Abstract
The phosphatidylinositol cycle (PI-cycle) has a central role in cell signaling. It is the major pathway for the synthesis of phosphatidylinositol and its phosphorylated forms. In addition, some lipid intermediates of the PI-cycle, including diacylglycerol and phosphatidic acid, are also important lipid signaling agents. The PI-cycle has some features that are important for the understanding of its role in the cell. As a cycle, the intermediates will be regenerated. The PI-cycle requires a large amount of metabolic energy. There are different steps of the cycle that occur in two different membranes, the plasma membrane and the endoplasmic reticulum. In order to complete the PI-cycle lipid must be transferred between the two membranes. The role of the Nir proteins in the process has recently been elucidated. The lipid intermediates of the PI-cycle are normally highly enriched with 1-stearoyl-2-arachidonoyl molecular species in mammals. This enrichment will be retained as long as the intermediates are segregated from other lipids of the cell. However, there is a significant fraction (>15 %) of lipids in the PI-cycle of normal cells that have other acyl chains. Phosphatidylinositol largely devoid of arachidonoyl chains are found in cancer cells. Phosphatidylinositol species with less unsaturation will not be as readily converted to phosphatidylinositol-3,4,5-trisphosphate, the lipid required for the activation of Akt with resulting effects on cell proliferation. Thus, the cyclical nature of the PI-cycle, its dependence on acyl chain composition and its requirement for lipid transfer between two membranes, explain many of the biological properties of this cycle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Lipid Synthesis and Signaling Lipids
Lipids serve many roles in biology. The synthesis of lipids to form cell membranes is often the rate-limiting step in cell proliferation. Phospholipids are major constituents of cell membranes and they have roles in the formation of membrane domains and in the modulation of the activity of membrane proteins. Triglycerides form fat bodies that can be later oxidized as a source of metabolic energy. There are many pathways for lipid synthesis and many species of signaling lipids.
In the present review we will focus on the phosphatidylinositol cycle (PI-cycle) that is the major pathway for the synthesis of phosphatidylinositol (PI). This cycle also plays a major role in lipid signaling. We will discuss some of the properties of the PI-cycle that are important for signal transduction. These features include the fact that this is a metabolic cycle with the consequence that intermediates of the cycle will perform a catalytic role and will tend to remain at a constant concentration. Thermodynamics requires that the cycle function in only one direction since it consumes a large amount of energy. As a metabolic cycle it may be unique in requiring two different membranes. Hence the transfer of lipids between these two membranes becomes an important step in the cycle. In normal cells the cycle enriches lipid intermediates with 1-stearoyl-2-arachidonoyl acyl chains, but it can also produce other molecular species of PI. In order to maintain a particular acyl chain composition, the lipid intermediates of the PI-cycle must be isolated from other lipids of the same type that are present in the cell.
The Phosphatidylinositol Cycle
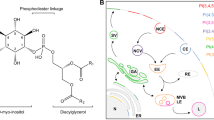
The PI-cycle is a series of enzyme-catalyzed biochemical reactions that form a cyclical process, such that all of the intermediates of the cycle are regenerated each time the cycle goes around once. There are many known biochemical metabolic cycles and as cycles they all have the property that each intermediate in the cycle has a catalytic role in accelerating the cycle, while the intermediate itself is never depleted or increased in amount. The particular example of the PI-cycle is shown in Fig. 1.
The PI-cycle is unique among biochemical cycles in that it is not located in a single organelle or membrane within the cell. Phospholipase C (PLC) (1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase (EC:3.1.4.11)) and phosphatidylinositol-4-phosphate 5-kinase (PI4P5K) (EC:2.7.1.68) that are involved in the PI-cycle are located in the plasma membrane, while the enzymes CDP-DAG synthase (CDS) [Phosphatidate cytidylyltransferase (EC:2.7.7.41)] and PI synthase (PIS) [CDP-diacylglycerol-inositol 3-phosphatidyltransferase (EC:2.7.8.11)] are in the endoplasmic reticulum. This means that lipids have to be transferred between the endoplasmic reticulum and the plasma membrane in order to complete the cycle. Portions of the endoplasmic reticulum are juxtaposed closely to the plasma membrane (Fig. 2). The routes of lipid transfer will be discussed below.

Taken from English and Voeltz (2013) (Color figure online)
Close apposition between the endoplasmic reticulum and plasma membrane. a Schematic model showing the observed membrane contact sites between the endoplasmic reticulum and plasma membrane, as well as other organelles. b An electron microscope tomography of a yeast cell illustrating the close contact between the peripheral endoplasmic reticulum (shown in blue, labeled ER) and the plasma membrane (the dark edge, labeled as PM).
Each cycle of the PI-cycle converts 3 ATP + CTP + inositol to 3 ADP + CMP + pyrophosphate + inositol triphosphate. Thus the PI-cycle consumes a fair amount of energy and is required to “turn” in the clockwise direction, according to the way it is drawn in Fig. 1. Furthermore, one of the products of the PI-cycle, pyrophosphate, would be hydrolyzed by endogenous pyrophosphatases to inorganic phosphate, further making the cycle uni-directional.
The cycle also produces inositol triphosphate that is an important ligand in opening calcium channels in the endoplasmic reticulum. Hence, indirectly the PI-cycle contributes, in part, to the regulation of intracellular calcium levels. In addition to inositol triphosphate increasing cellular calcium levels by opening calcium channels in the endoplasmic reticulum, the loss of Ca2+ from the endoplasmic reticulum activates a Ca2+-sensor protein, Stim1 that in turn activates a store Orai1 Ca2+ channel in the plasma membrane, resulting in a further increase in the intracellular calcium through influx into the cell (Putney and Tomita 2012). PLC, an enzyme of the PI-cycle, can also activate certain transient receptor potential cation (TrpC) channels in the plasma membrane, likely as a result of changes in the PI-cycle intermediates PIP2 and DAG that are the substrate and product, respectively, of this enzyme. With some TrpC channels, DAG can act indirectly by activating PKC that in turn inhibits TrpC channels (Putney and Tomita 2012).
Lipids that are Derived from or form Intermediates of the PI-Cycle
Although there is no net synthesis or consumption of any of the lipid intermediates in the cycle, the lipid intermediates do have connections with other metabolic pathways that can consume or synthesize intermediates of the cycle (Fig. 3). For example, there are many sources of DAG including the cleavage of phospholipids by different isoforms of phospholipase C (PLC), the hydrolysis of PA by PA phosphohydrolases and lipid phosphate phosphatases, as a product in the synthesis of sphingomyelin from ceramide by sphingomyelin synthase and by the hydrolysis of triglycerides with lipases (Carrasco and Merida 2007). In addition, PA, which is an another intermediate in the PI-cycle, is the precursor for the formation of most phospholipids, including those not made through the PI-cycle. PA can also be hydrolyzed back to DAG, which is not the reverse of the step in the PI-cycle, since the hydrolysis of PA produces inorganic phosphate together with DAG and does not regenerate ATP. The lipid intermediate in the PI-cycle, CDP-DAG, is a precursor not only for the formation of PI but also for the formation of phosphatidylglycerol. In yeast CDP-DAG is also converted to phosphatidylserine. Many phosphorylated species of PI exist in the cell as lipid signaling agents. Only two of them, PI4P and PIP2, are intermediates in the PI-cycle. There can be interconversion among PI and its phosphorylated forms through the action of specific lipid kinases and phosphatases. Thus, in addition to the energy required to drive the whole PI-cycle, there can also be combinations of kinases and phosphatases that can catalyze the interconversion of PI or phosphorylated forms of PI with other more highly phosphorylated forms of PI. There is the possibility of futile cycling between any pair of phosphorylating and dephosphorylating reactions that will utilize additional ATP.
Some of the metabolic processes outside the PI-cycle in which lipid intermediates of the cycle participate. Lipids that are intermediates in the PI-cycle are in yellow ovals, lipids made from intermediates in the PI-cycle or lipids forming these intermediates are shown in pink rectangles. TAG triacylglycerol, SM sphingomyelin. CDP-DAG is synthesized by both CDS1 and CDS2. We suggest that the CDP-DAG formed by CDS1 results in the formation of phosphatidylglycerol, while that formed by CDS2 leads to the synthesis of PI. CDS does not catalyze the formation of these lipids, but is shown in brackets to distinguish which CDS isoform catalyzes the previous step (Color figure online)
An important connection with the PI-cycle for cell proliferation and cancer is the phosphorylation of PIP2 to PIP3 by the enzyme phosphatidylinositol 3-kinase (PI3K) (Phosphatidylinositol-4,5-bisphosphate 3-kinase, EC:2.7.11.1) and the hydrolysis of PIP3 to PIP2 by the enzyme Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) (Phosphatidylinositol-4,5-bisphosphate 3-kinase, EC 3.1.3.16, EC:3.1.3.48, EC:3.1.3.67). The interconversion among PI and phosphorylated forms of PI both within the PI-cycle and with reactions connected to the cycle is an important and complex aspect of lipid signaling and will be discussed in the next section in relation to the PI-cycle.
Phosphorylation of PI
The entire group of phosphorylated PI, having varying numbers and positions of phosphorylation on the inositol ring are referred to as PIP n . The concentration of PIP n species outside the PI-cycle is very low and does not remove a large fraction of lipid from the cycle (Table 1). Many species of PIP n play important roles in lipid signaling. Many forms of PIP n bind to specific sites on proteins (Hammond and Balla 2015). The parent molecule, PI, represents 10–20 mol% of the total cellular phospholipid. It is synthesized in the endoplasmic reticulum via the CDS/PIS pathway, but its distribution among cellular membranes is not yet well established. In the PI-cycle, PI is first converted to PI4P. At steady state in a typical mammalian cell, PI4P constitutes only 2–5 % of the PI. PI4P is then converted in the PI-cycle to PI(4,5)P2. PI(4,5)P2 is known to be present almost exclusively in the plasma membrane. However, the enzyme PI4K that catalyzes the conversion of PI to PI4P is located mainly in the Golgi and endosomal compartments (Balla and Balla 2006). There are two classes of PI4K, Type II and Type III, with each of these having two different isofomrs, α and β (Delage et al. 2013). Types II and III PI4K differ from one another in their size, Km values, and sensitivity to inhibitors. The major fraction of the product of this reaction, PI4P, has recently been found in the plasma membrane (Hammond et al. 2009, 2014; Sarkes and Rameh 2010). An explanation for different subcellular location of PI4K and the products of its catalysis came when it was found that different isoforms of PI4K are responsible for making PI4P in the two organelles. PI4KA is present in the plasma membrane where it generates PI4P, while PI4KB is present in the Golgi (Godi et al. 1999). PI4P is not detected in the endoplasmic reticulum but the enzyme PI4KA is present in that organelle. It is possible that PI4P is located in the endoplasmic reticulum exit sites (Blumental-Perry et al. 2006). The other PIP n in the PI-cycle is PI(4,5)P2. It represents 2–5 % of the total PI. The level of PI(4,5)P2 is maintained in spite of changes in the concentration of its precursor, PI4P (Bojjireddy et al. 2014; Hammond et al. 2012). This may be a result of increased movement of PI from the endoplasmic reticulum to the plasma membrane as a result of lipid exchange when the PI-cycle is activated (see discussion of lipid transfer, below). In addition to being hydrolyzed by PLC to form the secondary messengers DAG and inositol triphosphate, PI(4,5)P2 also has other important roles in signal transduction as well as in the regulation of enzymatic activities, membrane transport, the actin cytoskeleton, and nuclear signaling (Delage et al. 2013). PI(4,5)P2 affects the actin cytoskeleton by binding to specific actin-binding proteins (Moseley and Goode 2006; Saarikangas et al. 2010) as well as affecting membrane properties including membrane bending, fusion, fission, and affecting membrane trafficking and signaling (Ischebeck et al. 2010; Vicinanza et al. 2008). PI(4,5)P2 regulates a number of ion channels and transporters which are essential for many signal transduction pathways (Rohacs 2009; Suh and Hille 2008; Suh et al. 2010; Yaradanakul et al. 2007).
There are also several other PIP n that are present in very low amounts, generally only a few percent of the concentration of PI4P. Many of these PIP n have roles in signal transduction (Balla 2013). We will not review all of the species of PIP n , but will consider one of them that is in higher concentration. This lipid is PI(3,4,5)P3, whose concentration in the cell is 1–5 % that of PI(4,5)P2.
Because of their important roles in cell signaling, several mechanisms exist for the modulation of the activities of PI4K as well as PI4P5K. The activity of these enzymes is modulated by small G-proteins in response to extracellular signals (Balla and Balla 2006; Krauss and Haucke 2007; Santarius et al. 2006). There is also evidence for multimolecular complexes channeling substrate into particular pathways for end product formation (Balla et al. 2009 125/id; Lee et al. 2004 126/id). In addition to the kinases, lipid phosphatases exist that can reverse the direction of the metabolic flow. Both the kinases and the phosphatases also have their enzymatic activities regulated by post-translational protein phosphorylation and dephosphorylation. In addition, PI4P5K is activated by PA (Moritz et al. 1992; Jenkins and Frohman 2005). The acyl chain composition of the PA has a large effect on the extent of activation. There is a growing realization of the importance of the whole lipid molecule, including the acyl chains in the biological activity of certain lipids (Kimura et al. 2016). The presence of a saturated acyl chain at the sn-1 position of PA markedly lowers the extent of activation (Shulga et al. 2012). For example, dioleoyl phosphatidic acid is a good activator of PI4P5K, but 1-stearoyl-2-oleoyl phosphatidic acid hardly activates at all (Shulga et al. 2012), even though these two forms of PA differ by only one double bond.
Both PI4P and PA are intermediates in the PI-cycle. The major acyl chain composition of these lipids as intermediates in the PI-cycle in normal cells is 1-stearoyl-2-arachidonoyl. This provides the possibility that there could be positive feedback within the cycle, resulting in an increased rate of interconversions within the cycle (Oude Weernink et al. 2007). However, 1-stearoyl-2-arachidonoyl phosphatidic acid is not among the best activators of PI4P5K; diarachidonoyl phosphatidic acid is a much better activator (Shulga et al. 2012). Another indication that there is not a feedback regulation of the PI-cycle is that although the PA formed by DGKζ (Luo et al. 2004), DGKα (Jones et al. 2000) as well as by phospholipase D (PLD) (Pettitt et al. 2001) can all increase the activity of PI4P5K. The PA formed within the PI-cycle by DGKε catalysis, i.e., 1-stearoyl-2-arachidonoyl phosphatidic acid, does not produce a product that can activate PI4P5K (Jones et al. 2000). There may however be a positive augmentation in the rate of the PI-cycle by coupling with PLD. A product of the PI-cycle, PI(4,5)P2, activates PLD (Hammond et al. 1997; Liscovitch et al. 1994) that generates PA. This PA product of PLD can then activate PI4P5K resulting in the feedback acceleration of the PI-cycle.
Increased PIP3 in Cancer Cells
It has recently been shown that transformed mammalian cells contain little 1-stearoyl-2-arachidonoyl PI, but rather have species of PI with shorter and less unsaturated acyl chains compared with normal cells (Naguib et al. 2015). These transformed cells still have a normal content of PI but the specific species of PI are different. This demonstrates that the synthesis of PI can be altered so that other acyl chains besides 1-stearoyl-2-arachidonoyl can be incorporated into this lipid. There is also recent evidence that the positions of double bonds in acyl chains of the same molecular mass (isobaric) differ in cancer cells (Ma et al. 2016).
Cancer cells have more PIP3 than normal cells. This is often a consequence of the mutation of PI3K, resulting in increased activity, as well as the inactivation or gene deletion of PTEN, the enzyme that catalyzes the hydrolysis of PI(3,4,5)P3 to form PI(4,5)P2. In addition, there is another mechanism, through the mutation of the transcription factor p53, that can result in higher levels of PI(3,4,5)P3. Mutation of p53, which occurs in many cancer cells, results in an alteration of the acyl chain composition of several phospholipids. P53 is a repressor of the expression of stearoyl-CoA desaturase (Rueda-Rincon et al. 2015). Many cancer cells have inactivating mutations in p53 leading to an increased expression of stearoyl-CoA desaturase and an increased amount of monounsaturated phospholipid species (Igal 2011; Minville-Walz et al. 2010; Naguib et al. 2015; Rueda-Rincon et al. 2015). The rate determining step in the synthesis of PIP2 is the phosphorylation of PI4P to PI(4,5)P2 catalyzed by PI4P5K. There are three isoforms of PI4P5K, α, β, and γ. There is evidence that these isoforms may have different biological functions. PI4P5Kβ is involved in regulating the pool of PI(4,5)P2 that controls store-operated Ca2+ channels, while PI4P5Kγ catalyzes the formation of inositol triphosphate (Calloway et al. 2011; Vasudevan et al. 2009). For all three isoforms of this enzyme, PI4P with no unsaturation is a very poor substrate compared with PI4P containing one or more double bonds (Shulga et al. 2012). Increased expression of p53 causes a shift of phospholipid acyl chains in the cell from those composed of two monounsaturated acyl chains to those with one or no unsaturation in the two acyl chains (Rueda-Rincon et al. 2015). The PI4P with fully saturated acyl chains will block the conversion of PI4P to PI(4,5)P2 (Shulga et al. 2012). Thus, mutation of p53 in cancer cells causes an increase of stearoyl-CoA desaturase, in turn increasing the amount of phospholipids with two monounsaturated acyl chains, allowing more efficient conversion of PI4P to PIP2 and thus higher substrate concentration for PI3K to catalyze the formation of PIP3. The resulting increase in PIP3 affects oncogenesis through modulation of the activity of Akt. Akt is a protein kinase that is recruited to the cell membrane by interaction with PIP3, resulting in the activation of Akt by phosphorylation. Increased activation of Akt mediates downstream responses that are typical of cancer cells including cell survival, growth, proliferation, and cell migration (Fig. 4). The increased synthesis of PIP3 in cancer cells is indirectly a result of an increased level of the substrate PIP2 caused by changes in the acyl chain composition of PI4P (Rueda-Rincon et al. 2015). In support of this relationship are the findings that inhibition of stearoyl-CoA desaturase decreases the activity of Akt (Scaglia and Igal 2008) and that the addition of monounsaturated oleic acid to cells reverses the p53-induced repression of Akt activation by phosphorylation (Rueda-Rincon et al. 2015).
Mechanism connecting reduced p53 activity to increased cell proliferation as a consequence of changes in the acyl chain composition of lipid intermediates of the PI-cycle. Progression of steps indicated by horizontal red arrows. Vertical blue arrows indicate that as a result of a loss of p53 activity, there is a resultant increase in a series of enzyme activities or lipid products (Color figure online)
Acyl Chain Composition of Other Lipid Intermediates of the Phosphatidylinositol Cycle
The above discussion demonstrates the importance of the acyl chain composition of PIP n in determining the rate of formation of PIP3. The acyl chain composition of all of the lipid intermediates of the PI-cycle in non-transformed mammalian cells is predominantly 1-stearoyl-2-arachidonoyl. Some of the steps contributing to the enrichment of these lipids with 1-stearoyl-2-arachidonoyl chains in normal mammalian cells have been identified. It is at least in part, the result of the acyl chain dependence of substrate specificity of two enzymes of the PI-cycle. One of these enzymes is the epsilon isoform of DGK (DGKε). There are 10 isoforms of DGK, in addition to gene splicing variants in mammals, each having its own function, subcellular localization and organ distribution (Shulga et al. 2011). Among all of these variants of DGK, only one form exhibits specificity for DAG substrates having an arachidonoyl chain at the sn-2 position (Rodriguez de Turco et al. 2001; Milne et al. 2008; Tang et al. 1996) as well as a stearoyl chain at the sn-1 position (Lung et al. 2009). Other isoforms of DGK have similar activities against most DAG species, independent of their acyl chains. Another enzyme that exhibits specificity for a 1-stearoyl-2-arachidonoyl lipid substrate is CDS2. CDS has only two isoforms in mammals; CDS1 and CDS2. There is a marked contrast between the acyl chain specificities of these two isoforms. Only CDS2 is highly specific for 1-stearoyl-2-arachidonoyl phosphatidic acid, while CDS1 shows almost no effect of the acyl chain composition of the substrate on enzymatic activity (D’Souza et al. 2014).
The PI-cycle intermediate, CDP-DAG is a precursor for the synthesis of phosphatidylglycerol as well as PI. Unlike PI, phosphatidylglycerol is not enriched in 1-stearoyl-2-arachidonoyl, suggesting that CDS1 may be responsible for the synthesis of that lipid and CDS2 would pass the CDP-DAG product to PIS for the synthesis of PI. In the brain, an organ having a relatively high level of expression of DGKε, the arachidonoyl content in the sn-2 position of CDP-DAG, is 44.6 % while that for PI in this organ is 62.6 % (Thompson and MacDonald 1976). This increase in arachidonoyl content is not a result of the enzyme specificity in the last step of PI synthesis, catalyzed by PIS, since that enzyme shows no acyl chain specificity (D’Souza and Epand 2015). Remodeling of PI by the Lands cycle (see below) could contribute to the additional acyl chain enrichment. It is also possible that there is a direct transfer of 1-stearoyl-2-arachidonoyl-CDP-DAG from CDS2 to PIS, while other species of CDP-DAG are used for the synthesis of phosphatidylglycerol (Weeks et al. 1997) through the action of CDS1 (Fig. 3).
CDP-DAG is also a precursor for cardiolipin that is synthesized and located in the mitochondria. It is not known if a mitochondrial CDS1 or a mammalian homolog of the yeast enzyme Tam41 (Tamura et al. 2013) or a combination of these paths is involved in cardiolipin synthesis.
Although the major fraction of PI is the 1-stearoyl-2-arachidonoyl species, there is a minor fraction of PI (~25 %) that has other acyl chains. Transformed cells contain little of 1-stearoyl-2-arachidonoyl PI (Naguib et al. 2015). Thus there must also be pathways to synthesize PI with other acyl chain compositions. The simplest explanation for the incorporation of other acyl chains into PI is that other isoforms of DGK, besides DGKε and CDS1 in addition to CDS2 contributes to the respective steps in the PI-cycle. These other isoforms exhibit little or no acyl chain specificity for their substrates. The molecular mechanism controlling the relative contributions of these other isoforms to the synthesis of PI is yet to be determined.
Compartmentalization
There is a requirement for substrate channeling or compartmentalization in order to explain the observation that although there are many molecular species of PA in the cell, predominantly one of these species, the 1-stearoyl-2-arachidonoyl-PA, is normally converted into PI. Most cells express both CDS1 and CDS2. Why does so little of other molecular species of PA normally end up as PI with different acyl chains? Similarly, if CDS2 contributed to the synthesis of phosphatidylglycerol, why does this phospholipid not contain more of the 1-stearoyl-2-arachidonoyl-PA species?
Neither total cellular DAG nor PA is highly enriched with arachidonoyl-containing forms (Milne et al. 2008). This is because these lipids are not confined to the PI-cycle, but are involved in other pathways of lipid metabolism. Another question is how is the substrate selectivity of DGKε for acyl chains is maintained in the next step of the PI-cycle, catalyzed by CDS? If the 1-stearoyl-2-arachidonoyl phosphatidic acid synthesized by DGKε mixed with the large excess of PA from other sources in the cell, the enrichment with particular acyl chains through catalysis by DGKε would be lost. Hence there must be some segregation of the lipid intermediates of the PI-cycle from other lipids in the cell, either by physical isolation in different cellular structures or in membrane domains or by the formation of multiprotein complexes containing different enzymes of the PI-cycle. There has to be some mechanism to have the product of one step in the PI-cycle passed on directly to the enzyme catalyzing the next step, without allowing mixing with other species of the PI-cycle. There is also the feature that this is a cycle, so that each time the PI-cycle goes around, the lipid intermediates can become progressively enriched with specific acyl chains as a result of the substrate specificities of some of the enzymes in the cycle, provided that the cycle is largely isolated from other lipids of the cell. Additional factors contributing to the extent of acyl chain enrichment in PI are the levels of expression of DGKε and CDS2. DGKε is found mostly in the brain (Kohyama-Koganeya et al. 1997) but CDS2 is very widely expressed. In addition, there is remodeling of the PI that is formed (see below). In mammalian organs other than brain, in which the expression levels of DGKε are low, PI is still found to be highly enriched with the 1-stearoyl-2-arachidonoyl species. A contributing factor to determine which acyl chains are incorporated into PI may be compartmentalization. This is supported by the findings that using special resolution imaging mass spectrometry, both breast cancer tissue (Kawashima et al. 2013) as well as tissue from prostate cancer patients (Goto et al. 2014) have PI species with altered acyl chain compositions that are spatially clustered.
CDP-DAG is synthesized within the PI-cycle. In normal mammalian cells this lipid is already significantly enriched in the 1-stearoyl-2-arachidonoyl species (Thompson and MacDonald 1976), even though remodeling does not occur until PI is synthesized. This suggests that most of the CDP-DAG is synthesized via the reaction catalyzed by CDS2. If any CDP-DAG is made using CDS1, this CDP-DAG, without acyl chain enrichment, must be rapidly converted to other phospholipids so that it does not form a major fraction of the CDP-DAG intermediate. The species of CDP-DAG enriched in 1-stearoyl-2-arachidonoyl chains would be converted to PI by PIS, maintaining the high enrichment of 1-stearoyl-2-arachidonoyl acyl chains.
Non-Vesicular Lipid Transfer
As discussed above, the PI-cycle can only be completed if there is transfer of lipid intermediates of the cycle between the endoplasmic reticulum and the plasma membrane. The protein Nir2 has been shown to transfer PI between membranes in vitro (Garner et al. 2012). In addition to being required to complete the PI-cycle, it is possible that some of the lipid transfer processes preferentially transfer lipids with specific acyl chain compositions. The acyl chain specificity of this exchange has not yet been evaluated.
It has recently been found that Nir2 facilitates the exchange of PI in the endoplasmic reticulum for PA in the plasma membrane (Chang and Liou 2015; Kim et al. 2013, 2015). This exchange can account for the rapid replenishment of PIP2 subsequent to its hydrolysis on the stimulation of PLC (Chang and Liou 2015; Kim et al. 2015). Another reason that the concentration of PIP2 remains constant is because it is part of a metabolic cycle, the PI-cycle. Intermediates of a metabolic cycle are maintained at a constant level and are neither increased nor decreased in amount. This is particularly true for PIP2 that is found exclusively in the plasma membrane where it is likely to be a part of the PI-cycle. However, PI4P is found in many subcellular compartments, not all of which are directly associated with the PI-cycle. This could explain the observation that the concentration of PI4P varies to a greater extent than that of PIP2. For lipids that are part of the PI-cycle, each intermediate is buffered by other intermediates of the cycle which can all be interconverted through the cycle. When the cycle is stimulated, as for example by the activation of PLC, there can be a brief rise in the concentration of certain intermediates of the PI-cycle, but levels promptly return to the steady-state levels. However, when the cycle is broken, as for example by eliminating exchange of PA and PI between the endoplasmic reticulum and the plasma membrane, the concentration of intermediates of the cycle markedly fall for a more prolonged period of time.
Binding of Nir2 to the endoplasmic reticulum is also associated with another protein of the endoplasmic reticulum, VAP-B (Kim et al. 2015). VAP-B expression increased the association of Nir2 to the endoplasmic reticulum. Furthermore after stimulation, Nir2 and VAP-B showed colocalization in contact sites between the plasma membrane and endoplasmic reticulum. In addition, Nir2, that is difficult to detect by fluorescence microscopy in resting cells, rapidly forms puncta that localize between the endoplasmic reticulum and the plasma membrane, colocalizing with CDS2 after stimulation (Kim et al. 2015). These results provide evidence that both Nir2 and CDS2 are closely associated with the PI-cycle. There is also another isoform, Nir3, which is a homolog of Nir2. These two proteins appear to be related, but somewhat play different roles. While Nir2 functions to transfer PI so as to maintain PIP2 signaling capacity in the plasma membrane, Nir3 maintains the basal level of PIP2 in the plasma membrane in the resting state (Chang and Liou 2015). Both Nir2 and Nir3 are sensors for PA formed from the hydrolysis of PIP2 and the phosphorylation of the resulting DAG by DGK. Of the two Nir proteins, Nir3 has a stronger binding affinity for PA and may serve to maintain the basal level of PIP2. By contrast, Nir2 has a weaker translocation to the endoplasmic reticulum–plasma membrane junction following receptor stimulation and appears to have a higher capacity to transfer PI, while at the same time avoiding the formation of excess PIP2 at the plasma membrane. The importance of PI transfer from the endoplasmic reticulum membrane to the plasma membrane to enable continued PIP2 signaling is shown by the observation that depletion of PI at the plasma membrane results in defective PIP2 replenishment at the plasma membrane. However, initial signaling from PIP2 at the plasma membrane can occur even after acute depletion of PI from the endoplasmic reticulum. It is suggested that PIP2 can be regenerated from a small pool of precursors in the plasma membrane but this regeneration of PIP2 cannot be sustained without the presence of the Nir proteins. These studies also suggest that both Nir2 and Nir3 function by binding PA and translocating to the endoplasmic reticulum–plasma membrane contact sites, where they can facilitate the exchange of PA and PI. Thus receptor activation of PLC to hydrolyze PIP2 will produce PA that can then activate the movement of the Nir proteins to be non-vesicular lipid exchange proteins. It is possible that one of the Nir proteins functions to transfer lipids with 1-stearoyl-2-arachidonoyl chains, while the other is not specific for the acyl chain, as we have seen with other steps of the PI-cycle catalyzed by more than one isoform of the enzyme involved. Since Nir2 maintains the basal level of PIP2 in the plasma membrane, we suggest that it is the isoform specific for binding 1-stearoyl-2-arachidonoyl-PA. Its lower binding affinity may be a consequence of non-optimal species of PA being used for the binding studies.
It has been shown that the molecular species of PI and PA are similar in the endoplasmic reticulum and in the plasma membrane. However, the PI:PA ratio is >1 for 34:2, 36:1, 38:3, and 38:4, while for most other species it is <1 (Shulga et al. 2010). This demonstrates a selective enrichment of PI with 1-stearoyl-2-arachidonoyl (38:4) and related acyl chains from its precursor PA. There are also short chain PA species 30:1 and 30:0 making up 21 % of the PA in the endoplasmic reticulum, but apparently they do not get converted to PI since no PI with this acyl chain composition is found (Shulga et al. 2010). Furthermore, these species of PA are twice as abundant in the endoplasmic reticulum as in the plasma membrane, suggesting that not only they are not metabolized to PI, but they also are not as likely to be exchanged between the endoplasmic reticulum and the plasma membrane. It is possible that they are required to stabilize regions of high curvature in the endoplasmic reticulum. There are also species of PI for which there is no corresponding PA (Shulga et al. 2010). These PI species are found equally prevalent in the endoplasmic reticulum and in the plasma membrane. The most abundant of these species of PI is 38:5 PI that comprises about 17 % of the PI of both the endoplasmic reticulum and the plasma membrane. 38:5 PI is quite similar to the most abundant 1-stearoyl-2-arachidonoyl (38:4) species, but apparently is not acted on by PLC. These results also suggest that these species of PI for which there is no corresponding PA are readily exchanged between the two membranes. The endoplasmic reticulum is the site of synthesis of PI, so that lipid would be present in the endoplasmic reticulum for exchange with PA in the plasma membrane. In order to form PA in the plasma membrane as part of the PI-cycle, to complete the lipid exchange, would require the presence of DGK in the plasma membrane. There are several isoforms of DGK that partition to the plasma membrane, in some cases after stimulation (Shulga et al. 2011). However, for the acyl chain-specific formation of 1-stearoyl-2-arachidonoyl phosphatidic acid, the DGK isoform has to be DGKε. Most of the DGKε is found in the endoplasmic reticulum (Kobayashi et al. 2007; Matsui et al. 2014), although there is evidence for some of this isoform to be in the plasma membrane (Decaffmeyer et al. 2008). These findings would suggest that DGKε in the endoplasmic reticulum might function as a reservoir form to be transferred to the plasma membrane upon stimulation of PLC. An observation linking DGKε with the fraction of PA that participates in the in the PI-cycle is the finding that the content of PA in the plasma membrane is decreased by 3-fold in mouse embryo fibroblasts knocked out for DGKε (Shulga et al. 2010). This could be explained by the removal of DGKε disrupting the PI-cycle and the generation of PA in the plasma membrane. This change in lipid composition in the plasma membrane occurs despite the fact that the total cellular PA is unaffected in these cells (Milne et al. 2008).
Alternatively, in addition to the PA-PI transfer process there may also be some DAG that is transferred from the plasma membrane to the endoplasmic reticulum where it is phosphorylated by DGKε. In addition to the lipid transfer from the plasma membrane to the endoplasmic reticulum, there also has to be lipid transfer in the opposite direction to complete the PI-cycle. With the Nir proteins the reverse lipid movements are coupled by the lipid exchange process. PI synthesized in the endoplasmic reticulum is exchanged for PA in the plasma membrane. There may also be other ways to deliver PI to the plasma membrane. There are PI-specific lipid transfer proteins that could perform this function (Cockcroft and Garner 2011; Routt and Bankaitis 2004). It has also been shown that in cells that overexpress PIS, highly mobile membrane compartments form at the endoplasmic reticulum that can deliver PI to various membranes by vesicular transport (Kim et al. 2011). There are also contact sites between the endoplasmic reticulum and plasma membrane through which lipids can be transferred (Henne et al. 2015). At these sites, the movement of PI from the endoplasmic reticulum to the plasma membrane can be balanced by movement of DAG and/or PA in the opposite direction.
It has been shown that the lipid transfer protein Nir2 enhances the epithelial–mesenchymal transition and facilitates breast cancer metastasis mediated through the PI3K/Akt pathway (Keinan et al. 2014). We discussed above how the acyl chain composition of PI could affect cancer progression by affecting the phosphorylation of Akt. Nir2 may have a role in this process by completing the PI-cycle and allowing more rapid formation of PIP2, the substrate of PI3K to form PIP3 and activate Akt (final steps in Fig. 4).
Lipid Remodeling
All phospholipids undergo acyl chain remodeling through the actions of acyl transferases and phospholipases. This process is collectively known as the Land’s cycle (Lands 1958, 1960; Lands and Merkl 1963; Lands et al. 1982). Remodeling allows certain lipids to attain a specific acyl chain composition, which is important for signaling functions. Several classes of enzymes that are needed for remodeling have been characterized. Lysophospholipid acyltransferases are enzymes that transfer an acyl group from acyl-CoA to a lysophospholipid (Matsuda et al. 2008). Other enzymes required for remodeling include the phospholipase A1 and A2 families, which cleave acyl chains off the phospholipid to produce the lysophospholipid that can accept an acyl chain from an acyl-CoA (Aoki et al. 2002; Puttmann et al. 1993). The Land’s cycle could result in acyl chain enrichment through the selective incorporation and/or removal of acyl chains.
There is a specific mechanism for enriching PI with arachidonoyl chains. Lysophosphatidylinositol acyltransferase 1 (LPIAT1), also known as membrane bound O-acyltransferase containing domain 7 (also referred to as MBOAT7), catalyzes the transfer of an acyl group from acyl-CoA to lysoPI (Gijon et al. 2008). LPIAT1 has a high specificity for arachidonoyl-CoA (Gijon et al. 2008). Thus, LPIAT1’s arachidonoyl specificity contributes to the enrichment of PI with an arachidonoyl chain (Fig. 5).
The Land’s cycle of acyl chain remodeling of PI. The acyl chains of PI can be remodeled through phospholipases (PLA2) and acyltransferases (LPIAT1). PLA2 exhibits little acyl chain specificity, while LPIAT1 is highly specific for transferring an acyl chain from arachidonoyl-CoA to lysophosphatidylinositol (LPI). The combination of these two steps results in the enrichment of PI with arachidonoyl chains at the sn-2 position
Knocking out LPIAT1in mice with the same genetic background (C57/B16) was found independently by two groups to significantly decrease arachidonoyl-containing PI, and PI(4,5)P2 (Anderson et al. 2013; Lee et al. 2012). LPIAT1 is critical for neural development of mice; LPIAT−/− mice were viable up to 30 days after birth, but exhibited a smaller, atrophied cerebral cortex, and hippocampus. The laminal structure of the neocortex was also disordered due to delayed neural migration, which indicated a role for LPIAT1 in cortical lamination (Lee et al. 2012).
Other lipid products can form arachidonic acid that is released from the sn-2 position of lipid intermediates in the PI-cycle by the action of phospholipase A2. The arachidonic acid released can be further metabolized to form signaling eicosanoids. Another lipid signaling product could be formed by the hydrolysis of the stearoyl group of 1-stearoyl-2-arachidonoyl glycerol by phospholipase A1. The resulting product of this reaction is 2-arachidonoyl glycerol that is an endogenous endocannabinoid ligand. The resulting 2-arachidonoyl glycerol can no longer efficiently enter the PI-cycle as a lysophosphatidic acid (Gantayet et al. 2011). In addition to remodeling with an arachidonoyl group at the sn-2 position, the sn-1 position is also remodeled with a stearoyl group, catalyzed by the enzyme that was originally named lysocardiolipin acyl transferase (Imae et al. 2012).
Roles of the Acyl Chains of PI-Cycle Intermediates and Lipid Signaling
The predominant molecular species of PI(4,5)P2 has 1-stearoyl-2-arachidonoyl acyl chains in normal mammalian cells and tissues. PI(4,5)P2 has many functions, making it complex to assess the role of acyl chains in the signaling by PI(4,5)P2. One role of PI(4,5)P2 is as a precursor of PI(3,4,5)P3 that is important for the activation of Akt. The rate of formation of PI(4,5)P2 and PI(3,4,5)P3 is acyl chain dependent as described above. PI(4,5)P2 plays a major role in endocytosis in the synapse (Haucke 2005). To end this process, synaptojanin-1 dephosphorylates PI(4,5)P2 (Wenk and De Camilli 2004). In vitro studies have shown that natural PI(4,5)P2 (largely the 1-stearoyl-2-arachidonoyl form) is hydrolyzed more rapidly than the dipalmitoyl form of PI(4,5)P2 (Schmid et al. 2004), demonstrating a role for the acyl chains in modulating this process.
In addition to PI(4,5)P2, the PI-cycle also includes other lipid signaling intermediates such as DAG and PA. There are two major signaling routes for the formation of DAG and PA that are activated by the stimulation of cells with certain agonists. One is the hydrolysis of PI by PLC to produce DAG, as part of the PI-cycle (Fig. 1). The DAG formed by PLC hydrolysis uses predominantly inositol phospholipids as substrate and produces DAG enriched with 18:0/20:4 and 18:0/20:3 species (Pettitt and Wakelam 1993). This DAG can be converted to PA by most mammalian DGK isoforms, including DGKε, the isoform that has specificity for 1-stearoyl-2-arachidonoyl glycerol (D’Souza and Epand 2014). The other pathway for the formation of PA is by PLD hydrolysis of phospholipids, mostly phosphatidylcholine. There may be a crosstalk between the two pathways since PI(4,5)P2 from the PI-cycle activates PLD and PA from the PLD pathway activates PI4P5K, an enzyme of the PI-cycle, to form more PIP2. The PA can also be hydrolyzed by phosphatidate phosphohydrolase to produce DAG. The DAG species produced from PC through this pathway has distinct acyl chain compositions compared with the DAG produced from PI(4,5)P2 (Madani et al. 2001; Pettitt et al. 1997). It has been shown that in endothelial cells, activation of PLD by lysophosphatidic acid produces DAG that does not activate protein kinase C (PKC) (Pettitt et al. 1997).
In vitro studies of PKC activation with DAG confirms that the nature of the acyl chains on DAG determines its potency in stimulating PKC (Marignani et al. 1996; Madani et al. 2001). Sustained PKC activation has been shown to be oncogenic and contributes to malignant phenotypes seen in cancers (Koivunen et al. 2006; Rajotte et al. 1992). PKC-βII is maximally activated by 1-stearoyl-2-arachidonoyl glycerol (Deacon et al. 2002) and expression of this PKC isoform is required for the proliferation of human leukemic cells (Thompson and Fields 1996). PKC-βII is targeted to the nucleus during the G2/M phase of the cell cycle (Deacon et al. 2002). The translocation of PKC-βII to the nucleus during the G2/M is triggered in part by the buildup of 1-stearoyl-2-arachidonoyl glycerol in the nucleus (Deacon et al. 2002). This DAG species arises from the hydrolysis of PI(4,5)P2 in the nucleus (Cocco et al. 1987; Irvine and Divecha 1992) as part of the PI-cycle that is also known to be present in the nucleus, independently of the cycle occurring in the endoplasmic reticulum/plasma membrane (Cocco et al. 1989).
Other evidence of acyl chain specificity of DAG comes from recent studies of the activation of caged DAGs with different acyl chains (Nadler et al. 2013). It was demonstrated that the photoactivation to liberate 1-stearoyl-2-arachidonoyl glycerol, and not other DAGs, resulted in a massive increase of intracellular Ca2+ levels. This can be understood in terms of the intermediates of the PI-cycle having a catalytic role as well as the fact that a product of the cycle is inositol triphosphate that is a ligand capable of opening Ca2+ channels in the endoplasmic reticulum. Thus, these experiments tie in specific acyl chains of DAG with signaling resulting from the PI-cycle.
Another signaling lipid intermediate of the PI-cycle is PA. PA plays an important role in activating PI4P5K with some dependence on its acyl chain composition (Shulga et al. 2012). PA also activates a protein kinase in platelets (Khan et al. 1994); PA has modulatory effects on membrane fusion (Rogasevskaia and Coorssen 2015); PA regulates the formation of actin stress fibers (Cross et al. 1996). Less is known about the influence of the acyl chains of PA on its various functions. Recently it has been shown that PA binds to the transcriptional repressor Opi1 in a manner that distinguishes between PA with a C16 versus a C18 acyl chain (Hofbauer et al. 2014). In addition, the activation of DNA synthesis by lysophosphatidic acid is dependent on the acyl chain composition of this lipid (van Corven et al. 1992).
Conclusions
The PI-cycle has particular properties as a consequence of the fact that it is a cycle so that all of the lipid intermediates of the cycle are regenerated and the intermediates can act as catalysts. The PI-cycle consumes a large amount of energy and can thus proceed only in one direction. The PI-cycle is the major pathway for the synthesis of PI from which the signaling PIP n lipids are derived. In addition, some lipid intermediates of the cycle are also important in signaling, including DAG and PA. The PI-cycle requires transfer of lipid to and from the endoplasmic reticulum and the plasma membrane. This is facilitated by the Nir proteins that exchange PI formed in the endoplasmic reticulum with PA formed in the plasma membrane. The substrate specificity of two enzymes in the PI-cycle contributes to the enrichment of PI with 1-stearoyl-2-arachidonoyl acyl chains in normal mammalian cells. Some of the intermediates in the PI-cycle are common to other lipid metabolic pathways. In order for the acyl chain composition to be maintained by the intermediates of the cycle, they must be isolated from the same lipids having different acyl chains. This suggests that the PI-cycle is largely isolated from the lipid metabolic processes occurring outside the cycle. However, at least 15 % of PI in these cells has other acyl chains and with cancer cells, as a consequence of alterations in lipid metabolism, PI is even less enriched with the 1-stearoyl-2-arachidonoyl acyl species. The arachidonoyl content of PI in transformed cells is very low, yet the same amount of PI is still synthesized. This suggests that there can be alternative isoforms of DGK and CDS that allow the completion of the PI-cycle.
Abbreviations
- CDP-DAG:
-
Cytidine diphosphate-diacylglycerol
- CDS:
-
CDP-DAG synthase (Phosphatidate cytidylyltransferase)
- DAG:
-
Diacylglycerol
- DGK:
-
Diacylglycerol kinase
- LPIAT1:
-
Lysophosphatidylinositol acyltransferase 1 (O-acyltransferase containing domain 7) (MBOAT7)
- PA:
-
Phosphatidic acid
- PI:
-
Phosphatidylinositol
- PI-cycle:
-
Phosphatidylinositol cycle
- PI3K:
-
Phosphatidylinositol-4,5-bisphosphate 3-kinase
- PI4K:
-
Phosphatidylinositol-4-kinase
- PI4P:
-
Phosphatidylinositol-4-phosphate
- PIP2 :
-
Phosphatidylinositol-4,5-bisphosphate
- PIP3 :
-
Phosphatidylinositol-3,4,5-trisphosphate
- PIP n :
-
Phosphorylated forms of PI
- PI4P5K:
-
Phosphatidylinositol-4-phosphate 5-kinase
- PIS:
-
PI synthase (CDP-diacylglycerol-inositol 3-phosphatidyltransferase)
- PKC:
-
Protein kinase C
- PLC:
-
Phospholipase C (1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase)
- PLD:
-
Phospholipase D
- PTEN:
-
Phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase (Phosphatase and tensin homolog)
- TrpC channel:
-
Transient receptor cation channel
References
Anderson KE, Kielkowska A, Durrant TN, Juvin V, Clark J, Stephens LR, Hawkins PT (2013) Lysophosphatidylinositol-acyltransferase-1 (LPIAT1) is required to maintain physiological levels of PtdIns and PtdInsP(2) in the mouse. PLoS ONE 8:e58425
Aoki J, Nagai Y, Hosono H, Inoue K, Arai H (2002) Structure and function of phosphatidylserine-specific phospholipase A1. Biochim Biophys Acta 1582:26–32
Balla T (2013) Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev 93:1019–1137
Balla A, Balla T (2006) Phosphatidylinositol 4-kinases: old enzymes with emerging functions. Trends Cell Biol 16:351–361
Balla T, Szentpetery Z, Kim YJ (2009) Phosphoinositide signaling: new tools and insights. Physiology 24:231–244
Blumental-Perry A, Haney CJ, Weixel KM, Watkins SC, Weisz OA, Aridor M (2006) Phosphatidylinositol 4-phosphate formation at ER exit sites regulates ER export. Dev Cell 11:671–682
Bojjireddy N, Botyanszki J, Hammond G, Creech D, Peterson R, Kemp DC, Snead M, Brown R, Morrison A, Wilson S, Harrison S, Moore C, Balla T (2014) Pharmacological and genetic targeting of the PI4KA enzyme reveals its important role in maintaining plasma membrane phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate levels. J Biol Chem 289:6120–6132
Calloway N, Owens T, Corwith K, Rodgers W, Holowka D, Baird B (2011) Stimulated association of STIM1 and Orai1 is regulated by the balance of PtdIns(4,5)P(2) between distinct membrane pools. J Cell Sci 124:2602–2610
Carrasco S, Merida I (2007) Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci 32:27–36
Chang CL, Liou J (2015) Phosphatidylinositol 4,5-bisphosphate homeostasis regulated by Nir2 and Nir3 proteins at endoplasmic reticulum–plasma membrane junctions. J Biol Chem 290:14289–14301
Cocco L, Gilmour RS, Ognibene A, Letcher AJ, Manzoli FA, Irvine RF (1987) Synthesis of polyphosphoinositides in nuclei of Friend cells. Evidence for polyphosphoinositide metabolism inside the nucleus which changes with cell differentiation. Biochem J 248:765–770
Cocco L, Martelli AM, Gilmour RS, Ognibene A, Manzoli FA, Irvine RF (1989) Changes in nuclear inositol phospholipids induced in intact cells by insulin-like growth factor I. Biochem Biophys Res Commun 159:720–725
Cockcroft S, Garner K (2011) Function of the phosphatidylinositol transfer protein gene family: is phosphatidylinositol transfer the mechanism of action? Crit Rev Biochem Mol Biol 46:89–117
Cross MJ, Roberts S, Ridley AJ, Hodgkin MN, Stewart A, Claesson-Welsh L, Wakelam MJ (1996) Stimulation of actin stress fibre formation mediated by activation of phospholipase D. Curr Biol 6:588–597
Deacon EM, Pettitt TR, Webb P, Cross T, Chahal H, Wakelam MJO, Lord JM (2002) Generation of diacylglycerol molecular species through the cell cycle: a role for 1-stearoyl, 2-arachidonyl glycerol in the activation of nuclear protein kinase C-βII at G2/M. J Cell Sci 115:983–989
Decaffmeyer M, Shulga YV, Dicu AO, Thomas A, Truant R, Topham MK, Brasseur R, Epand RM (2008) Determination of the topology of the hydrophobic segment of mammalian diacylglycerol kinase epsilon in a cell membrane and its relationship to predictions from modeling. J Mol Biol 383:797–809
Delage E, Puyaubert J, Zachowski A, Ruelland E (2013) Signal transduction pathways involving phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate: convergences and divergences among eukaryotic kingdoms. Prog Lipid Res 52:1–14
D’Souza K, Epand RM (2014) Enrichment of phosphatidylinositols with specific acyl chains. Biochim Biophys Acta 1838:1501–1508
D’Souza K, Epand RM (2015) The phosphatidylinositol synthase catalyzed formation of phosphatidylinositol does not exhibit acyl chain specificity. Biochemistry 54:1151–1153
D’Souza K, Kim YJ, Balla T, Epand RM (2014) Distinct properties of the two isoforms of CDP-diacylglycerol synthase. Biochemistry 53:7358–7367
English AR, Voeltz GK (2013) Endoplasmic reticulum structure and interconnections with other organelles. Cold Spring Harb Perspect Biol 5:a013227
Gantayet A, Jegatheswaran J, Jayakumaran G, Topham MK, Epand RM (2011) Endocannabinoids and diacylglycerol kinase activity. Biochim Biophys Acta 1808:1050–1053
Garner K, Hunt AN, Koster G, Somerharju P, Groves E, Li M, Raghu P, Holic R, Cockcroft S (2012) Phosphatidylinositol transfer protein, cytoplasmic 1 (PITPNC1) binds and transfers phosphatidic acid. J Biol Chem 287:32263–32276
Gijon MA, Riekhof WR, Zarini S, Murphy RC, Voelker DR (2008) Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils. J Biol Chem 283:30235–30245
Godi A, Pertile P, Meyers R, Marra P, Di TG, Iurisci C, Luini A, Corda D, De Matteis MA (1999) ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat Cell Biol 1:280–287
Goto T, Terada N, Inoue T, Nakayama K, Okada Y, Yoshikawa T, Miyazaki Y, Uegaki M, Sumiyoshi S, Kobayashi T, Kamba T, Yoshimura K, Ogawa O (2014) The expression profile of phosphatidylinositol in high spatial resolution imaging mass spectrometry as a potential biomarker for prostate cancer. PLoS ONE 9:e90242
Hammond GRV, Balla T (2015) Polyphosphoinositide binding domains: key to inositol lipid biology. Biochim Biophys Acta (BBA) Mol Cell Biol Lipids 1851:746–758
Hammond SM, Jenco JM, Nakashima S, Cadwallader K, QM Gu, Cook S, Nozawa Y, Prestwich GD, Frohman MA, Morris AJ (1997) Characterization of two alternately spliced forms of phospholipase D1: activation of the purified enzymes by phosphatidylinositol 4,5-bisphosphate, ADP-ribosylation factor, and rho family monomeric gtp-binding proteins and protein kinase C-α. J Biol Chem 272:3860–3868
Hammond GR, Schiavo G, Irvine RF (2009) Immunocytochemical techniques reveal multiple, distinct cellular pools of PtdIns4P and PtdIns(4,5)P(2). Biochem J 422:23–35
Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF (2012) PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science 337:727–730
Hammond GR, Machner MP, Balla T (2014) A novel probe for phosphatidylinositol 4-phosphate reveals multiple pools beyond the Golgi. J Cell Biol 205:113–126
Haucke V (2005) Phosphoinositide regulation of clathrin-mediated endocytosis. Biochem Soc Trans 33:1285–1289
Henne WM, Liou J, Emr SD (2015) Molecular mechanisms of inter-organelle ER-PM contact sites. Curr Opin Cell Biol 35:123–130
Hofbauer HF, Schopf FH, Schleifer H, Knittelfelder OL, Pieber B, Rechberger GN, Wolinski H, Gaspar ML, Kappe CO, Stadlmann J, Mechtler K, Zenz A, Lohner K, Tehlivets O, Henry SA, Kohlwein SD (2014) Regulation of gene expression through a transcriptional repressor that senses acyl-chain length in membrane phospholipids. Dev Cell 29:729–739
Igal RA (2011) Roles of stearoylCoA desaturase-1 in the regulation of cancer cell growth, survival and tumorigenesis. Cancers 3:2462–2477
Imae K, Inoue, T, Nakasaki Y, Uchida Y, Ohba Y, Kno N, Nakanishi H, Sasaki T, Mitani S, Arai H (2012) LYCAT, a homologue of C. elegans acl-8, acl-9, and acl-10, determines the fatty acid composition of phosphatidylinositol in mice. J Lipid Res 53:335–347
Irvine RF, Divecha N (1992) Phospholipids in the nucleus—metabolism and possible functions. Semin Cell Biol 3:225–235
Ischebeck T, Seiler S, Heilmann I (2010) At the poles across kingdoms: phosphoinositides and polar tip growth. Protoplasma 240:13–31
Jenkins GM, Frohman MA (2005) Phospholipase D: a lipid centric review. Cell Mol Life Sci 62:2305–2316
Jones DR, Sanjuan MA, Merida I (2000) Type Ialpha phosphatidylinositol 4-phosphate 5-kinase is a putative target for increased intracellular phosphatidic acid. FEBS Lett 476:160–165
Kawashima M, Iwamoto N, Kawaguchi-Sakita N, Sugimoto M, Ueno T, Mikami Y, Terasawa K, Sato TA, Tanaka K, Shimizu K, Toi M (2013) High-resolution imaging mass spectrometry reveals detailed spatial distribution of phosphatidylinositols in human breast cancer. Cancer Sci 104:1372–1379
Keinan O, Kedan A, Gavert N, Selitrennik M, Kim S, Karn T, Becker S, Lev S (2014) The lipid-transfer protein Nir2 enhances epithelial-mesenchymal transition and facilitates breast cancer metastasis. J Cell Sci 127:4740–4749
Khan WA, Blobe GC, Richards AL, Hannun YA (1994) Identification, partial purification, and characterization of a novel phospholipid-dependent and fatty acid-activated protein kinase from human platelets. J Biol Chem 269:9729–9735
Kim YJ, Guzman-Hernandez ML, Balla T (2011) A highly dynamic ER-derived phosphatidylinositol-synthesizing organelle supplies phosphoinositides to cellular membranes. Dev Cell 21:813–824
Kim S, Kedan A, Marom M, Gavert N, Keinan O, Selitrennik M, Laufman O, Lev S (2013) The phosphatidylinositol-transfer protein Nir2 binds phosphatidic acid and positively regulates phosphoinositide signalling. EMBO Rep 14:891–899
Kim YJ, Guzman-Hernandez ML, Wisniewski E, Balla T (2015) Phosphatidylinositol-phosphatidic acid exchange by Nir2 at ER-PM contact sites maintains phosphoinositide signaling competence. Dev Cell 33:549–561
Kimura T, Jennings W, Epand RM (2016) Roles of specific lipid species in the cell and their molecular mechanism. Prog Lipid Res 62:75–92
Kobayashi N, Hozumi Y, Ito T, Hosoya T, Kondo H, Goto K (2007) Differential subcellular targeting and activity-dependent subcellular localization of diacylglycerol kinase isozymes in transfected cells. Eur J Cell Biol 86:433–444
Kohyama-Koganeya A, Watanabe M, Hotta Y (1997) Molecular cloning of a diacylglycerol kinase isozyme predominantly expressed in rat retina. FEBS Lett 409:258–264
Koivunen J, Aaltonen V, Peltonen J (2006) Protein kinase C (PKC) family in cancer progression. Cancer Lett 235:1–10
Krauss M, Haucke V (2007) Phosphoinositide-metabolizing enzymes at the interface between membrane traffic and cell signalling. EMBO Rep 8:241–246
Lands WE (1958) Metabolism of glycerolipides; a comparison of lecithin and triglyceride synthesis. J Biol Chem 231:883–888
Lands WE (1960) Metabolism of glycerolipids 2. The enzymatic acylation of lysolecithin. J Biol Chem 235:2233–2237
Lands WE, Merkl I (1963) Metabolism of glycerolipids. III. Reactivity of various acyl esters of coenzyme a with alpha’-acylglycerophosphorylcholine, and positional specificities in lecithin synthesis. J Biol Chem 238:898–904
Lands WE, Inoue M, Sugiura Y, Okuyama H (1982) Selective incorporation of polyunsaturated fatty acids into phosphatidylcholine by rat liver microsomes. J Biol Chem 257:14968–14972
Lee SY, Wenk MR, Kim Y, Nairn AC, De Camilli P (2004) Regulation of synaptojanin 1 by cyclin-dependent kinase 5 at synapses. Proc Natl Acad Sci USA 101:546–551
Lee HC, Inoue T, Sasaki J, Kubo T, Matsuda S, Nakasaki Y, Hattori M, Tanaka F, Udagawa O, Kono N, Itoh T, Ogiso H, Taguchi R, Arita M, Sasaki T, Arai H (2012) LPIAT1 regulates arachidonic acid content in phosphatidylinositol and is required for cortical lamination in mice. Mol Biol Cell 23:4689–4700
Liscovitch M, Chalifa V, Pertile P, Chen CS, Cantley LC (1994) Novel function of phosphatidylinositol 4,5-bisphosphate as a cofactor for brain membrane phospholipase D. J Biol Chem 269:21403–21406
Lung M, Shulga YV, Ivanova PT, Myers DS, Milne SB, Brown HA, Topham MK, Epand RM (2009) Diacylglycerol kinase epsilon is selective for both acyl chains of phosphatidic acid or diacylglycerol. J Biol Chem 284:31062–31073
Luo B, Prescott SM, Topham MK (2004) Diacylglycerol kinase zeta regulates phosphatidylinositol 4-phosphate 5-kinase Ialpha by a novel mechanism. Cell Signal 16:891–897
Ma X, Chong L, Tian R, Shi R, Hu TY, Ouyand Z, Xia Y (2016) Identification and quantitation of lipid C=C location isomers: a shotgun lipidomics approach enabled by photochemical reaction. Proc Natl Acad Sci USA 113:2573–2578
Madani S, Hichami A, Legrand A, Belleville J, Khan NA (2001) Implication of acyl chain of diacylglycerols in activation of different isoforms of protein kinase C. FASEB J 15:2595–2601
Marignani PA, Epand RM, Sebaldt RJ (1996) Acyl chain dependence of diacylglycerol activation of protein kinase C activity in vitro. Biochem Biophys Res Commun 225:469–473
Matsuda S, Inoue T, Lee HC, Kono N, Tanaka F, Gengyo-Ando K, Mitani S, Arai H (2008) Member of the membrane-bound O-acyltransferase (MBOAT) family encodes a lysophospholipid acyltransferase with broad substrate specificity. Genes Cells 13:879–888
Matsui H, Hozumi Y, Tanaka T, Okada M, Nakano T, Suzuki Y, Iseki K, Kakehata S, Topham MK, Goto K (2014) Role of the N-terminal hydrophobic residues of DGKepsilon in targeting the endoplasmic reticulum. Biochim Biophys Acta 1841:1440–1450
Milne SB, Ivanova PT, Armstrong MD, Myers DS, Lubarda J, Shulga YV, Topham MK, Brown HA, Epand RM (2008) Dramatic differences in the roles in lipid metabolism of two isoforms of diacylglycerol kinase. Biochemistry 47:9372–9379
Minville-Walz M, Pierre AS, Pichon L, Bellenger S, Fevre C, Bellenger J, Tessier C, Narce M, Rialland M (2010) Inhibition of stearoyl-CoA desaturase 1 expression induces CHOP-dependent cell death in human cancer cells. PLoS ONE 5:e14363
Moritz A, De Graan PN, Gispen WH, Wirtz KW (1992) Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem 267:7207–7210
Moseley JB, Goode BL (2006) The yeast actin cytoskeleton: from cellular function to biochemical mechanism. Microbiol Mol Biol Rev 70:605–645
Nadler A, Reither G, Feng S, Stein F, Reither S, Muller R, Schultz C (2013) The fatty acid composition of diacylglycerols determines local signaling patterns. Angew Chem Int Ed Engl 52:6330–6334
Naguib A, Bencze G, Engle DD, Chio II, Herzka T, Watrud K, Bencze S, Tuveson DA, Pappin DJ, Trotman LC (2015) p53 mutations change phosphatidylinositol acyl chain composition. Cell Rep 10:8–19
Oude Weernink PA, de Lopez JM, Schmidt M (2007) Phospholipase D signaling: orchestration by PIP2 and small GTPases. Naunyn Schmiedebergs Arch Pharmacol 374:399–411
Pettitt TR, Wakelam MJ (1993) Bombesin stimulates distinct time-dependent changes in the sn-1,2-diradylglycerol molecular species profile from Swiss 3T3 fibroblasts as analysed by 3,5-dinitrobenzoyl derivatization and h.p.l.c. separation. Biochem J 289(Pt 2):487–495
Pettitt TR, Martin A, Horton T, Liossis C, Lord JM, Wakelam MJ (1997) Diacylglycerol and phosphatidate generated by phospholipases C and D, respectively, have distinct fatty acid compositions and functions. Phospholipase D-derived diacylglycerol does not activate protein kinase C in porcine aortic endothelial cells. J Biol Chem 272:17354–17359
Pettitt TR, McDermott M, Saqib KM, Shimwell N, Wakelam MJ (2001) Phospholipase D1b and D2a generate structurally identical phosphatidic acid species in mammalian cells. Biochem J 360:707–715
Putney JW, Tomita T (2012) Phospholipase C signaling and calcium influx. Adv Biol Regul 52:152–164
Puttmann M, Aufenanger J, von OE, Durholt S, Harenberg J, Harenberg J, Hoffmann GE (1993) Increased phospholipase A activities in sera of intensive-care patients show sn-2 specificity but no acyl-chain selectivity. Clin Chem 39:782–788
Rajotte D, Haddad P, Haman A, Cragoe EJ Jr, Hoang T (1992) Role of protein kinase C and the Na+/H+ antiporter in suppression of apoptosis by granulocyte macrophage colony-stimulating factor and interleukin-3. J Biol Chem 267:9980–9987
Rodriguez de Turco EB, Tang W, Topham MK, Sakane F, Marcheselli VL, Chen C, Taketomi A, Prescott SM, Bazan NG (2001) Diacylglycerol kinase epsilon regulates seizure susceptibility and long-term potentiation through arachidonoyl- inositol lipid signaling. Proc Natl Acad Sci USA 98:4740–4745
Rogasevskaia TP, Coorssen JR (2015) The role of phospholipase D in regulated exocytosis. J Biol Chem 290:28683–28696
Rohacs T (2009) Phosphoinositide regulation of non-canonical transient receptor potential channels. Cell Calcium 45:554–565
Routt SM, Bankaitis VA (2004) Biological functions of phosphatidylinositol transfer proteins. Biochem Cell Biol 82:254–262
Rueda-Rincon N, Bloch K, Derua R, Vyas R, Harms A, Hankemeier T, Khan NA, Dehairs J, Bagadi M, Binda MM, Waelkens E, Marine JC, Swinnen JV (2015) p53 attenuates AKT signaling by modulating membrane phospholipid composition. Oncotarget 6:21240–21254
Saarikangas J, Zhao H, Lappalainen P (2010) Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev 90:259–289
Santarius M, Lee CH, Anderson RA (2006) Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem J 398:1–13
Sarkes D, Rameh LE (2010) A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5P and other phosphoinositides. Biochem J 428:375–384
Scaglia N, Igal RA (2008) Inhibition of Stearoyl-CoA Desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int J Oncol 33:839–850
Schmid AC, Wise HM, Mitchell CA, Nussbaum R, Woscholski R (2004) Type II phosphoinositide 5-phosphatases have unique sensitivities towards fatty acid composition and head group phosphorylation. FEBS Lett 576:9–13
Shulga YV, Myers DS, Ivanova PT, Milne SB, Brown HA, Topham MK, Epand RM (2010) Molecular species of phosphatidylinositol-cycle intermediates in the endoplasmic reticulum and plasma membrane. Biochemistry 49:312–317
Shulga YV, Topham MK, Epand RM (2011) Regulation and functions of diacylglycerol kinases. Chem Rev 111:6186–6208
Shulga YV, Anderson RA, Topham MK, Epand RM (2012) Phosphatidylinositol-4-phosphate 5-kinase isoforms exhibit acyl chain selectivity for both substrate and lipid activator. J Biol Chem 287:35953–35963
Suh BC, Hille B (2008) PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys 37:175–195
Suh BC, Leal K, Hille B (2010) Modulation of high-voltage activated Ca(2+) channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron 67:224–238
Tamura Y, Harada Y, Nishikawa S, Yamano K, Kamiya M, Shiota T, Kuroda T, Kuge O, Sesaki H, Imai K, Tomii K, Endo T (2013) Tam41 is a CDP-diacylglycerol synthase required for cardiolipin biosynthesis in mitochondria. Cell Metab 17:709–718
Tang W, Bunting M, Zimmerman GA, McIntyre TM, Prescott SM (1996) Molecular cloning of a novel human diacylglycerol kinase highly selective for arachidonate-containing substrates. J Biol Chem 271:10237–10241
Thompson LJ, Fields AP (1996) betaII protein kinase C is required for the G2/M phase transition of cell cycle. J Biol Chem 271:15045–15053
Thompson W, MacDonald G (1976) Cytidine diphosphate diglyceride of bovine brain. Positional distribution of fatty acids and analysis of major molecular species. Eur J Biochem 65:107–111
van Corven EJ, van Rijswijk A, Jalink K, van der Bend RL, van Blitterswijk WJ, Moolenaar WH (1992) Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J 281(Pt 1):163–169
Vasudevan L, Jeromin A, Volpicelli-Daley L, De CP, Holowka D, Baird B (2009) The beta- and gamma-isoforms of type I PIP5K regulate distinct stages of Ca2+ signaling in mast cells. J Cell Sci 122:2567–2574
Vicinanza M, D’Angelo G, Di CA, De Matteis MA (2008) Phosphoinositides as regulators of membrane trafficking in health and disease. Cell Mol Life Sci 65:2833–2841
Weeks R, Dowhan W, Shen H, Balantac N, Meengs B, Nudelman E, Leung DW (1997) Isolation and expression of an isoform of human CDP-diacylglycerol synthase cDNA. DNA Cell Biol 16:281–289
Wenk MR, De Camilli P (2004) Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci USA 101:8262–8269
Yaradanakul A, Feng S, Shen C, Lariccia V, Lin MJ, Yang J, Kang TM, Dong P, Yin HL, Albanesi JP, Hilgemann DW (2007) Dual control of cardiac Na+Ca2+ exchange by PIP(2): electrophysiological analysis of direct and indirect mechanisms. J Physiol 582:991–1010
Acknowledgments
This work was supported by the Natural Sciences and Engineering Council of Canada, Grant 9848.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Epand, R.M. Features of the Phosphatidylinositol Cycle and its Role in Signal Transduction. J Membrane Biol 250, 353–366 (2017). https://doi.org/10.1007/s00232-016-9909-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-016-9909-y