Abstract
Purpose
In acute cerebral ischemia, the assessment of irreversible injury is crucial for treatment decisions and the patient’s prognosis. There is still uncertainty how imaging can safely differentiate reversible from irreversible ischemic brain tissue in the acute phase of stroke.
Methods
We have searched PubMed and Google Scholar for experimental and clinical papers describing the pathology and pathophysiology of cerebral ischemia under controlled conditions.
Results
Within the first 6 h of stroke onset, ischemic cell injury is subtle and hard to recognize under the microscope. Functional impairment is obvious, but can be induced by ischemic blood flow allowing recovery with flow restoration. The critical cerebral blood flow (CBF) threshold for irreversible injury is ~15 ml/100 g × min. Below this threshold, ischemic brain tissue takes up water in case of any residual capillary flow (ionic edema). Because tissue water content is linearly related to X-ray attenuation, computed tomography (CT) can detect and measure ionic edema and, thus, determine ischemic brain infarction. In contrast, diffusion-weighted magnetic resonance imaging (DWI) detects cytotoxic edema that develops at higher thresholds of ischemic CBF and is thus highly sensitive for milder levels of brain ischemia, but not specific for irreversible brain tissue injury.
Conclusion
CT and MRI are complimentary in the detection of ischemic stroke pathology and are valuable for treatment decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The invention of computed tomography (CT) and of magnetic resonance imaging (MRI) about 40 years ago has enabled us to image the human brain’s tissue and vessels on macroscopic and microscopic levels, to study its pathology, and to measure cerebral perfusion and neuronal activity. Though the diagnostic and therapeutic impact of CT and MRI is obvious for ischemic stroke patients, still uncertainty exists how to interpret imaging findings associated with acute ischemic stroke symptoms like hypoattenuation or impaired proton diffusion. Focal X-ray hypoattenuation and other CT findings are imprecisely, vaguely, and falsely addressed as “hypodensity, early ischemic changes, early stroke signs, early infarct signs, insular ribbon sign (loss of the insular ribbon [1]), obscuration of the lentiform nucleus [2], loss of gray/white matter contrast, infarct core, effacement of sulci, or dense artery sign [3].” The language reveals the uncertainty of radiologists and stroke experts how “early ischemic changes” on CT and MRI translate to acute ischemic brain pathology like brain edema and infarction. In particular, there is uncertainty how to safely differentiate reversible from irreversible ischemic injury on CT and MRI in the acute phase of stroke when treatment decisions are urgent. This differentiation is of utmost importance for ischemic stroke management, because brain tissue with reversible changes can recover with reperfusion whereas irreversibly injured tissue cannot. There is conviction that CT cannot reliably demonstrate irreversibly damaged brain tissue in the acute stage of ischemic stroke [4] and that diffusion-weighted MRI (DWI) has proved to be more sensitive than CT for the detection of infarction [5]. Both statements suffer, however, from the lack of a reference standard for irreversible ischemic damage (infarction) the indispensable requirement for assessing and comparing the diagnostic accuracy of CT and DWI for brain infarction. Moreover, our understanding of acute stroke pathology is limited, because thousands of ischemic stroke patients have been included into clinical trials without assessing basic pathological conditions like arterial occlusion and recanalization or cerebral blood flow (CBF) [6]. Moreover, patients rarely die from ischemic stroke within the first hours of symptom onset. Consequently, autopsy data are lacking. We have to rely on animal experiments to understand what X-ray hypoattenuation and diffusion impairment of ischemic brain tissue mean in terms of tissue pathology, survival, and recovery. We provide here a review of experimental studies on ischemic brain ischemia, edema, and neuronal death that may help avoiding the misinterpretation of imaging findings in the acute phase of ischemic stroke.
Cerebral blood flow in experimental middle cerebral artery occlusion
In experimental animals, global or focal brain ischemia can be induced. We will discuss here mainly focal brain ischemia because it best mirrors ischemic stroke in humans. Permanent or transient occlusion of the proximal middle cerebral artery (MCA) is regarded as the most relevant and reproducible model of focal ischemic stroke or transient ischemic attack (TIA) [7]. Avoiding open-skull surgery by transorbital approach, occlusion of the MCA was achieved by means of a clip or snare ligature or—in smaller experimental animals—by introducing a nylon suture via the internal carotid artery into the orifice of the MCA [8, 9]. Measurement of local CBF with microelectrodes and hydrogen clearance or with microspheres enabled the study of the brain’s physiological and pathophysiological reactions to different levels of ischemia in the brain’s cortex and basal ganglia of experimental animals [10,11,12,13,14,15,16]. Brain ischemia after MCA occlusion was controlled by varying the cerebral perfusion pressure in the non-human primate, cat, or rat. After MCA occlusion, CBF dropped heterogeneously to different degrees within the MCA territory in non-human primates varying between 6.5 and 146.6% of basal cortical CBF [17]. This variation in focal CBF allowed the study of electrophysiological and metabolic reactions of the brains cortex to different levels of ischemia.
Thresholds of ischemia in relation to brain structure and function
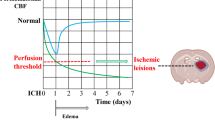
The concept of thresholds of ischemia in relation to brain structure and function was introduced in 1977 [18, 19] and became the rationale for reperfusion therapy by showing that the critical cerebral blood flow (CBF) threshold for irreversible injury is lower than for neuronal dysfunction. Cortical evoked potentials’ amplitude diminished immediately with a residual CBF of <16 ml/100 g × min and became zero with lower CBF values. Animals with permanent MCA occlusion were followed during 3 years and then sacrificed. It turned out that the tissue volume with initial loss of cortical function exceeded the tissue volume of final brain infarction. The authors concluded that “the ultimate area of infarction is confined to areas where blood flow reduction in the acute stage of the stroke is a great deal more intense and certainly below values of 10 ml/100 g × min” [18]. Astrup et al. showed that massive release of intra-cellular potassium ions into the extra-cellular space does not explain the failure of neuronal function occurring at lower cortical CBF at about 6 ml/100 g × min [19]. They proposed the concept of “ischemic penumbra during which the neurons remain structurally intact but functionally inactive” after finding that an increase in CBF can restore evoked potential and normalize extra-cellular potassium activity [20]. This concept was widened by studies showing that neuronal survival under ischemic conditions depends on time [21, 22]. At CBF below 10 ml/100 g × min, neurons die within 30 min, but survive indefinitely at a CBF above 15 ml/100 g × min. Awake non-human primates can tolerate brain ischemia of 10 to 15 ml/100 g × min for 2 to 3 h without irreversible injury. The concept of the viability thresholds of brain ischemia became more complex after the study of additional metabolic disturbances in brain ischemia. Table 1 summarizes the most important electro-physiological and metabolic changes at different ischemic CBF thresholds according to Hossmann [14].
Though the penumbra concept is promising in telling that neuronal dysfunction in ischemic stroke is reversible in principle and does not necessarily result in permanent disability, it does not provide clues how to reliably identify brain tissue that is irreversibly damaged under clinical conditions. Dysfunction is obvious to the stroke patient and his/her physician, but the proportion of the functionally inactive brain tissue that will not recover with CBF restoration is obscure. Searching for an imaging marker for ischemic tissue damage, we aim to find out when tissue necrosis becomes visible after arterial occlusion in histological studies.
Histology of evolving ischemic brain infarction
Garcia et al. extensively studied the evolution of ischemic brain infarction after MCA occlusion in animal models [7, 9, 23,24,25,26]. He differentiated between necrotic neurons (pyknosis, karyorrhexis, karyolysis, cytoplasmatic eosinophilia, loss of hematoxylin affinity), infarction (pannecrosis involving all cell types within a defined vascular territory), and selective neuronal necrosis (irreversible injury limited to specific brain neuron populations) [25]. After MCA occlusion in rats, microscopic changes appear first after 30 min as small lesions in the preoptic area involve then the striatum and, finally, the cerebral cortex. Up to 6 h, the ischemic injury induces neuronal scalloping, shrinkage, and swelling in less than 20% of neurons in the affected territories [25]. Eosinophylia and karyolysis appeared not before 12 h. Astrocytes responded to ischemia with cytoplasmatic disintegration in the preoptic area and with nuclear and plasmatic swelling in striatum and cortex at a time and place where neurons appeared only minimally injured. Focal brain ischemia does not produce coagulation necrosis (pannecrosis) within 6 h of arterial occlusion [26]. It became obvious that histological changes are subtle after MCA occlusion within the acute phase of ischemic stroke (6 h) and unlikely to be detected by CT or MRI. Because of their relatively low spatial resolution, both imaging modalities have no chance to directly identify selective neuronal necrosis. Moreover, it remained unclear whether and which such subtle changes—detected early after stroke onset—are reversible or irreversible ischemic injuries. A better imaging marker for ischemic tissue damage may be the shrinkage of extracellular space due to cellular swelling and tissue water content.
Types of ischemic edema
Molecular pathophysiology differentiates three types of edema in focal brain ischemia: cytotoxic, ionic, and vasogenic [27].
Cytotoxic edema or cellular edema involves oncotic swelling of glial and neuronal cells due to a shift of ions—mainly Na+ and Cl−—and water molecules from the extracellular to the intracellular space as a consequence of ischemic adenosintriphosphate depletion. Without any new constituent from the intravascular space, tissue swelling does not occur. Pure cytotoxic edema develops in brain tissue being removed from the living brain. Remote from blood supply, such a piece of ischemic brain will not swell or develop other types of edema [27]. Oncotic swelling of astrocytes and neurons means shrinkage of extracellular space that is already observed at relatively mild ischemia with a CBF below 30 ml/100 g × min (Table 1) [28].
Ionic edema means net uptake of water by dense ischemic brain tissue from perfused or re-perfused capillaries through an intact blood-brain-barrier. Depletion of extracellular Na+ due to cytotoxic edema creates a concentration gradient between intravascular and extracellular compartments across the blood-brain-barrier. Along this osmotic pressure gradient, Na+, Cl−, and water molecules are transported into the extracellular space through special endothelium channels (permeability pores) that can be blocked with low-dose glibenclamide [29]. Net water uptake starts immediately after MCA occlusion and was related to the degree of ischemia in that region if CBF was reduced below 15–20 ml/100 g × min [30]. Cerebral water content increased from 78 to 80.5% within 3 h of permanent arterial occlusion and up to 83% within 9 h. Restoration of blood flow following 30-min occlusion stopped the increase in cerebral water content and reached the control value after 3 days [31]. In a rat model of global ischemia down to 5.8 ml/100 g × min, cortical specific gravity decreased from 1.0484 to 1.0445 within 60 min and recovered with restoration of CBF after 15 min of ischemia, but not after 30 or 60 min of ischemia [32]. After MCA occlusion in the cat, cortical water content increased from 80.5 to 84% in regions with CBF below 10–15 ml/100 g × min. Ionic edema was associated with further decrease in CBF below the threshold of 10 ml/100 g × min defined as “critical ischemia” below which functional recovery is impossible [15]. Ionic edema is, therefore, an early marker of irreversible ischemic injury.
Ischemic vasogenic edema is the consequence of blood-brain-barrier breakdown with leakage of plasma proteins into extracellular space starting 4 to 12 h after arterial occlusion [7]. Hydrostatic and osmotic pressure gradients contribute to the increase in tissue water content that may cause severe brain tissue swelling, further decrease in perfusion pressure, mass effects, and fatal herniation (malignant infarction).
Imaging of brain edema
Cytotoxic edema
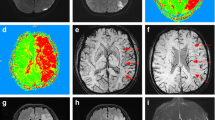
Computed tomography cannot detect pure cytotoxic edema (shrinkage of extracellular space and oncotic cell swelling without net water uptake), because cytotoxic edema does not affect X-ray attenuation or induce tissue swelling (Fig. 1). It is evident that cytotoxic edema is associated with impairment of proton diffusion detected by diffusion-weighted MRI (DWI) and measured on apparent diffusion coefficient (ADC) maps. A decrease in ADC 90 min after MCA occlusion was associated with extracellular fluid loss, swelling of various cellular compartments, and neuronal shrinkage [33]. Apparent diffusion coefficient declines immediately when CBF falls below 20 to 40 ml/100 g × min in animals and humans [34, 35] corresponding well with the CBF threshold for the shrinkage of extracellular space [28]. High-signal lesions on DWI representing cytotoxic edema can disappear with reperfusion, but are closely associated with infarction if persistent as shown in animal and clinical studies [36,37,38]. Detecting cytotoxic edema at the time of stroke symptom onset immediately after arterial occlusion with high sensitivity, DWI identifies brain regions suffering from CBF below 40 ml/100 g × min and, thus, tissue regions that may not survive without blood flow restoration. In patients with persistent MCA occlusion, a DWI lesion >145 cm3 detected within 14 h of stroke onset is accurately predictive for the development of life-threatening (malignant) MCA infarction [39]. Diffusion-weighted MRI does not, however, specifically identify brain tissue being prone to die even with reperfusion.
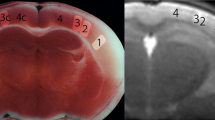
Embolic pattern of cytotoxic edema not detected by CT. a–d Four sections of cranial CT of a 62-year-old man 87 min after onset of left-sided hemiparesis. CT angiography (not presented) does not show arterial occlusion. Subtle small lesion in both cerebellar hemispheres not explaining the symptoms. e–h DWI at 3 h and 10 min after symptom onset shows an embolic pattern of small lesions in both cerebral and cerebellar hemispheres with predominance in the right central region explaining the stroke symptoms. The patient had a dissection of his aorta
Ionic edema
Ionic edema in contrast to cytotoxic edema means increase in water content and swelling of severely ischemic tissue (Fig. 2). In vitro measurements of X-ray attenuation and T1 and T2 relaxation in gelatin gels and eggs showed a linear relationship between CT attenuation and specific gravity/water content of the gel (r = 0.992; p < 0.0001) and no effect on X-ray attenuation by egg hardening with water content kept constant. A 1% increase in gel water content was associated with a decrease in X-ray attenuation of 2.6 Hounsfield units (HU). The relationship between T1 and T2 relaxation and gel water content was linear as well. A 6% increase in gel water content resulted in a 19% increase in T2 signal intensity in contrast to a 25% change in CT attenuation in the same specimens [40]. Moreover, cooking egg white while keeping water content stable considerably shortened T1 and T2 relaxation and impaired MR signal intensity showing the impact of bulk water transfer into bound water on MR signal intensity and demonstrating the complexity of MR signal intensity. Computed tomography can measure and follow ionic edema following MCA occlusion in the experimental animal [41,42,43]. Ischemic brain tissue water content increased steadily within 6 h of MCA occlusion from 77.9 to 79.3%, whereas X-ray attenuation declined from 75.6 to 71.7 HU meaning that a 1% increase in hemispheric tissue water content caused an attenuation decrease of 1.8 HU (linear correlation r = 0.55, p < 0.0001) [41]. In a reperfusion experiment, CT attenuation remained stable after initial decline after 1 h of MCA occlusion, but further decreased with reperfusion after 2, 3, and 4 h of occlusion [42]. Occlusion of the MCA in non-human primates decreased CBF below 10 ml/100 g × min for 4 to 6 h that was associated with an attenuation decrease of 4–5 HU [43]. A linear relationship between ischemic CBF and decrease in CT attenuation was also observed in ischemic stroke patients within 4.5 h of symptom onset [44].
Development of malignant infarction. a CT within 6 h of stroke onset. Subtle hypoattenuation of the total frontal lobe, insular cortex, temporal lobe, caudate, and lentiform nucleus (territories of middle and anterior cerebral arteries). b 12 h later: well-demarcated MCA and ACA infarction with effacement of sulci, but no mass effect. c At 24 h: mass effect with shift of midline structures and herniation of the right medial temporal lobe
Based on these experimental and clinical observations, it is evident that CT can detect and even quantify ionic brain edema and, thus, the volume of ischemic brain tissue that is irreversibly injured. This evidence was strongly supported by the prospective expert CT reading in the European Cooperative Acute Stroke Study II (ECASS II), a randomized controlled trial on the effect of alteplase in 786 ischemic stroke patients [45]. In this study, experienced neuroradiologists from three European countries assessed areas of CT hypoattenuation within 6 h of ischemic stroke onset blinded to follow-up CTs at 22–96 h and 2–36 days that served as reference standard for the measurement of diagnostic accuracy [46]. Six hundred seventy-nine patients had infarctions on follow-up CT. The positive predictive values for these infarctions were 98 and 95% for the alteplase and placebo-treated patients. The experts detected CT hypoattenuation in 433 out of 679 patients (64%) on baseline CT compared to 271 patients (40%) identified by the study sites. The authors incorrectly calculated CT sensitivity for “early infarct signs” from these numbers neglecting that follow-up imaging is an inappropriate reference for the assessment of baseline CT sensitivity, because ionic edema has possibly not yet developed within the first 6 h of stroke with focal brain ischemia below the CBF threshold for neuronal function impairment, but above the threshold for irreversible injury. Nevertheless, the local investigators at ECASS 2 study sites missed CT hypoattenuation in 162 patients adjudicated positive by expert neuroradiologists demonstrating that non-experts can miss subtle gray matter hypoattenuation caused by early stages of ionic edema, e.g., an increase in tissue water content of less than 1%. Furthermore, low-contrast resolution due to wide window settings impairs the recognition of early ionic edema with subtle hypoattenuation. Window and level settings on CT should provide a clear differentiation of gray and white matter allowing substantial inter-observer agreement [47, 48]. Post-processing of CT images with special software can further enhance gray-white matter contrast and increase CT sensitivity for ionic edema [49, 50].
Ionic edema may be accompanied by brain tissue swelling, but brain tissue swelling may not be associated with ionic edema. Brain tissue swelling without edema may develop with low perfusion pressure and consecutive compensatory arterial dilatation (Fig. 3). Computed tomography shows effacement of cerebro-spinal-fluid spaces, but no tissue hypoattenuation. There is no visible lesion on DWI. Perfusion imaging shows prolonged transit times, increased cerebral blood volume, but stable CBF [51, 52]. This so-called isolated focal brain swelling is reversible with an increase in cerebral perfusion pressure and not an “infarct sign,” but is at risk of ischemic damage if perfusion pressure is not enhanced. The Alberta Stroke Program Early CT Score (ASPECTS) originally semi-quantified CT hypoattenuation and focal swelling within the MCA territory [53]. After a few years of experience, the score was revised and only areas with tissue hypoattenuation are now counted making ASPECTS the ionic edema score [54].
Brain swelling without tissue hypoattenuation due to compensatory vasodilation. CT of a patient with left-sided hemiparesis since 12 h caused by right MCA occlusion. a No ionic edema visible, but severe swelling of the right hemisphere (arrow). b Six days later: thrombectomy not attempted with view to “therapeutic time-window.” Development of subcortical, central MCA infarction with persistent tissue swelling in the remaining MCA territory
Vasogenic edema
Extravasation of protein-rich fluids into ischemic brain tissue may cause severe swelling with mass effects like shift of midline structures and herniation of the medial temporal lobe with brain stem compression easily detected on CT and MRI. Leakage of proteins and blood affects CT attenuation and MR signal and may even obscurate hypoattenuating lesions that may be mistaken as tissue recovery [55].
Clinical implications
Based on retrospective observations, more than 20 years ago, it was hypothesized that ionic edema covering more than 50% of the MCA territory may be predictive for fatal clinical outcome [56]. In order to avoid patients with such poor prognosis, the ECASS investigators excluded patients with hypoattenuation >33% of the MCA territory. A post hoc analysis of ECASS showed that 52 falsely included patients with extended ionic edema (>33% MCA territory) did not benefit from alteplase in contrast to 215 patients with small volumes of ionic edema [57]. In a cohort of 156 stroke patients treated with alteplase within 3 h of symptom onset, 89% of patients with ionic edema >33% MCA territory were disabled or death compared to 24% of patients with smaller ionic edema. Symptomatic hemorrhages occurred in 14% of patients with extended ionic edema vs. 1% in patients without or smaller ionic edema [53]. A retrospective analysis of the Pro-Urokinase for Acute Cerebral Thromboembolism II (PROACT-II) trial showed that only patients with an ASPECTS >7 had a benefit from intra-arterial treatment [58]. More recently, a meta-analysis of five randomized trials on thrombectomy showed no beneficial effect of reperfusion therapy in patients with ASPECTS <6 [59]. These observations favor the view that ischemic brain tissue with ionic edema cannot be rescued with restoration of blood flow. Reperfusion therapy is likely ineffective and perhaps even risky if the volume of ionic edema exceeds 33% of the MCA territory or the ASPECTS is lower than 6.
Summary
Focal brain ischemia with CBF <25 ml/100 g × min causes neuronal dysfunction and cytotoxic edema being detected by DWI with high sensitivity. As long as CBF does not fall below ~15 ml/100 g × min (“critical ischemia”), brain tissue can recover with restoration of CBF. In areas with critical ischemia, cytotoxic edema is associated with ionic edema meaning net uptake of water if capillaries provide residual flow. Ionic edema with the increase in tissue water content is the specific marker of brain tissue with almost no chances to survive even with reperfusion. Moreover, delayed reperfusion of brain tissue with critical ischemia may enhance ionic and later vasogenic edema and mass effect. No randomized controlled trial has shown so far that patients with extended ionic edema benefit from reperfusion therapy. Computed tomography is accurate in detecting ionic and vasogenic edema and capable of quantifying changes in brain tissue water content. The comparison of the diagnostic accuracies of CT and MRI requires an exact definition of the object under observation. Both modalities do not diagnose the clinical entity “stroke,” but aspects of the underlying pathology. Moreover, both modalities are complementary in imaging two different aspects of acute ischemic brain tissue, the potentially reversible cytotoxic edema and the ionic edema indicating irreversible damage.
References
Truwit C, Barkovich A, Gean-Marton A, Hibri N, Norman D (1990) Loss of the insular ribbon: another early ct sign of acute middle cerebral artery infarction. Radiology 176:801–806
Tomura N, Uemura K, Inugami A, Fujita H, Higano S, Shishido F (1988) Early ct finding in cerebral infarction. Radiology 168:463–467
Schuierer G, Huk W (1988) The unilateral hyperdense middle cerebral artery: an early ct-sign of embolism or thrombosis. Neuroradiology 30:120–122
Tomandl BF, Klotz E, Handschu R, Stemper B, Reinhardt F, Huk WJ et al (2003) Comprehensive imaging of ischemic stroke with multisection ct. Radiographics 23:565–592
Zimmerman R (2004) Stroke wars: episode iv ct strikes back. AJNR. Am J Neuroradiol 25:1304–1309
Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E et al (2014) Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 384:1929–1935
Garcia J (1984) Experimental ischemic stroke: a review. Stroke; a journal of cerebral circulation. 15:5–14
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke; a journal of cerebral circulation. 20:84–91
Garcia JH, Mitchem HL, Briggs L, Morawetz R, Hudetz AG, Hazelrig JB et al (1983) Transient focal ischemia in subhuman primates. Neuronal injury as a function of local cerebral blood flow. J Neuropathol Exp Neurol 42:44–60
Pasztor E, Symon L, Dorsch NW, Branston NM (1973) The hydrogen clearance method in assessment of blood flow in cortex, white matter and deep nuclei of baboons. Stroke; a journal of cerebral circulation. 4:556–567
Symon L, Pasztor E, Dorsch NW, Branston NM (1973) Physiological responses of local areas of the cerebral circulation in experimental primates determined by the method of hydrogen clearance. Stroke; a journal of cerebral circulation. 4:632–642
von Kummer R (1984) Local vascular response to change in carbon dioxide tension. Long term observation in the cat's brain by means of the hydrogen clearance technique. Stroke; a journal of cerebral circulation. 15:108–114
von Kummer R, Herold S (1986) Hydrogen clearance method for determining local cerebral blood flow. I. Spatial resolution. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 6:486–491
Hossmann KA (1994) Viability thresholds and the penumbra of focal ischemia. Ann Neurol 36:557–565
Hossmann KA, Schuier FJ (1980) Experimental brain infarcts in cats. I. Pathophysiological observations. Stroke; a journal of cerebral circulation. 11:583–592
Schuier FJ, Hossmann KA (1980) Experimental brain infarcts in cats. Ii. Ischemic brain edema. Stroke; a journal of cerebral circulation. 11:593–601
Symon L, Pasztor E, Branston NM (1974) The distribution and density of reduced cerebral blood flow following acute middle cerebral artery occlusion: an experimental study by the technique of hydrogen clearance in baboons. Stroke; a journal of cerebral circulation. 5:355–364
Symon L, Branston N, Strong A, Hope T (1977) The concept of thresholds of ischaemia in relation to brain structure and function. J Clin Pathol 30:149–154
Astrup J, Symon L, Branston NM, Lassen NA (1977) Cortical evoked potential and extracellular k+ and h+ at critical levels of brain ischemia. Stroke; a journal of cerebral circulation. 8:51–57
Astrup J, Siesjö B, Symon L (1981) Thresholds in cerebral ischemia—the ischemic penumbra. Stroke; a journal of cerebral circulation. 12:723–725
Heiss W, Rosner G (1983) Fuctional recovery of cortical neurons as related to degree and duration of ischemia. Ann Neurol 14:294–301
Jones T, Morawetz R, Crowell R, Marcoux F, FitzGibbon S, DeGirolami R et al (1981) Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg 54:773–782
Garcia J (1988) Morphology of global cerebral ischemia: a review. Crit Care Med 16:979–987
Garcia J, Liu K-F, Ho K-L (1995) Neuronal necrosis after middle cerebral artery occlusion in wistar rats progresses at different time intervals in the caudoputamen and the cortex. Stroke; a journal of cerebral circulation. 26:636–643
Garcia JH, Liu KF, Ye ZR, Gutierrez JA (1997) Incomplete infarct and delayed neuronal death after transient middle cerebral artery occlusion in rats. Stroke; a journal of cerebral circulation. 28:2303–2309 discussion 2310
Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J et al (1993) Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol 142:623–635
Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V (2007) Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Lancet Neurol 6:258–268
Hossmann KA (2006) Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol 26:1057–1083
Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L et al (2006) Newly expressed sur1-regulated nc(ca-atp) channel mediates cerebral edema after ischemic stroke. Nat Med 12:433–440
Symon L, Branston N, Chikovani O (1979) Ischemic brain edema following middle cerebral artery occlusion in baboons: relationship between regional cerebral water content and blood flow at 1 to 2 hours. Stroke; a journal of cerebral circulation. 10:184–191
Ito U, Ohno K, Nakamura R, Suganuma F, Inaba Y (1979) Brain edema during ischemia and after restoration of blood flow. Measurement of water, sodium, potassium content and plasma protein permeability. Stroke; a journal of cerebral circulation. 10:542–547
Todd N, Picozzi P, Crockard A, Ross RR (1986) Duration of ischemia influences the development and resolution of ischemic brain edema. Stroke; a journal of cerebral circulation. 17:466–471
Liu KF, Li F, Tatlisumak T, Garcia JH, Sotak CH, Fisher M et al (2001) Regional variations in the apparent diffusion coefficient and the intracellular distribution of water in rat brain during acute focal ischemia. Stroke; a journal of cerebral circulation. 32:1897–1905
Lin W, Lee J, Lee Y, Vo K, Pilgram T, Hsu C (2003) Temporal relationship between apparent diffusion coefficient and absolute measurements of cerebral blood flow in acute stroke patients. Stroke; a journal of cerebral circulation. 34:64–70
Wang Y, Hu W, Perez-Trepichio A, Ng T, Furlan A, Majors A et al (2000) Brain tissue sodium is a ticking clock telling time after arterial occlusion in rat focal cerebral ischemia. Stroke; a journal of cerebral circulation. 31:1386–1392
Li F, Liu K, Silva M, Omae T, Sotak C, Fenstermacher J et al (2000) Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats. Stroke; a journal of cerebral circulation. 31:946–954
Fiehler J, Knudsen K, Kucinski T, Kidwell C, Alger J, Thomalla G et al (2004) Predictors of apparent diffusion coefficient normalization in stroke patients. Stroke; a journal of cerebral circulation. 35:514–519
Kidwell C, Saver J, Mattielloo J, Starkman S, Vinuela F, Duckwiler G et al (2000) Thrombolytic reversal of acute human cerebral ischemic injury shown by diffusion/perfusion magnetic resonance imaging. Ann Neurol 47:462–469
Oppenheim C, Samson Y, Manai R, Lalam T, Vandamme X, Crozier S et al (2000) Prediction of malignant middle cerebral artery infarction by diffusion-weighted imaging. Stroke; a journal of cerebral circulation. 31:2175–2181
Unger E, Littlefield J, Gado M (1988) Water content and water structure in ct and mr signal changes: possible influence in detection of early stroke. AJNR. Am J Neuroradiol 9:687–691
Dzialowski I, Weber J, Doerfler A, Forsting M, von Kummer R (2004) Brain tissue water uptake after middle cerebral artery occlusion assessed with ct. Journal of neuroimaging : official journal of the American Society of Neuroimaging 14:42–48
Dzialowski I, Weber J, Klotz E, Göricke S, Dörfler A, Forsting M et al (2007) Ct monitoring of ischemic brain tissue water content during middle cerebral artery occlusion and reperfusion. Radiology 243:720–726
Nemoto EM, Mendez O, Kerr ME, Firlik A, Stevenson K, Jovin T et al (2012) Ct density changes with rapid onset acute, severe, focal cerebral ischemia in monkeys. Translational stroke research 3:369–374
Kucinski T, Majumder A, Knab R, Naumann D, Fiehler J, Vaterlein O et al (2004) Cerebral perfusion impairment correlates with the decrease of ct density in acute ischaemic stroke. Neuroradiology 46:716–722
Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D et al (1998) Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ecass ii). Lancet 352:1245–1251
von Kummer R, Bourquain H, Bastianello S, Bozzao L, Manelfe C, Meier D et al (2001) Early prediction of irreversible brain damage after ischemic stroke by computed tomography. Radiology 219:95–100
von Kummer R, Holle R, Grzyska U, Hofmann E, Jansen O, Petersen D et al (1996) Interobserver agreement in assessing early ct signs of middle cerebral artery infarction. AJNR Am J Neuroradiol 17:1743–1748
von Kummer R, Nolte PN, Schnittger H, Thron A, Ringelstein EB (1996) Detectability of hemispheric ischemic infarction by computed tomography within 6 hours after stroke. Neuroradiology 38:31–33
Bier G, Bongers MN, Ditt H, Bender B, Ernemann U, Horger M (2016) Enhanced gray-white matter differentiation on non-enhanced ct using a frequency selective non-linear blending. Neuroradiology 58:649–655
Bier G, Bongers MN, Ditt H, Bender B, Ernemann U, Horger M (2016) Accuracy of non-enhanced ct in detecting early ischemic edema using frequency selective non-linear blending. PLoS One 11:e0147378
Butcher K, Lee S, Parsons M, Allport L, Fink J, Tress B et al (2007) Differential prognosis of isolated cortical swelling and hypoattenuation on ct in acute stroke. Stroke; a journal of cerebral circulation. 38:941–947
Na D, Kim E, Ryoo J, Lee K, Roh H, Kim S et al (2005) Ct sign of brain swelling without concomitant parenchymal hypoattenuation: comparison with diffusion- and perfusion-weighted mr imaging. Radiology 235:992–998
Barber P, Demchuk A, Zhang J, Buchan A (2000) Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet 355:1670–1674
Puetz V, Dzialowski I, Hill MD, Demchuk AM (2009) The alberta stroke program early ct score in clinical practice: what have we learned? International journal of stroke : official journal of the International Stroke Society 4:354–364
Becker H, Desche H, Hacker H, Pencz A (1979) Ct fogging effect with ischemic cerebral infarcts. Neuroradiology 18:185–192
von Kummer R, Meyding-Lamadé U, Forsting M, Rosin L, Rieke K, Hacke W et al (1994) Sensitivity and prognostic value of early computed tomography in middle cerebral artery trunk occlusion. AJNR Am J Neuroradiol 15:9–15
von Kummer R, Allen K, Holle R, Bozzao L, Bastianello S, Manelfe C et al (1997) Acute stroke: usefulness of early ct findings before thrombolytic therapy. Radiology 205:327–333
Hill M, Rowley H, Adler F, Eliasziew M, Furlan A, Higashida R et al (2003) Selection of acute ischemic stroke patients for intra-arterial thrombolysis with pro-urokinase by using aspects. Stroke; a journal of cerebral circulation 34:1925–1931
Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM et al (2016) Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387:1723–1731
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This review reports on many studies with animals and human participants performed and already published by the authors and by others. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
Statement of informed consent was not applicable since the manuscript does not contain any patient data.
Rights and permissions
About this article
Cite this article
von Kummer, R., Dzialowski, I. Imaging of cerebral ischemic edema and neuronal death. Neuroradiology 59, 545–553 (2017). https://doi.org/10.1007/s00234-017-1847-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1847-6