Abstract
We have previously shown that 21-benzylidene digoxin (21-BD) increases the total cholesterol and phospholipid content on the membrane of HeLa cells. Lipid modulation caused by cardiotonic steroids (CTS) is still unexplored. Therefore, the aim of the present study was to evaluate the cholesterol and phospholipid modulation of the cell membrane caused by ouabain and 21-BD and the possible involvement of the caveolae on this modulation. For this, one cell line containing caveolae (HeLa) and other not containing (Caco-2) were used. The modulation of the lipid profile was evaluated by total cholesterol and phospholipids measurements, and identification of membrane phospholipids by HPTLC. The cholesterol distribution was evaluated by filipin staining. The caveolin-1 expression was evaluated by Western Blotting. Ouabain had no effect on the total membrane lipid content in both cell lines. However, 21-BD increased total membrane phospholipid content and had no effect on the membrane cholesterol content in Caco-2 cells. CTS were not able to alter the specific phospholipids content. In the filipin experiments, 21-BD provoked a remarkable redistribution of cholesterol to the perinuclear region of HeLa cells. In Caco-2 cells, it was observed only a slight increase in cholesterol, especially as intracellular vesicles. The caveolin-1 expression was not altered by any of the compounds. Our data mainly show different effects of two cardiotonic steroids. Ouabain had no effect on the lipid profile of cells, whereas 21-BD causes important changes in cholesterol and phospholipid content. Therefore, the modulation of cholesterol content in the plasma membrane of HeLa cells is not correlated with the expression of caveolin-1.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell structure and function depend on the integrity of the plasma membrane and organelles that are essential in many biochemical processes. In 1972, Singer and Nicolson developed a model to characterize biological membranes in a unifying manner, since the knowledge about the plasma membrane composition and organization was very limited. This model, also known as the fluid mosaic model, states that membranes are asymmetric and composed of a phospholipid bilayer with several protein molecules floating around and embedded within it (Singer and Nicolson 1972).
Subsequently, a model was postulated demonstrating the existence of lipid rafts, membrane microdomains enriched in cholesterol and sphingolipids. Caveolin-1 (Cav-1) is present in some of these microdomains, which results in the formation of membrane invaginations producing specific structures called caveolae (Simons and Ikonen 1997; Simons and van Meer 1988). Caveolin-1 is essential for the formation of caveolae in cells since the loss of this important protein results in cells with no production of this structure. Moreover, exogenous expression of Cav-1 in cells with apparent no expression of this protein, and consequently, absence of caveolae structures, results in caveolae formation (Drab et al. 2001; Fra et al. 1995). Caveolae regions of the plasma membrane have specialized functions in cells, such as signal transduction due to the interaction of Cav-1 with cellular signaling proteins (Couet et al. 1997; Okamoto et al. 1998). In addition, Cav-1 takes part on the transport of cholesterol to the membrane, and its interaction with cholesterol is necessary to form and stabilize the caveolae structure (Murata et al. 1995; Parton and del Pozo 2013).
Lipid composition of the cell membrane is asymmetric and heterogeneous. Usually, the inner monolayer contains a higher concentration of phosphatidylserine (PS) and phosphatidylethanolamine (PE), whereas the outer monolayer is enriched in phosphatidylcholine (PC) and sphingomyelin (SM), which allows lipophilic or electrostatic interactions among different phospholipids permanently interchanging their partners through lateral diffusion along the cell membrane. Alterations in the distribution of phospholipids from the lipid bilayer may signal several cell processes, such as aggregation, adhesion, and apoptosis (Engelman 2005; Marquardt et al. 2015; Nicolson 2014).
Cardiotonic steroids (CTS) are well-known compounds used in the treatment of congestive heart diseases for centuries. The cellular target for CTS is the membrane enzyme Na,K-ATPase, responsible for maintaining the cellular osmotic gradient and controlling the intracellular concentrations of sodium and potassium (Stucky and Goldberger 2015; Thomas et al. 1990). CTS are able to bind to the α-subunit of the Na,K-ATPase causing its inhibition and leading to an increase of intracellular Na+, and consequently, an increase of intracellular Ca2+. This effect promotes an increase of the cardiac contraction force In addition, CTS interaction with the Na,K-ATPase may trigger intracellular signaling pathways that are involved with the regulation of gene expression, protein synthesis, and cell proliferation (Chen et al. 2006; Xie and Askari 2002).
21-Benzylidene digoxin (21-BD), a semi-synthetic CTS derivative of digoxin, consists of an additional styrene group on the lactone ring, specifically at C21 position. This change provides different characteristics compared to other CTS, probably because the additional aromatic group might promote a steric hindrance at the Na,K-ATPase catalytic site. The Na,K-ATPase demonstrates a lower affinity for 21-BD, compared to digoxin and ouabain, and its activity when isolated from cell membrane preparations is not affected by 21-BD. Interestingly, cells treated with 10 μM 21-BD have an increase of Na,K-ATPase activity. Moreover, 21-BD induces apoptosis of cancer cells, and increases the sealing degree of tight junctions (Rocha et al. 2014). More recently, it has been demonstrated in HeLa cells that 21-BD decreases cell proliferation by reducing epidermal growth factor receptor (EGFR) and extracellularly regulated kinase (ERK) phosphorylation and promoted apoptosis by activating intrinsic and extrinsic pathways (Pessôa et al. 2018). Thus, the biological effects of 21-BD are different from those observed for classical CTS, such as ouabain and digoxin, thus being an interesting compound to study the diversity of biological effects modulated by CTS.
There are few studies in the literature concerning the effect of CTS on modulation of cellular lipids. CTS are able to modify the lipid metabolism of cells (Campia et al. 2009, 2012), altering the cholesterol and phospholipid content (Garcia et al. 2015, 2018, 2019; Silva et al. 2017), as well as the plasma membrane fluidity (Manna et al. 2006).
We have demonstrated that HeLa cells treated with 21-BD undergo lipid alterations. An increase in the total phospholipid and cholesterol content of the plasma membrane was observed after treatment of the cells with 50 μM of 21-BD. This treatment also caused a decrease in the Na,K-ATPase activity, possibly due to modulations of the lipid microenvironment around the enzyme, since Na,K-ATPase has low affinity for 21-BD and no changes in the enzyme expression have been observed. Lipid modulation could lead to alterations in the membrane fluidity resulting in changes of the enzyme conformation that could inhibit the catalytic activity of Na,K-ATPase by exposing its binding site to 21-BD (Silva et al. 2017).
It has been described that caveolae could be important for the modulation of CTS in activating signaling pathways (de Souza et al. 2014; Liang et al. 2007; Liu et al. 2011). Although several reports have shown the association of the Na,K-ATPase with the caveolae for signaling events, there are still some debate if the presence of caveolae is crucial for the Na,K-ATPase effects. We have previously demonstrated that ouabain treatment in Caco-2 cells was able to activate ERK1/2 signaling pathway (de Souza et al. 2014). Since caveolae is a cellular microdomain of the plasma membrane enriched in cholesterol, it is of great importance to the maintenance of lipid homeostasis in the cell. Moreover, Cav-1 is able to regulate the Na,K-ATPase endocytosis, which can result in changes of its activity and signaling properties (Chen et al. 2009). Therefore, the expression pattern of Cav-1 can modulate several cellular responses, such as cell proliferation (Quintas et al. 2010), migration (Grande-Garcia et al. 2007; Nunez-Wehinger et al. 2014), apoptosis and cell survival (Gargalovic and Dory 2003; Han et al. 2015; Torres et al. 2006).
Through all those important effects, we can assume that the presence of caveolae could be crucial for the cellular effects of CTS, especially concerning the regulation of cholesterol and phospholipids. The objective of this study was to evaluate the effect of 21-BD and ouabain in the lipid modulation and to analyze if caveolae is involved in the CTS modulation of the lipid profile from HeLa (a caveolae-containing cell line) (Hirama et al. 2017) and Caco-2 (cells with no caveolae) cell lines (Breuza et al. 2002; Vogel et al. 1998).
Methods
Cell Culture
HeLa (cervix adenocarcinoma—ATCC CCL-2) and Caco-2 (colorectal adenocarcinoma—ATCC HTB-37) cancer cells were kindly provided by Fundação Ezequiel Dias (FUNED) from Belo Horizonte, MG, Brazil. Cells were cultured in a humidified incubator at 37 °C and 5% CO2. DMEM cell culture medium was used with fetal bovine serum (FBS) 10% and penicillin/streptomycin 0.1%. Medium was changed every 48 h and cells were rinsed with sterile phosphate buffered saline (PBS—[pH 7.4], NaCl 137 mM, KCl 2.7 mM, and PO43− 10 mM). For each experiment, cells were seeded in a density specified in each section.
Treatment
Ouabain was obtained from Sigma-Aldrich and 21-benzylidene digoxin (21-BD) was synthesized by the Laboratory of Organic Synthesis and Nanostructures of the Federal University of São João del-Rei, Minas Gerais, Brazil (Rocha et al. 2014). After 80% of confluence, cells were treated with the compounds for 48 h. Stock solutions were prepared by dilution of the compounds in DMSO and working concentrations were obtained by dilution in DMEM on the day of each experiment. Maximum concentration of DMSO on cells was 1%.
Cell Viability Assay
MTT assay was used to evaluate the cell viability. Viable cells can metabolize a yellow tetrazolium salt into a water-insoluble purple formazan crystal. The cytotoxic effect of CTS on the cells was observed to determine specific concentrations for the next assays. HeLa and Caco-2 cells were seeded in 96-well plates at a density of 1 × 104 cells/well, and after 24 h they were treated with increasing concentrations (2 nM to 500 µM) of ouabain or 21-BD. After 48 h of treatment, the culture medium was discarded and 100 µL of MTT salt (0.5 mg/mL) was added to each well. Formazan crystals formed after 3 h were diluted with 50 µL of DMSO for 15 min. Finally, a microplate reader Biotech Instruments, Inc. Winooski, VT, USA was used to record the absorbance at 550 nm.
Plasma Membrane Preparation
An amount of 2.25 × 106 cells were cultured in a 75 cm2 culture flask up to 80% confluence. Then, cells were treated for 48 h with ouabain (10 nM or 100 nM) or 21-BD (5 µM or 50 µM). Afterward, cells were rinsed with PBS and harvested in 3 mL of preparation buffer (Tris–HCl 6 mM [pH 6.8], imidazole 20 mM, sucrose 250 mM, sodium dodecyl sulfate 0.01%, EDTA 3 mM, and protease inhibitor cocktail 1:100). Samples were submitted to a Potter tissue homogenizer for 20 times, and subsequently, centrifuged at 10,000×g for 20 min. The pellet was discarded, and the supernatant was centrifuged at 70,000×g for 1 h using a WTi 45 ultracentrifuge rotor. The pellet was suspended in 300 µL of preparation buffer. Samples were stored at − 20 °C.
Protein Measurement
Protein measurement was evaluated using the Bradford method (Bradford 1976) in 96-well plates. Samples were diluted in ultrapure water and an aliquot of 40 µL/well was used. Afterward, 200 µL of Bradford reagent was added, and 15 min later a microplate reader BioTek Instruments, Inc. Winooski, VT, USA was used to record the absorbance at 595 nm. Albumin was used to create a standard curve.
Lipid Extraction
Membrane fractions from HeLa and Caco-2 cell lines were submitted to a solvent-based extraction using an adaption of the Folch method (Folch et al. 1957). After protein measurement, samples were evenly diluted to the same protein concentration. Five milliliters of chloroform/methanol (2:1) were added to the samples and they were kept under agitation for 1 h at room temperature. Afterward, 1 mL of NaCl 0.9% was added, samples were mixed using a vortex, and centrifuged at 670×g for 20 min. To improve phase separation, samples were kept resting for 30 min at room temperature. The organic phase was separated from the water-soluble phase, followed by the addition of methanol/water (1:1) (v/v) to the organic phase and chloroform (v/v) to the water-soluble phase. Samples were mixed again in a vortex and kept at 4 °C overnight. The water-soluble phase was discarded, and the organic phase was concentrated in rotary evaporator, followed by suspension in 300 µL of chloroform. Lipid extracts were stored at − 20 °C.
Cholesterol Measurement
Cholesterol content was determined using the Higgins method (1987) based on cholesterol complexation with ferric chloride (FeCl3⋅6H2O) (Higgins 1987). Forty microliters of lipid extract were dried under a nitrogen stream, followed by addition of 750 µL of acetic acid and 500 µL of reagent B composed of reagent A (2.5 g of FeCl3·6H2O in 100 mL of orthophosphoric acid 85%) in 46 mL of sulfuric acid. Samples were mixed in a vortex and kept at room temperature for 10 min. Finally, the absorbance was recorded in a spectrophotometer at 550 nm. The cholesterol content was calculated based on a cholesterol standard curve.
Phospholipid Measurement
Phospholipid content was determined using the Chen method (Chen et al. 1956). Forty microliters of lipid extract were dried under a nitrogen stream, followed by digestion with 500 µL of nitric acid 65% (v/v) in an incubator at 120 °C. Then, 500 µL of distilled water and 1 mL of Chen reagent were added to the samples. Afterward, samples were kept in a water bath at 45 °C for 20 min, followed by the spectrophotometry reading at 820 nm. A standard curve was created using sodium dihydrogen phosphate for calculating phospholipid content. Chen reagent was prepared by adding ascorbic acid to the solution B (1:6). Solution B was prepared diluting 1.25 g of ammonium molybdate in 30 mL of ultrapure water, followed by addition of 7.3 mL of sulfuric acid, and finally the volume was completed to 300 mL with ultrapure water.
Identification of Membrane Phospholipids
After the solvent-based lipid extraction from plasma membrane, samples were dried under a nitrogen stream, and suspended to 20 µL of chloroform/methanol (1:1). Phospholipid identification was performed using a one-dimensional high performance thin-layer chromatography (HPTLC) in silica gel 60 plates. Samples and phospholipid standards were applied to the plates at 1 cm from the margin (origin) and 0.5 cm from each other. The solvent system was composed of acetone:methanol:acetic acid:chloroform:water (15:13:12:40:8) and the plates were kept in a chamber for approximately 30 to 40 min (Ruiz and Ochoa 1997). The run was performed up to 1 cm of the superior margin (ending). The plates were developed using the Charring reagent (CuSO4 10% in H3PO4 8%) for 10 min at 200 °C. Then, plates were digitalized in a scanner and submitted to a densitometry analysis using the software Image Master Total Lab version 1.11 (Amersham Pharmacia Biotech). Phospholipids were analyzed according to the migration pattern of their corresponding standards (phosphatidylethanolamine, phosphatidylcholine, phosphatidylinositol, sphingomyelin).
Filipin Staining
Cholesterol localization was evaluated by filipin staining, a cholesterol probe (Carozzi et al. 2000). An amount of 2 × 105 cells was cultured in coverslips up to 80% confluence, followed by treatment with ouabain 10 nM or 21-BD 50 µM. Afterward, cells were fixed with 4% paraformaldehyde in PBS—Ca2+/Mg2+ (Ca2+ 0.1 mM and Mg2+ 1 mM) for 30 min at room temperature and the aldehyde groups quenched with NH4Cl 50 mM in PBS for 10 min. Then, cells were permeabilized with saponin 0.1% in PBS for 10 min and incubated in the dark with the filipin probe 0.05 mg/mL at room temperature for 1 h. Finally, coverslips were mounted onto slides, rinsed with PBS to remove filipin probe excess, and analyzed using a fluorescence microscope (Axio Vert. A1—Zen Imaging Software—ZEISS, Oberkochen, Germany).
Caveolin-1 Expression
Samples of membrane preparation, total extract, and cell fractionation were diluted in sample buffer (120 mM Tris–HCl [pH 6.8], 0.02% Bromophenol Blue, 10% 2-Mercaptoethanol, 20% Glycerol, and SDS 4%) for the same protein concentration. Subsequently, the samples were applied and separated by polyacrylamide gel electrophoresis. After running the samples, the proteins were transferred from the polyacrylamide gel to the nitrocellulose membrane in transfer buffer. The transfer efficiency was evaluated by staining the nitrocellulose membrane with Ponceau-Xylidine red solution. To block unspecific binding of the antibodies used, the membrane was blocked for 1 h with 5% BSA diluted in T-TBS (Composition of T-TBS-100 mM Tris-Base, 0.9% NaCl, and 0.1% Tween). Caveolin-1 was detected with primary anti-caveolin-1 antibody (ab 2910, Abcam) diluted in T-TBS (1:1000), and tubulin (T5168, Sigma Aldrich) diluted in T-TBS (1:3000). Then the membrane was incubated with secondary antibody and developed by chemiluminescence method. The bands corresponding to each sample were quantified by densitometry using the program Image J 1.47.
Statistical Analysis
One-way ANOVA followed by Tukey’s post hoc test was used to compare the sample means. The significance was set to p < 0.05. All analyses and graphs were performed using the software GraphPad Prism 5.
Results
Cell Viability After Ouabain and 21-BD Treatment
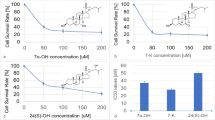
In previous studies, we have determined the IC50 of 21-BD for HeLa cells as 50 μM (Rocha et al. 2014). To define the concentrations to be used in the next experiments, we performed a cell viability assay after treatment of HeLa cells with increasing concentrations of ouabain, and after treatment of Caco-2 cells with increasing concentrations of ouabain or 21-BD. Ouabain showed a higher cytotoxicity on both cells when compared to 21-BD (Fig. 1), demonstrating a lower IC50 (50 nM in HeLa cells and 227 nM in the Caco-2 cells). For Caco-2 cells 21-BD was cytotoxic only with 500 μM.
Based on these results and considering that most studies involving signaling triggered by binding of CTS to Na,K-ATPase use concentrations of 10 and 100 nM ouabain, we have defined to use these concentrations in this study. Our previous studies, in different cell lines, have demonstrate that the significant effects caused by 21-BD occur at a concentration of 50 μM, even though each cell line has a different IC50 for 21-BD (Rocha et al. 2014; Silva et al. 2017). Therefore, we decided to continue the work using 50 μM of 21-BD for both cells.
Evaluation of Lipid Modulation on the Plasma Membrane
In vitro and in vivo experiments have shown that CTS may alter the lipid composition of plasma membrane (Garcia et al. 2015, 2018, 2019; Silva et al. 2017). We have demonstrated that treatment of HeLa cells with 21-BD caused a significant increase in the total phospholipid and cholesterol content of plasma membrane (Silva et al. 2017). For this reason, we investigated here whether treatment with ouabain or 21-BD for 48 h would have the same effect in Caco-2 cells. No significant alterations were observed in the total content of phospholipids and cholesterol in plasma membrane after treatment with ouabain (Fig. 2A and B).
Total lipid content of the cell membrane from Caco-2 cells. Total phospholipid (a) and cholesterol content (b) after treatment with different concentrations of ouabain for 48 h. Total phospholipid (c) and cholesterol content (d) after treatment with different concentrations of 21-BD for 48 h. *p < 0.05 n = 3
On the other hand, 21-BD (5 μM) treatment showed a significant increase in total phospholipid content in Caco-2 cell membrane compared to control group. The membrane cholesterol content was not altered after treatment with 21-BD (Fig. 2C and D). Moreover, ouabain treatment on HeLa cells did not cause changes in the lipid profile of plasma membrane (Fig. 3).
Thus, our data show different effects for two CTS on the membrane lipid profile of human cells. Only 21-BD demonstrated significant changes in total cellular lipid content of the plasma membrane. Interestingly, the cholesterol content was altered only in the caveolae-containing HeLa cell line.
Even though ouabain did not provoke an alteration in total membrane phospholipid, changes of specific phospholipids could occur. Therefore, considering changes in phospholipids caused by the treatment with 21-BD on both cell lines, we assessed the contents of specific phospholipids, such as phosphatidylethanolamine, phosphatidylcholine, phosphatidylinositol, and sphingomyelin (Figs. 4 and 5). We did not identify any significant differences in plasma membrane phospholipids from both Caco-2 and HeLa cell lines after treatment with CTS.
Specific phospholipid composition of cell membrane from Caco-2 cells treated with different concentrations of ouabain or 21-BD for 48 h. Membrane fractions were submitted to a lipid extraction and analyzed using HPTLC. Phosphatidylethanolamine (A), phosphatidylcholine (B), phosphatidylinositol (C), sphingomyelin (D). p > 0.05 n = 3
Specific phospholipid composition of cell membrane from HeLa cells treated with different concentrations of ouabain or 21-BD for 48 h. Membrane fractions were submitted to a lipid extraction and analyzed using HPTLC. Phosphatidylethanolamine (A), phosphatidylcholine (B), phosphatidylinositol (C), sphingomyelin (D). p > 0.05 n = 3
Cholesterol Distribution
One important effect to consider is whether an increment or redistribution of cholesterol would induce caveolae formation and, therefore, a stronger signaling. We investigated the cellular distribution of cholesterol using filipin, a fluorescent antimycotic that binds to membrane domains rich in cholesterol, in HeLa and Caco-2 cells, after treatment with ouabain or 21-BD for 48 h. In non-treated cells we observed a high fluorescent signal on the plasma membrane, reflecting the normal subcellular localization of cholesterol. Treatment with ouabain 10 nM affected neither the intensity nor the distribution of cholesterol in both cell lines. On the other hand, treatment with 50 μM of 21-BD on HeLa cells markedly increased the fluorescence intensity in the perinuclear region. In Caco-2 cells, fluorescence was also detected in the intracellular vesicles displaying a characteristic ‘‘chicken fence’’-like pattern, while fluorescence on the plasma membrane was similar to that in non-treated cells (Fig. 6).
CTS effect on cholesterol distribution. HeLa and Caco-2 cells were treated with ouabain 10 nM or 21-BD 50 µM for 48 h. Images were obtained at ×40 magnification by fluorescence microscopy after incubation with filipin. Arrows point to intracellular filipin signals. Images were edited using the software Image J 1.51 k. n = 3
Caveolin-1 Expression
21-BD increases the plasma membrane cholesterol of the caveolae-containing HeLa cells. Therefore, Cav-1 may be involved in regulating metabolism and cholesterol trafficking to the plasma membrane and caveolae in these cells. Thus, we evaluated the expression of this protein in HeLa cells treated with 21-BD (Fig. 7). Our data show that neither 21-BD nor ouabain alter the expression of Cav-1. It is important to note that this data were obtained through the analysis of the total extract of these cells and not membrane preparations.
Discussion
Changes in the composition of phospholipids and cholesterol can lead to alterations in plasma membrane fluidity and may impair crucial cellular functions. There are few studies about the lipid modulation caused by CTS. Oleandrin has been shown to induce changes in membrane fluidity of different cell lines (Manna et al. 2006; Raghavendra et al. 2007). In vivo studies with male Wistar rats have demonstrated that ouabain was able to cause an increase in the total phospholipid content from the hippocampus (Garcia et al. 2015). Interestingly, the ouabain effect appears to be different depending on the region of the brain, since it caused a decrease in the levels of total membrane phospholipids in the cerebellum (Garcia et al. 2018). In these neuronal tissues, no changes in membrane cholesterol levels were found (Garcia et al. 2015, 2018). In a previous study by our research group, we have demonstrated that 21-BD significantly increased the total phospholipids and membrane cholesterol contents in HeLa cells. As a consequence of this modulation, 21-BD decreased the Na,K-ATPase activity most likely due to alterations of the membrane fluidity of HeLa cells (Silva et al. 2017).
The present data demonstrated that ouabain treatment had no effect on the phospholipid content of plasma membrane from HeLa and Caco-2 cells. However, 21-BD caused a significant increase in total phospholipid content of Caco-2 cells (Figs. 2 and 3) and the same effect has also been previously demonstrated in HeLa cells (Silva et al. 2017).
We identified the main classes of plasma membrane phospholipids in HeLa and Caco-2 cells, such as phosphatidylethanolamine, phosphatidylcholine, phosphatidylinositol, and sphingomyelin. Surprisingly, we did not find any significant changes in the content of these specific phospholipids (Figs. 4 and 5). Reports in the literature have demonstrated that CTS can alter the content of specific phospholipids. For instance, an ouabain treatment caused alterations of the phosphatidylinositol and sphingomyelin content in prostate cancer cells (PC-3) (Gasper et al. 2011). In vivo experiments have demonstrated that ouabain increased the turnover of phosphatidic acid in the brain from guinea pigs (Yoshida et al. 1961) and rabbits (Nicholls et al. 1962). Moreover, ouabain was able to increase the contents of phosphatidylinositol, phosphatidylethanolamine, and phosphatidylcholine in rabbit cerebral cortex slices (Nicholls et al. 1962). Another study has described that an ouabain injection increased the content of phosphatidylethanolamine, phosphatidylcholine, and phosphatidylinositol in the hippocampus of Wistar rats (Garcia et al. 2019).
In our study, the treatment with ouabain caused no changes in the total or specific phospholipid contents. On the other hand, 21-BD caused an increase of total membrane phospholipid content of Caco-2 cells in the same extent as reported in our previous work for HeLa cells (Silva et al. 2017). We expected to find differences in the content of specific phospholipids after treatment with 21-BD; however, no significant alterations were observed. In addition to the phospholipids that we have analyzed, there are other subtypes present in the cell membrane, such as phosphatidylserine, lysophosphatidylcholine, phosphatidylglycerol, and phosphatidic acid that are important for cellular functions (Zegarlinska et al. 2018). We believe that the high phospholipid content induced by 21-BD described in the present study could be the effect of those other phospholipids. For example, 21-BD treatment could be causing an increase of activity of enzymes involved in the hydrolysis of membrane phospholipids, such as phospholipase D and lysophospholipases, leading to an increase of lysophospholipid levels that we were unable to identify in this study.
Ouabain is the most used CTS in kinetic studies and cell signaling involving the Na,K-ATPase. Moreover, it has been described that Na,K-ATPase function depends on “annular lipids”, and also, the presence of sites of interaction between the membrane lipids and the enzyme (Contreras et al. 2011; Cornelius 2001; Cornelius et al. 2003). There are specific binding sites within the enzyme able to interact with phosphatidylserine, phosphatidylcholine, phosphatidylethanolamine, sphingomyelin, and cholesterol, all of them required to maintain the enzyme stability, and to stimulate and inhibit its catalytic activity (Cornelius et al. 2015; Habeck et al. 2015, 2017). Thus, alterations in the lipid environment of the enzyme could modify its function (Garcia et al. 2015, 2018, 2019; Silva et al. 2017). Therefore, ouabain at the concentrations used in this study does not cause lipid changes in the plasma membrane that could impair kinetic and signaling studies.
By using a cholesterol probe (filipin), we demonstrated here that ouabain does not alter the cholesterol composition of the plasma membrane of HeLa and Caco-2 cells. However, in both cell lines treated with 21-BD, we could observe changes in cholesterol distribution compared to non-treated cells (Fig. 6). In Caco-2 cells, intracellular vesicles containing cholesterol were observed, whereas in HeLa cells, an increase of the cholesterol in the perinuclear region could be observed, and perhaps in the total cellular cholesterol content. However, in both of the cells, the cholesterol content of the plasma membrane seems to be similar to that in non-treated cells.
The differences in the effect of ouabain and 21-BD in HeLa and Caco-2 cells, in relation to the changes caused in the content of cholesterol and phospholipids, are probably due to differences in the interaction of the molecules with the Na,K-ATPase. In molecular docking studies it was shown that 21-BD interacts at sites other than ouabain in the α1 subunit of Na,K-ATPase. The aromatic ring attached to the lactone ring in 21-BD helps the molecule to interact with a hydrophobic pocket formed by Ala330, Glu786, Ph790, Leu800, Ile807. The lactone ring of ouabain interacts with Val329 and Gly803 by electrostatic interactions. Hydrogen bonds are formed between C12 of the molecule and residue Thr804, while ouabain interacts through hydrogen bonds between C11α, C19α, and C14β with residues Arg118, Asp128, and Thr804, respectively. The presence of 2 extra distal sugar units in the molecule causes it to establish new polar interactions with Asp892 and Arg893, as well as hydrogen bonds with Trp984. Thus, all these different features observed at the molecular level about the interaction of 21-BD with Na,K-ATPase, compared to ouabain, could be the reason why the effects observed with 21-BD are different from those observed with ouabain. Furthermore, these differences are evidenced by the absence of any modulatory effect of 21-BD, at nanomolar concentrations, on the Na,K-ATPase activity (Pessôa et al. 2018).
In HeLa cells, an important increase in fluorescence that would correspond to an increase in intracellular cholesterol in the perinuclear region was observed. This finding confirms the increase of total cholesterol in cell lysates and the increase of the plasma membrane cholesterol in plasma membrane fractions, demonstrated previously by us in HeLa cells (Silva et al. 2017). These cells present caveolae, in contrast with Caco-2 cells that do not. Therefore, it is important to address whether caveolae could be involved in the CTS modulation of the lipid environment of the cells.
Caveolin-1 plays an important role in the cholesterol homeostasis, being involved in the trafficking of newly synthesized cholesterol to the membrane (Smart et al. 1996) and modulating the activity of other proteins that are involved in the regulation of intracellular cholesterol. Caveolin-1 binds directly to cholesterol and transports it to the membrane. This interaction, of caveolin-1 with cholesterol, is important to form the caveolae microdomains (Cai et al. 2008).
In fact, the formation and maintenance of caveolae depends on the membrane and intracellular cholesterol levels. The plasma membrane cholesterol levels can regulate Cav-1 expression, where the cholesterol depletion can disrupt the formation of caveolae and, consequently, reduce the number of these structures and the expression of caveolin-1 in cells (Hailstones et al. 1998). In addition, the inhibition of cholesterol biosynthesis leads to a marked reduction in cholesterol content causing disruption of the lipid rafts, with redistribution of caveolin-1 (Sánchez-Wandelmer et al. 2009). On the other hand, intracellular accumulation of cholesterol promotes Cav-1 expression and Cav-1 directs the trafficking of cholesterol to caveolae, in response to increased intracellular cholesterol uptake or synthesis (Frank et al. 2006). Therefore, Cav-1 expression can be regulated by cholesterol and this regulation is reciprocal (Fu et al. 2004; Hailstones et al. 1998). It has been observed an increase in cholesterol in cells overexpressed with exogenous Cav-1, whereas Cav-1 knockout mouse embryonic fibroblasts (MEFs) and mouse peritoneal macrophages (MPMs) was associated with reduced free cholesterol synthesis but increased acyl-CoA:cholesterol acyl-transferase (ACAT) activity (Frank et al. 2006). Thus, differences in the caveolar content in the two cells analyzed in this work could be one of the reasons why these cells present different responses in relation to cholesterol content after treatment with 21-BD.
Another relevant point is that the interaction between Na,K-ATPase and Cav-1 is important for the maintenance of the membrane cholesterol homeostasis (Cai et al. 2008; Chen et al. 2009, 2011). Downregulation of Na,K-ATPase redistributes the cholesterol from the plasma membrane to the cytosol, and decreased expression of caveolin-1 from LLC-PK1 cells also alters the cholesterol metabolism in mice α1+/– (Chen et al. 2009). Cholesterol depletion stimulates the endocytosis and degradation of α1 through a Src-dependent pathway, and consequently decreases the expression of α1 Na,K-ATPase (Chen et al. 2011). This data shows that this regulation between α1 expression and the cholesterol content is mutual. The interaction of Na,K-ATPase and Src is important for the regulation of the trafficking of Cav-1 and cholesterol to the cell membrane. Cholesterol specifically regulates the expression of Na,K-ATPase α1 subunit and the complex Na,K-ATPase/Src/caveolin-1 is essential for this regulation (Zhang et al. 2020).
Our data show that Cav-1 expression was not altered after treating HeLa cells with 21-BD (Fig. 7); however, this was observed in total cell lysates and not membrane fractions. In a previous study, we have observed an increase in the levels of α1 subunit mRNA in HeLa cells treated with 50 μM of 21-BD (Rocha et al. 2014). However, we have demonstrated that the expression of the Na,K-ATPase in HeLa cells after treatment with 21-BD remained unchanged in the plasma membrane (Silva et al. 2017). Therefore, interestingly in our hands, the increasing cholesterol levels in HeLa cells did not alter the Na,K-ATPase/Cav-1 expression.
The mechanism by which 21-BD affects cell lipids is not well understood. We know that the lipid changes caused by 21-BD, is not due to a direct action of the compound on the membrane. This was demonstrated in a previous study where human erythrocyte membrane fractions were treated with 21-BD. In this case, the content of cholesterol and phospholipids was not changed (Silva et al. 2017). Perhaps, treatment of HeLa cells with 21-BD alters the cholesterol homeostasis by increasing the synthesis or uptake of this lipid. CTS, such as digoxin and ouabain, can modulate the cholesterol content by interfering with the lipid metabolism. Studies have shown that CTS stimulated the cholesterol synthesis in different cell types (liver cells—HepG2, colon cancer cells—HT29, leukemia cells—THP-1, and cardiomyoblasts—H9c2). Both CTS increased the cholesterol synthesis by increasing the activity and expression of HMG-CoA reductase (Campia et al. 2009, 2012).
Some studies have shown that endogenous and exogenous CTS can interact with nuclear receptors causing different effects on processes regulated by gene transcription (Karaś et al. 2020). For instance, RORγ has been found to be involved in the control of several metabolic pathways, including lipid metabolism by positively regulating the transcription of genes related to lipid metabolism (Takeda et al. 2014; Urlep et al. 2017). It has been shown that some CTS, such as digoxin and synthetic analogues derived from digoxin, can interact with RORγ (Karaś et al. 2018), but not ouabain. CTS can act as receptor antagonists (Huh et al. 2011; Wu et al. 2013) or agonists as demonstrated by other authors (Huh et al. 2011). In addition, intermediaries in the cholesterol biosynthesis pathway can bind to RORγ and increase its activity (Zou et al. 2021). Thus, we can also raise the possibility of involvement of the orphan receptor related to retinoid acid (RORγ) in the modulation of lipid content in HeLa cells, which would be interesting to investigate to better understand the mechanisms of 21-BD to increase cholesterol levels.
Conclusion
21-BD caused important lipid changes in cells, although the major changes in the lipid content were observed predominantly in HeLa cells that produce caveolae, even though the Cav-1 expression was not increased in these cells. Therefore, 21-BD does not increase the cholesterol content by increasing the expression of Cav-1 and the increase in cholesterol does not cause an increase in the expression of Cav-1.
21-BD was able to increase phospholipids in Caco-2 cells. In addition, ouabain was not able to cause modification in the lipid content of any cell line in this study. We could observe the same effect in a previous study using digoxin in HeLa cells. Apparently, the additional styrene group on the lactone ring of 21-BD structure promotes its ability to affect the lipid content of these cells. This could be of relevance to develop new compounds focused on the modulation of cholesterol and phospholipids as a mechanism of action to prevent diseases.
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Breuza L, Corby S, Arsanto JP, Delgrossi MH, Scheiffele P, Le Bivic A (2002) The scaffolding domain of caveolin 2 is responsible for its Golgi localization in Caco-2 cells. J Cell Sci 115:4457–4467
Cai T, Wang H, Chen Y, Liu L, Gunning WT, Quintas LE, Xie ZJ (2008) Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J Cell Biol 182:1153–1169
Campia I, Gazzano E, Pescarmona G, Ghigo D, Bosia A, Riganti C (2009) Digoxin and ouabain increase the synthesis of cholesterol in human liver cells. Cell Mol Life Sci 66:1580–1594
Campia I, Sala V, Kopecka J, Leo C, Mitro N, Costamagna C, Caruso D, Pescarmona G, Crepaldi T, Ghigo D, Bosia A, Riganti C (2012) Digoxin and ouabain induce the efflux of cholesterol via liver X receptor signalling and the synthesis of ATP in cardiomyocytes. Biochem J 447:301–311
Carozzi AJ, Ikonen E, Lindsay MR, Parton RG (2000) Role of cholesterol in developing T-tubules: analogous mechanisms for T-tubule and caveolae biogenesis. Traffic 1:326–341
Chen PS, Toribara TY, Warner H (1956) Microdetermination of phosphorus. Anal Chem 28:1756–1758
Chen JQ, Contreras RG, Wang R, Fernandez SV, Shoshani L, Russo IH, Cereijido M, Russo J (2006) Sodium/potassium ATPase (Na+, K+-ATPase) and ouabain/related cardiac glycosides: a new paradigm for development of anti-breast cancer drugs? Breast Cancer Res Treat 96:1–15
Chen Y, Cai T, Wang H, Li Z, Loreaux E, Lingrel JB, Xie Z (2009) Regulation of intracellular cholesterol distribution by Na/K-ATPase. J Biol Chem 284:14881–14890
Chen Y, Li X, Ye Q, Tian J, Jing R, Xie Z (2011) Regulation of alpha1 Na/K-ATPase expression by cholesterol. J Biol Chem 286:15517–15524
Contreras FX, Ernst AM, Wieland F, Brugger B (2011) Specificity of intramembrane protein-lipid interactions. Cold Spring Harb Perspect Biol 3
Cornelius F (2001) Modulation of Na, K-ATPase and Na-ATPase activity by phospholipids and cholesterol I. Steady-state kinetics. Biochemistry 40:8842–8851
Cornelius F, Turner N, Christensen HR (2003) Modulation of Na, K-ATPase by phospholipids and cholesterol II. Steady-state and presteady-state kinetics. Biochemistry 42:8541–8549
Cornelius F, Habeck M, Kanai R, Toyoshima C, Karlish SJ (2015) General and specific lipid-protein interactions in Na,K-ATPase. Biochim Biophys Acta 1848:1729–1743
Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP (1997) Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem 272:6525–6533
de Souza WF, Barbosa LA, Liu L, de Araujo WM, de-Freitas-Junior, JC, Fortunato-Miranda, N, Fontes, CF, Morgado-Diaz, JA, (2014) Ouabain-induced alterations of the apical junctional complex involve alpha1 and beta1 Na, K-ATPase downregulation and ERK1/2 activation independent of caveolae in colorectal cancer cells. J Membr Biol 247:23–33
Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV (2001) Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293:2449–2452
Engelman DM (2005) Membranes are more mosaic than fluid. Nature 438:578–580
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Fra AM, Williamson E, Simons K, Parton RG (1995) De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci U S A 92:8655–8659
Frank GP, Cheung MWC, Pavlides S, Llaverias G, Park DS, Michael PL (2006) Caveolin-1 and regulation of cellular cholesterol homeostasis. Am J Physiol Heart Circ Physiol 291(2):667–686
Fu Y, Hoang A, Escher G, Parton RG, Krozowski Z, Sviridov D (2004) Expression of caveolin-1 enhances cholesterol efflux in hepatic cells. J Biol Chem 279:14140–14146
Garcia IJ, Kinoshita PF, Scavone C, Mignaco JA, Barbosa LA, Santos Hde L (2015) Ouabain modulates the lipid composition of hippocampal plasma membranes from rats with LPS-induced neuroinflammation. J Membr Biol 248:1191–1198
Garcia IJP, Kinoshita PF, de Oliveira Braga I, Parreira GM, Mignaco JA, Scavone C, Barbosa LA, de Lima Santos H (2018) Ouabain attenuates the oxidative stress induced by lipopolysaccharides in the cerebellum of rats. J Cell Biochem 119:2156–2167
Garcia IJP, Kinoshita PF, Silva L, De Souza Busch M, Atella GC, Scavone C, Cortes VF, Barbosa LA, De Lima Santos H (2019) Ouabain attenuates oxidative stress and modulates lipid composition in hippocampus of rats in lipopolysaccharide-induced hypocampal neuroinflammation in rats. J Cell Biochem 120:4081–4091
Gargalovic P, Dory L (2003) Cellular apoptosis is associated with increased caveolin-1 expression in macrophages. J Lipid Res 44:1622–1632
Gasper R, Vandenbussche G, Goormaghtigh E (2011) Ouabain-induced modifications of prostate cancer cell lipidome investigated with mass spectrometry and FTIR spectroscopy. Biochim Biophys Acta 1808:597–605
Grande-Garcia A, Echarri A, de Rooij J, Alderson NB, Waterman-Storer CM, Valdivielso JM, del Pozo MA (2007) Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J Cell Biol 177:683–694
Habeck M, Haviv H, Katz A, Kapri-Pardes E, Ayciriex S, Shevchenko A, Ogawa H, Toyoshima C, Karlish SJ (2015) Stimulation, inhibition, or stabilization of Na, K-ATPase caused by specific lipid interactions at distinct sites. J Biol Chem 290:4829–4842
Habeck M, Kapri-Pardes E, Sharon M, Karlish SJ (2017) Specific phospholipid binding to Na, K-ATPase at two distinct sites. Proc Natl Acad Sci USA 114:2904–2909
Hailstones D, Sleer LS, Parton RG, Stanley KK (1998) Regulation of caveolin and caveolae by cholesterol in MDCK cells. J Lipid Res 39:369–379
Han F, Zhang L, Zhou Y, Yi X (2015) Caveolin-1 regulates cell apoptosis and invasion ability in paclitaxel-induced multidrug-resistant A549 lung cancer cells. Int J Clin Exp Pathol 8:8937–8947
Higgins JA (1987) Separation and analysis of membrane lipid components. In: Findlay JBC, Evans WH (eds) Biological membranes: a practical approach. IRL Press, Oxford, pp 103–137
Hirama T, Das R, Yang Y, Ferguson C, Won A, Yip CM, Kay JG, Grinstein S, Parton RG, Fairn GD (2017) Phosphatidylserine dictates the assembly and dynamics of caveolae in the plasma membrane. J Biol Chem 292:14292–14307
Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, Chow J, Manel N, Ciofani M, Kim SV, Cuesta A, Santori FR, Lafaille JJ, Xu HE, Gin DY, Rastinejad F, Littman DR (2011) Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 472:486–490
Karaś K, Sałkowska A, Walczak-Drzewiecka A, Ryba K, Dastych J, Bachorz RA, Ratajewski M (2018) The cardenolides strophanthidin, digoxigenin and dihydroouabain act as activators of the human RORγ/RORγT receptors. Toxicol Lett 295:314–324
Karaś K, Sałkowska A, Dastych J, Bachorz RA, Ratajewski M (2020) Cardiac glycosides with target at direct and indirect interactions with nuclear receptors. Biomed Pharmacother 127:110106
Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, Xie ZJ (2007) Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem 282:10585–10593
Liu L, Ivanov AV, Gable ME, Jolivel F, Morrill GA, Askari A (2011) Comparative properties of caveolar and noncaveolar preparations of kidney Na+/K+-ATPase. Biochemistry 50:8664–8673
Manna SK, Sreenivasan Y, Sarkar A (2006) Cardiac glycoside inhibits IL-8-induced biological responses by downregulating IL-8 receptors through altering membrane fluidity. J Cell Physiol 207:195–207
Marquardt D, Geier B, Pabst G (2015) Asymmetric lipid membranes: towards more realistic model systems. Membranes (Basel) 5:180–196
Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K (1995) VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A 92:10339–10343
Nicholls D, Kanfer J, Titus E (1962) The effect of ouabain on the incorporation of inorganic P32 into phospholipid. J Biol Chem 237:1043–1049
Nicolson GL (2014) The Fluid-Mosaic Model of Membrane Structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim Biophys Acta 1838:1451–1466
Nunez-Wehinger S, Ortiz RJ, Diaz N, Diaz J, Lobos-Gonzalez L, Quest AF (2014) Caveolin-1 in cell migration and metastasis. Curr Mol Med 14:255–274
Okamoto T, Schlegel A, Scherer PE, Lisanti MP (1998) Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 273:5419–5422
Parton RG, del Pozo MA (2013) Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol 14:98–112
Pessôa MTC, Alves SLG, Taranto AG, Villar JAFP, Blanco G, Barbosa LA (2018) Selectivity analyses of γ-benzylidene digoxin derivatives to different Na, K-ATPase α isoforms: a molecular docking approach. J Enzyme Inhib Med Chem 33:85–97
Quintas LE, Pierre SV, Liu L, Bai Y, Liu X, Xie ZJ (2010) Alterations of Na+/K+-ATPase function in caveolin-1 knockout cardiac fibroblasts. J Mol Cell Cardiol 49:525–531
Raghavendra PB, Sreenivasan Y, Manna SK (2007) Oleandrin induces apoptosis in human, but not in murine cells: dephosphorylation of Akt, expression of FasL, and alteration of membrane fluidity. Mol Immunol 44:2292–2302
Rocha SC, Pessoa MT, Neves LD, Alves SL, Silva LM, Santos HL, Oliveira SM, Taranto AG, Comar M, Gomes IV, Santos FV, Paixao N, Quintas LE, Noel F, Pereira AF, Tessis AC, Gomes NL, Moreira OC, Rincon-Heredia R, Varotti FP, Blanco G, Villar JA, Contreras RG, Barbosa LA (2014) 21-Benzylidene digoxin: a proapoptotic cardenolide of cancer cells that up-regulates Na, K-ATPase and epithelial tight junctions. PLoS ONE 9:e108776
Ruiz JI, Ochoa B (1997) Quantification in the subnanomolar range of phospholipids and neutral lipids by monodimensional thin-layer chromatography and image analysis. J Lipid Res 38:1482–1489
Sánchez-Wandelmer J, Dávalos A, Herrera E, Giera M, Cano S, de la Peña G, Lasunción MA, Busto R (2009) Inhibition of cholesterol biosynthesis disrupts lipid raft/caveolae and affects insulin receptor activation in 3T3-L1 preadipocytes. Biochim Biophys Acta 1788(9):1731–1739
Silva LND, Pessoa MTC, Alves SLG, Venugopal J, Cortes VF, Santos HL, Villar J, Barbosa LA (2017) Differences of lipid membrane modulation and oxidative stress by digoxin and 21-benzylidene digoxin. Exp Cell Res 359:291–298
Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387:569–572
Simons K, van Meer G (1988) Lipid sorting in epithelial cells. Biochemistry 27:6197–6202
Singer SJ, Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175:720–731
Smart EJ, Ying Y, Donzell WC, Anderson RG (1996) A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem 271:29427–29435
Stucky MA, Goldberger ZD (2015) Digoxin: its role in contemporary medicine. Postgrad Med J 91:514–518
Takeda Y, Kang HS, Lih FB, Jiang H, Blaner WS, Jetten AM (2014) Retinoid acid-related orphan receptor γ, RORγ, participates in diurnal transcriptional regulation of lipid metabolic genes. Nucleic Acids Res 42:10448–10459
Thomas R, Gray P, Andrews J (1990) Digitalis: its mode of action, receptor, and structure-activity relationships. In: Testa B (ed) Advances in drug research. Academic Press, Philadelphia, pp 311–562
Torres VA, Tapia JC, Rodriguez DA, Parraga M, Lisboa P, Montoya M, Leyton L, Quest AF (2006) Caveolin-1 controls cell proliferation and cell death by suppressing expression of the inhibitor of apoptosis protein survivin. J Cell Sci 119:1812–1823
Urlep Ž, Lorbek G, Perše M, Jeruc J, Juvan P, Matz-Soja M, Gebhardt R, Björkhem I, Hall JA, Bonneau R, Littman DR, Rozman D (2017) Disrupting hepatocyte Cyp51 from cholesterol synthesis leads to progressive liver injury in the developing mouse and decreases RORC signalling. Sci Rep 7:40775
Vogel U, Sandvig K, van Deurs B (1998) Expression of caveolin-1 and polarized formation of invaginated caveolae in Caco-2 and MDCK II cells. J Cell Sci 111(Pt 6):825–832
Wu J, Zhou C, Chen W, Xie A, Li J, Wang S, Ye P, Wang W, Xia J (2013) Digoxin attenuates acute cardiac allograft rejection by antagonizing RORγt activity. Transplantation 95:434–441
Xie Z, Askari A (2002) Na(+)/K(+)-ATPase as a signal transducer. Eur J Biochem 269:2434–2439
Yoshida H, Nukada T, Fujisawa H (1961) Effect of ouabain on ion transport and metabolic turnover of phospholipid of brain slices. Biochim Biophys Acta 48:614–615
Zegarlinska J, Piascik M, Sikorski AF, Czogalla A (2018) Phosphatidic acid: a simple phospholipid with multiple faces. Acta Biochim Pol 65:163–171
Zhang J, Li X, Yu H, Larre I, Dube P, Kennedy DJ, Tang WHW, Westfall K, Pierre SV, Xie Z, Chen Y (2020) Regulation of Na/K-ATPase expression by cholesterol: isoform specificity and the molecular mechanism. Am J Physiol Cell Physiol 319:1107
Zou H, Yang N, Zhang X, Chen HW (2021) RORγ is a context-specific master regulator of cholesterol biosynthesis and an emerging therapeutic target in cancer and autoimmune diseases. Biochem Pharmacol 9:114725
Acknowledgements
This work was funded by FAPEMIG (Fundação de Amparo a Pesquisa do Estado de Minas Gerais) APQ-00290-16, PPM-00307-18, APQ-00855-19; CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) Finance Code 01, and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) 305173/2018-9, 409436/2016-0.
Author information
Authors and Affiliations
Contributions
LNDS, IJPG wrote the main manuscript text. LNDS and IJPG performed the lipids and filipin experiments, JMMV and MMT performed the caveolae blotting, MTCP performed MTT experiments, MVM performed the 21-BD synthesis, MSB and IR performed the quantification of phospholipids. JAFPV, GCA, VFC, HLS, and LAB reviewed all the experiments, mentored the students, and reviewed the manuscript. GCA and LAB supported the paper with grants.
Corresponding authors
Ethics declarations
Conflict of interest
No conflicts of interest.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Silva, L.N.D., Garcia, I.J.P., Valadares, J.M.M. et al. Evaluation of Cardiotonic Steroid Modulation of Cellular Cholesterol and Phospholipid. J Membrane Biol 254, 499–512 (2021). https://doi.org/10.1007/s00232-021-00203-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-021-00203-z