlikely to have caused the decreased development of gonads, gills, and mantles in contaminated clams. For the same reasons, lower food quality or quantity is also an unlikely cause of the reduction in gonad, gill, and mantle weights. Exposure to elevated levels of metals inhibited gonad development and gamete production in scallops, clams, and mussels (Gauthier-Clerc et al. 2002; Regoli et al. 2001; Siah et al. 2003). Reduced weights of gonads observed in A. umbonella from the contaminated site compared to similarly sized clams from the reference site are most likely due to the effects of elevated metal concentrations on gonad tissue development. Gill and mantle development may have been similarly affected.
Bivalves are known to accumulate, detoxify, and immobilize a diversity of metals in their kidneys (Denton and Burdon-Jones 1986; Langston et al. 1998; Ahn et al. 2001; Podgurskaya and Kavun 2005). In the present study, there were no signs of visible stress in kidney size or weight in contaminated A. umbonella clams, and the relatively high metal concentrations in A. umbonella kidneys did not affect kidney growth. Thus, kidneys provide a biological reservoir of stable mass for use as a monitor of environmental metal contamination. In addition, in A. umbonella, kidneys appear to have the best dynamic range with respect to metal concentrations among clam organs. For example, while the digestive gland was the site of the highest concentration of Cu in A. umbonella clams from both the contaminated and reference sites in Kuwait Bay, the relative increase in Cu concentration in the digestive gland of contaminated clams relative to clams from the reference site was smaller (factor of 2) than that in the kidneys (factor of 4). Our results show that the choice of bivalve organ for use as a biomonitor of environmental contamination must consider the variability of organ weight under contaminant stress and the dynamic range of metal concentrations each organ records.
References
Ahn, I.-Y., Lee, S. H., Kim, K. T., Shim, J. H., & Kim, D. Y. (1996). Baseline heavy metal concentrations in the Antarctic clam Laternula elliptica (King and Broderip) in Maxwell Bay, King George Island. Antarctica Marine Pollution Bulletin, 32, 592–598.
Ahn, I.-Y., Kang, J., & Kim, K.-W. (2001). The effect of body size on metal accumulations in the bivalve Laternula elliptica. Antarctica Science Bulletin, 13, 355–362.
Al-Bakri, D., & Kittaneh, W. (1998). Physicochemical characteristics and pollution indicators in the intertidal zone of Kuwait: implications for benthic ecology. Environmental Management, 22, 415–442.
Alyahya, H., El-Gendy, A. H., Al Farraj, S., & El-Hedeny, M. (2011). Evaluation of heavy metal pollution in the Arabian Gulf using the clam Meretrix meretrix Linnaeus, 1758. Water, Air, and Soil Pollution, 214, 499–507.
Amiard-Triquet, C., Berthet, B., & Metayer, C. (1986). Contribution to the ecotoxicological study of cadmium, lead, copper and zinc in the mussel Mytilus edulis. Marine Biology, 90, 425–431.
Balls, P. W. (1985). Copper, lead, and cadmium in coastal waters of the western North Sea. Marine Chemistry, 15, 363–378.
Baudrimont, M., Metivaud, J., Maury-Brachet, R., Ribeyre, F., & Boudou, A. (1997). Bioaccumulation and metallothionein response in the asiatic clam (Corbicula fluminea) after experimental exposure to cadmium and inorganic mercury. Environmental Toxicology and Chemistry, 16, 2096–2105.
Bebianno, M. J., Nott, J. A., & Langston, W. J. (1993). Cadmium metabolism in the clam Ruditapes decussata: the role of metallothioneins. Aquatic Toxicology, 27, 315–334.
Bruland, K. W., Coale, K., & Mart, H. L. (1985). Analysis of seawater for dissolved cadmium, copper, and lead: an intercomparison of voltammetric and atomic absorption methods. Marine Chemistry, 17, 285–300.
Bu-Olayan, A. H., & Al-Yakoob, S. (1998). Lead, nickel and vanadium in seafood: an exposure assessment for Kuwaiti consumers. Science of the Total Environment, 223, 81–86.
Bu-Olayan, & Thomas, B. V. (2006). Assessment on biocides bioaccumulation in mullet Liza klunzingeri in Kuwaiti waters, off the Arabian Gulf. American Journal of Environmental Science, 2, 109–113.
Bu-Olayan, A. H., Thomas, B. V., & Husaini, M. S. (2008). Trace metals toxicity to the body structures of mullet Liza klunzingeri. International Journal of Environmental Research, 2, 249–254.
Chung, K. W., Fulton, M. H., & Scott, G. I. (2007). Use of the juvenile clam, Mercenaria mercenaria, as a sensitive indicator of aqueous and sediment toxicity. Ecotoxicology and Environmental Safety, 67, 333–340.
Cooper, S., Hare, L., & Campbell, P. G. C. (2010). Subcellular partitioning of cadmium in the freshwater bivalve, Pyganodon grandis, after separate short-term exposures to waterborne or diet-borne metal. Aquatic Toxicology, 100, 303–312.
Dauer, D. M., Maybury, C. A., & Ewing, R. M. (1981). Feeding behaviour and general ecology of several spionid polychaetes from the Chesapeake Bay. Journal of Experimental Marine Biology and Ecology, 54, 21–38.
Denton, G. R. W., & Burdon-Jones, C. (1986). Trace metals in fish from the Great Barrier Reef. Marine Pollution Bulletin, 17, 201–209.
Dragun, Z., Erk, M., Ivanković, D., Žaja, R., Marijić, V. F., & Raspor, B. (2010). Assessment of low-level metal contamination using the Mediterranean mussel gills as the indicator tissue. Environmental Science and Pollution Research, 17, 977–986.
Escobedo-Fregoso, C., Mendez-Rodriguez, L. C., Monsalvo-Spencer, P., Llera-Herrera, R. A., Zenteno-Savin, T., & Acosta-Vargas, B. (2010). Assessment of metallothioneins in tissues of the clam Megapitaria squalida as biomarkers for environmental cadmium pollution from areas enriched in phosphorite. Archives of Environmental Contamination and Toxicology, 59, 255–263.
Fisher, N. S., Teyssié, J.-L., Fowler, S. W., & Wang, W.-X. (1996). Accumulation and retention of metals in mussels from food and water: a comparison under field and laboratory conditions. Environmental Science and Technology, 30, 3232–3242.
Gauthier-Clerc, G., Pellerin, J., Blaise, C., & Gagné, F. (2002). Delayed gametogenesis of Mya arenaria in the Saguenay fjord (Canada): a consequence of endocrine disruptors? Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 131, 457–467.
Giguere, A., Couillard, Y., Campbell, P. G. C., Perceval, O., Hare, L., Pinel-Alloul, B., et al. (2003). Steady-state distribution of metals among metallothionein and other cytosolic ligands and links to cytotoxicity in bivalves living along a polymetallic gradient. Aquatic Toxicology, 64, 185–200.
Goldberg, E. D., Koide, M., Holdge, V., Flegal, A. R., & Martin, J. (1983). U.S. Mussel Watch: 1977–1978 results on trace metals and radionuclides. Estuarine, Coastal and Shelf Science, 16, 69–93.
Inza, B., Ribeyre, F., Maury-Brachet, R., & Boudou, A. (1997). Tissues distribution of inorganic mercury and cadmium in the Asiatic clam (Corbicula fluminae) in relation to the contamination levels of the water column and sediment. Chemosphere, 35, 2817–2836.
Kavun, V. Y., & Podgurskaya, O. V. (2009). Adaptation strategy of bivalve Modiolus modiolus from upwelling regions of the Kuril Islands shelf (Sea of Okhotsk) to heavy metal effects. Continental Shelf Research, 29, 1597–1604.
King, C. K., Dowse, M. C., & Simpson, S. L. (2010). Toxicity of metals to the bivalve Tellina deltoidalis and relationship between metal bioacumulation and metal partitioning between seawater and marine sediments. Archives of Environmental Contamination and Toxicology, 58, 657–665.
Klumpp, D. W., & Burdon-Jones, C. (1982). Investigations of the potential of bivalve molluscs as indicators of heavy metal levels in tropical marine waters. Australian Journal of Marine & Freshwater Research, 33, 285–300.
Langston, W. J., & Zhou, M. (1987). Cadmium accumulation, distribution and elimination in the bivalve Macoma balthica: neither metallothionein nor metallothionein-like proteins are involved. Marine Environmental Research, 21, 225–237.
Langston, W. J., Bebianno, M. J., & Burt, G. R. (1998). Metal handling strategies in molluscs. In W. J. Langston & M. J. Bebianno (Eds.), Metal metabolism in aquatic environments (pp. 219–272). London: Chapman and Hall.
Marina, M. O., & Enzo, O. (1983). Variability of zinc and manganese concentrations in relation to sex and season in the bivalve Donax trunculus. Marine Pollution Bulletin, 4, 342–346.
Mouneyrac, C., Amiard, J. C., & Amiard-Triquet, C. (1998). Effects of natural factors (salinity and body weight) on cadmium, copper, zinc and metallothionein-like protein levels in resident populations of oysters Crassostrea gigas from a polluted estuary. Marine Ecology Progress Series, 162, 125–135.
Nigro, M., Regoli, F., Rocchi, R., & Orlando, E. (1997). Heavy metals in Antarctic mollusks. In B. Battaglia, J. Valencia, & D. W. H. Walton (Eds.), Antarctic communities: species, structure and survival (pp. 409–412). Cambridge: Cambridge University Press.
Nørum, U., Lai, V. W. M., & Cullen, W. R. (2005). Trace element distribution during the reproductive cycle of female and male spiny and Pacific scallops, with implications for biomonitoring. Marine Pollution Bulletin, 50, 175–184.
O’Connor, T. P. (2004). Trends in chemical concentrations in mussels and oysters collected along the US coast from 1986 to 1993. Marine Environmental Research, 41, 183–201.
Odzak, N., Martinic, D., Zvonaric, T., & Branica, M. (1994). Bioaccumulation rate of Cd and Pb in M. galloprovincialis foot and gills. Marine Chemistry, 46, 119–131.
Okazaki, R. K., & Panietz, M. H. (1981). Depuration of twelve trace metals in tissues of the oysters Crassostrea gigas and C. virginica. Marine Biology, 63, 113–120.
Phillips, D. J. H., & Rainbow, P. S. (1989). Strategies of trace metal sequestration in aquatic organisms. Marine Environment Research, 28, 207–210.
Phillips, D. J. H., & Rainbow, P. S. (1995). Biomonitoring of heavy metal availability in the marine environment. Marine Pollution, 31, 183–192.
Podgurskaya, O. V., & Kavun, V. Y. (2006). Subcellular distribution of heavy metals in organs of bivalve Modiolus modiolus living along a metal contamination gradient. Ocean Science Journal, 41, 43–51.
Podgurskaya, O. V., & Kavun, V. Y. (2005). Comparison analysis of subcellular distribution of heavy metals in organs of the bivalve mollusks Crenomytilus grayanus and Modiolus modiolus from a continuously polluted environment. Russian Journal of Marine Biology, 31, 373–381.
Podgurskaya, O. V., Kavun, V. Y., & Lukyanova, O. N. (2004). Heavy metal accumulation and distribution in organs of the mussel Crenomytilus grayanus from upwelling areas of the Okhotsk Sea and Sea of Japan. Russian Journal of Marine Biology, 30, 219–226.
Rainbow, P. S., & Phillips, D. J. H. (1993). Cosmopolitan biomonitors of trace metals. Marine Pollution Bulletin, 26, 593–601.
Regoli, L. H., Chan, M., De Lafontaine, Y., & Mikaelian, I. (2001). Organotins in zebra mussel (Dreissena polymorpha) and sediments of the Quebec City Harbour area of the St. Lawrence River. Aquatic Toxicolology, 53, 115–126.
Reinfelder, J. R., Wang, W.-X., Luoma, S. N., & Fisher, N. S. (1997). Assimilation efficiencies and turnover rates of trace elements in marine bivalves: a comparison of oysters, clams, and mussels. Marine Biology, 129, 443–452.
Reinfelder, J. R., Fisher, N. S., Wang, W.-X., Nichols, J., & Luoma, S. N. (1998). Trace element trophic transfer in aquatic organisms: a critique of the kinetic model approach. Science of the Total Environment, 219, 117–135.
Roesijadi, G., & Robinson, W. E. (1994). Metal regulation in aquatic animals: mechanisms of uptake, accumulation and release. In D. C. Malins & G. K. Ostrander (Eds.), Aquatic toxicology: molecular, biochemical and cellular perspectives (pp. 387–420). Boca Raton: Lewis Publishers.
Siah, A., Pellerin, J., Amiard, J. C., Pelletier, E., & Viglino, L. (2003). Delayed gametogenesis and progesterone levels in soft- shell clams (Mya arenaria) in relation to in situ contamination to organotins and heavy metals in the St. Lawrence River (Canada). Toxicology and Pharmacology, 135, 145–156.
Simkiss, K., & Mason, A. Z. (1983). Metal ions: metabolic and toxic effects. In K. M. Wilbur (Ed.), Biology of Mollusca (pp. 101–164). New York: Academic.
Taghon, G. L., Nowell, A. R. M., & Jumars, P. A. (1980). Induction of suspension feeding in spionid polychaetes by high particulate fluxes. Science, 210, 562–564.
Tessier, A., Campbell, P. G. C., Auclair, J. C., & Bisson, M. (1984). Relationships between the partitioning of trace metals in sediments and their accumulation in the tissues of the freshwater mollusc Elliptio complanata in a mining area. Canadian Journal of Fisheries and Aquatic Sciences, 41, 1463–1472.
Yap, C. K., Ismail, A., Cheng, W. H., et al. (2006). Crystalline style and tissue redistribution in Perna viridis as indicators of Cu and Pb bioavailabilities and contamination in coastal waters. Ecotoxicology and Environmental Safety, 63, 413–423.
Acknowledgments
We thank the former Director of the Environmental Public Authority (EPA) of Kuwait, Dr. Mohammed Sarawi, for his support and invaluable assistance in collecting and processing specimens, data management, and laboratory analysis. I would like to express my gratitude to Dr. Michael Gochfeld and Dr. Keith Cooper for their constructive comments and Dr. Manaf Behbehani and Dr. Salim Al-Mohanna of the University of Kuwait for their helpful discussions regarding this research. I would also like to thank NIEHS (P 30ES005022) for their partial support for this project. This research was conducted under approved Rutgers University protocol. The results, conclusions, and interpretations reported herein are the sole responsibility of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarique, Q., Burger, J. & Reinfelder, J.R. Metal Concentrations in Organs of the Clam Amiantis umbonella and Their Use in Monitoring Metal Contamination of Coastal Sediments. Water Air Soil Pollut 223, 2125–2136 (2012). https://doi.org/10.1007/s11270-011-1009-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-1009-0