Abstract
Gap junctions and gap junction communication have long been recognized to play roles in tissue organization and remodeling through both cell autonomous and intercellular means. We hypothesized that these processes become dysregulated during pancreas cancer progression. Molecular and histological characterization of the gap junction protein, connexin43, during progression of pancreatic ductal adenocarcinoma could yield insight into how these events may contribute to or be modulated during carcinogenesis. In a mouse model of pancreatic ductal adenocarcinoma generated through targeted endogenous expression of KrasG12D in the murine pancreas, we examined the evolving expression and localization of connexin43. Overall, connexin43 expression increased over time, and its localization became more widespread. At early stages, connexin43 is found almost exclusively in association with the basolateral membrane of duct cells found in invasive lesions. Connexin43 became increasingly associated with the surrounding stroma over time. Connexin43 phosphorylation was also altered during tumorigenesis, as assessed by migrational changes of the protein in immunoblots. These data suggest a potential role for gap junctions and connexin43 in mediating interactions between and amongst the stromal and epithelial cells in pancreatic ductal adenocarcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
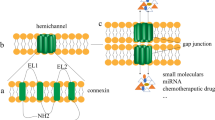
Alterations in gap junction biology have long been recognized to contribute to tumor promotion and progression (Cronier et al. 2009; Naus and Laird 2010). Gap junctions are specialized membrane domains containing channels, made up of connexins, that allow exchange of small molecules (<1,000 Da) including ions, metabolites, and second messengers (e.g., Ca2+ and IP3) between neighboring cells (Loewenstein 1981; Saez et al. 2003; Willecke et al. 2002). A wealth of correlative evidence in vivo and in cell lines indicates that gap junctional intercellular communication (GJC) and connexin expression can regulate proliferation and play key tumor prevention roles (Cronier et al. 2009). Loss of GJC is a common feature of transformed cells and tissues (Cronier et al. 2009; Hossain et al. 1989; Naus and Laird 2010). In fact, modulation or loss of connexin expression and localization have been correlated with degree of carcinogenesis in several human tissues including breast, ovary and cervix (Hanna et al. 1999; King et al. 2000; Laird et al. 1999). Mice lacking Cx32 are 25 times more susceptible to carcinogen-induced liver tumors (Moennikes et al. 1999; Temme et al. 1997) and 3 times more susceptible to radiation-induced liver tumors (King and Lampe 2004). Tumor promoters, activated oncogenes (e.g., src, ras, mos, v-raf), growth factors (e.g., EGF, PDGF, FGF) and carcinogens all decrease GJC (Fitzgerald and Yamasaki 1990; Jou et al. 1995; Kihara et al. 1990; Trosko et al. 1990). For example, down-regulation of metabolic coupling through gap junctions after treatment with various tumor promoters, such as 12-O-tetradecanoylphorbol-13-acetate (TPA), has been observed in a variety of cell systems. Viral infection or transfection of viral encoded oncogenes, such as v-src and polyomavirus middle T-antigen into GJC-competent cells results in decreased GJC (Lau et al. 1996). Importantly, connexin mutations do not appear to be common in human tumors (Yamasaki et al. 1999). Rather, two epigenetic events, silencing of expression via gene methylation (King et al. 2002; Piechocki et al. 1999) and loss of connexin localization from cell-cell interfaces (Krutovskikh et al. 1994), have been observed to correlate with carcinogenesis. Thus, there is the potential to reverse these effects during tumorigenesis. In fact, a chemopreventive role for GJC has been established both in vitro and in vivo by observations that connexin expression and GJC function are up-regulated by several cancer preventive agents including various retinoids and carotenoids (Hossain et al. 1989). Ectopic expression of connexin43 (Cx43) in some tumor cells and cell lines can restore growth control (Chen et al. 1995; Cronier et al. 2009). Paradoxically some tumors, particularly at later stages, can actually begin to overexpress Cx43. For example, malignant gliomas, a tumor which, like pancreatic ductal adenocarcinoma (PDA), is highly aggressive, metastatic and resistant to treatment, shows Cx43 upregulation (Cronier et al. 2009; Zhang et al. 1999). Recently, several studies have raised questions as to whether connexins can play key roles in tumorigenesis independent of gap junction formation (Naus and Laird 2010).
PDA, the most common cancer of the pancreas, is the fourth leading cause of cancer-related death in the United States (Siegel et al. 2012) with an annual incidence and mortality of >40,000 people and a 5 year overall survival rate of <3 % (Hruban 2007). The extremely high mortality occurs primarily because symptoms do not present until tumors are either locally unresectable or widely metastatic. The highly metastatic nature of this cancer is apparent as surgical resection with clean margins in patients diagnosed at “early stages” still inevitably leads to recurrent or metastatic disease (Allison et al. 1998; Yeo et al. 2002). These tumors are also highly resistant to virtually all chemical and radiotherapies (Hruban 2007).
Interestingly and frustratingly, effective treatments have been difficult to achieve despite a fairly good understanding of many of the mutational events present in PDA. Pancreas cancer exhibits activating mutations in KRAS in over 90 % of cases (Almoguera et al. 1988) leading to the belief that this is an initiating event in PDA (Hingorani et al. 2003). In addition, overexpression of ERBB2/HER2, a member of the family of epidermal growth factor receptors, is a common early event in PDA progression (Hansel et al. 2003; Hingorani et al. 2005). Animal models of preinvasive and invasive PDA (Aguirre et al. 2003; Hingorani et al. 2003, 2005) have been generated through the targeted physiologic expression of oncogenic Kras G12D to the mouse pancreas (hereafter termed K* mouse). These models faithfully mimic the clinical syndrome, histopathology and genetic progression of PDA found in humans. Resected pancreata from these animals demonstrate the full spectrum of preinvasive lesions seen in patients, and the lesions progress histologically over time culminating in fully invasive and metastatic disease. During PDA progression, the cellular makeup of the pancreas changes dramatically; there is a loss of acinar cells with a concomitant increase in glandular epithelial cells. In addition there is a robust fibroinflammatory or desmoplastic reaction in which the stromal components can outnumber the tumor epithelial cells in both the K* model and human cancer. The relevance of the K* mouse models to human pancreas cancer has been validated by an independent panel of human pancreas cancer pathologists assembled by the NCI/MMHCC (Hruban et al. 2006).
Recently, there has been an increased appreciation for the role of the tumor microenvironment in disease progression (Erkan et al. 2010). In particular, increasing attention is being paid to the interaction of pancreatic tumor epithelial cells with the surrounding stroma. Indeed, paracrine interaction between these cell compartments has been observed (Bailey et al. 2008; Brentnall et al. 2012; Tian et al. 2009; Yauch et al. 2008). Cx43 expression in the normal pancreas is quite low and other connexins are responsible for GJC in this tissue. The endocrine cells of the islet express Cx36 (Serre-Beinier et al. 2000), while the exocrine acinar cells are coupled by Cx26 and Cx32 (Meda et al. 1993). The acinar and islet cells make up the mass majority of a normal pancreas. Cx43 is found at low levels in association with endothelial cells or the epithelial duct cells and associated fibroblasts, which only sparsely populate the normal pancreas (Theis et al. 2004). However, as discussed above, these are the cells that expand during PDA. Interestingly, a paracrine-like role for Cx43 in fibroblast activation has recently been suggested, where mast cells could activate fibroblasts via GJC (Pistorio and Ehrlich 2011). It is intriguing to consider that gap junctions and Cx43 could be involved in epithelial: stromal interactions during PDA progression. Here, we present preliminary data indicating that overall Cx43 expression increases and that its localization increasingly shifts to the stromal compartment during cancer progression. This model could help us determine whether increased Cx43 expression during progression is playing a tumor suppressive role and/or a stromal-tumor communication role.
Materials and Methods
Antibodies and Reagents
All general chemicals, unless otherwise noted, were purchased from Fisher Scientific. The rabbit anti-Cx43 antibody (C6219) and anti-α-smooth muscle actin were purchased from Sigma (St. Louis, MO). The mouse antibody NT1 was raised against amino acids 1–20 of Cx43 at the Fred Hutchinson Cancer Research Center Hybridoma Development Facility (Seattle, WA).
Mouse Strains and Tissue Processing
Mice expressing a single allele of KrasG12D in the pancreas were generated as described previously (Hingorani et al. 2003). Animals were euthanized at the appropriate time points using an overdose of isofluorane followed by cervical dislocation and necropsies were performed. Pancreata were bisected laterally, one half was flash frozen and the other was fixed in 10 % neutral buffered formalin, paraffin embedded and processed for tissue sectioning.
Histology and Immunofluorescence
8 μm sections were analyzed by hematoxylin and eosin staining or immunofluorescence. For immunofluorescence experiments, deparaffinized sections underwent antigen retrieval in a solution of Tris/EDTA pH9.0, were blocked with 10 % normal goat serum (Sigma) and 1 % IgG-free BSA (Jackson Labs, Westgrove, PA). Slides were incubated with primary antibody overnight. Samples were then incubated with anti-rabbit secondary antibodies conjugated to AlexaFluor 635 or anti-mouse IgG2a conjugated to AlexaFluor 546 (Invitrogen, Carlsbad, CA), counterstained with DAPI (4′,6-diamidino-2-phenylindole) and mounted in ProLong Antifade (Invitrogen). Slides were analyzed using a Nikon Eclipse 80i bright field/fluorescence microscope and images collected with a Nikon DS-U1 color camera controlled with NIS Elements (Nikon, v3.1) software.
Immunoblotting
Frozen tissue was weighed and lysed with 5 volumes of lysis buffer [0.5 % deoxycholate, 0.5 % Triton X-100, 10 mM NaF, 1 mM Na3VO4, 5 % β-mercaptoethanol, 1 mM PMSF and 1× complete protease inhibitors (Roche Molecular Biochemicals, Alameda, CA) in TBS] and sonicated. We have found that tissue lysates, especially from tumor tissue, often contain large amounts of IgG that can make it difficult to resolve and detect Cx43 phosphoforms by SDS-PAGE. To minimize this effect tissue lysates were incubated with protein G agarose to remove IgG. Protein assays were performed on these clarified lysates and 100 μg loaded per sample. However, because the IgG content likely varied from animal to animal, it may be difficult to make direct comparisons of Cx43 levels across these samples. This depletion of IgG also allowed the use of anti-mouse secondary antibodies on mouse tissue. For the MDCK cell lysate control, cells were lysed directly in sample buffer. After sonication, samples were separated by sodium dodecylsulfate-10 % polyacrylamide gel electrophoresis (SDS-PAGE). After immunoblotting, protein was detected with rabbit and mouse primary antibodies. Primary antibodies were sequentially visualized with fluorescent dye-labeled secondary antibodies AlexaFluor 680 goat anti-rabbit (Invitrogen) followed by IRDye800-conjugated rabbit anti-mouse IgG1 (Rockland Immunochemicals, Gilbertsville, PA) using the LI-COR Biosciences Odyssey infrared imaging system (Omaha, NE) and associated software.
Results
Cx43 Expression and Localization Change During Pancreatic Cancer Progression
Pancreata from wild-type (WT) mice are made up predominantly of acinar cells but ducts and vessels can be also be visualized by the distinct organization of nuclei when stained with DAPI. In Fig. 1, sections of pancreas from WT and K* mice are shown labeled with DAPI and an antibody to Cx43. In Fig. 1, a typical small duct in WT pancreas can be seen with the characteristic punctate gap junction signal for Cx43 on the basolateral side of the duct. The remaining panels show staining from pancreata of K* mice taken at 6, 12 and 16 months of age. Previous studies have shown there is a progressive loss of acinar cells and an increase in both number and grade of preinvasive lesions as these mice age (Hingorani et al. 2003). This is reflected in Fig. 1 by the organization of the DAPI labeled nuclei, where the glandular structures become more numerous and show increasing cellular and architectural atypia over time. These data also show Cx43 expression increasing during PDA progression. At 6 months, increased Cx43 expression can be found tightly associated with some, but not all, ducts on the basolateral membrane. For example, in the 6 months panel the duct on the right shows Cx43 puncta circumscribing the duct structure, while the one on the left shows very little Cx43 labeling. By 12 months Cx43 can be detected circumscribing many ducts but the association between Cx43 and ductal structures appear looser and less organized (Fig. 1, 12 months). This could reflect changes in the ducts as they become less organized during cancer progression or a change in the cell population expressing Cx43 as the stromal content evolves. By 16 months, Cx43 expression is higher, is much less organized and is found both in association with ducts (Fig. 1, 16 months, lower right) and in the surrounding stroma (Fig. 1, 16 months).
Cx43 Phosphorylation Changes During Pancreatic Cancer Progression
Cx43 normally migrates as several distinct bands that reflect the phosphorylation state of the protein; these are often named P0, P1, P2, etc., with P0 co-migrating with Cx43 that has been dephosphorylated with phosphatases (Musil et al. 1990). Immunoblots were probed with Cx43 antibodies to both the C and N-termini: Rb Cx43, a rabbit polyclonal antibody made to the C terminus and NT1, a mouse monoclonal antibody made to the N terminus. Use of two reliable Cx43 antibodies that yielded overlaying signal gave us confidence that the low signals we observed were, in fact, Cx43.
Expression was very low in WT pancreas though both Rb Cx43 and NT1 detected a faint doublet that may include the P0 form of Cx43 (Fig. 2, WT compared to cell lysate). At 6 months, pancreata from duplicate K* animals show Cx43 migrating as apparent P2, a phosphoisoform associated with functional junctions. At 12 and 16 months an even slower migrating form of Cx43 appears and is maintained. This slow migrating form is also found in the lysate from cells containing activated src, a condition associated with gap junction closure. The samples from the K* mice at 6–16 months shown in this immunoblot demonstrate considerable variation in the levels of Cx43. Because the K* mice need to develop subsequent mutations for tumor progression to proceed and these events are stochastic as demonstrated by their wide ranging lifespan, we believe that this variation in Cx43 expression could be related to the extent of cancer progression. We speculate that these changes in Cx43 phosphorylation and migration are associated with a functional change in gap junction communication between or amongst cancer and stromal cells.
Cx43 phosphorylation changes during PDA progression. Pancreatic tissue lysates from 1 normal (WT) mouse and 2 each K* mice taken at 6, 12, and 16 months were analyzed by SDS-PAGE and immunoblot. A lysate from MDCK cells expressing Cx43 and activated src is included as a control (cell lysate). Blots were probed with antibodies to the C terminus (Rb Cx43) and N terminus (NT1) of Cx43. Protein marker molecular weights (from top): 64, 50, and 38 kDa
Cx43 Expression Is Increasingly Heterogeneous and Associated with Stromal Cells During PDA Progression
As discussed above, PDA exhibits a distinct and robust stromal response containing a heterogeneous mixture of immune and connective cells and extracellular material (Cubilla and Fitzgerald 1976; Hruban et al. 2001; Klimstra and Longnecker 1994; Tian et al. 2009). To examine which cells are making apparent gap junctions, we performed Cx43 immunofluorescence and hematoxylin and eosin staining on serial sections from pancreatic tissue harvested at 6 and 16 months. At 6 months Cx43 is closely associated with ducts (Fig. 3a), as described above, but hematoxylin and eosin staining indicates gap junctions are also closely associated with cells surrounding the precursor lesions (Fig. 3c, arrowheads). In 16 month-old animals, Cx43 expression is still found closely associated with some ducts (Fig. 3b, arrowheads) but is also highly expressed in the stroma, seemingly independent of the ducts. Figure 3e, f shows colabeling of tissue with antibodies to Cx43 in green and α-smooth muscle actin (SMA) in red. SMA is used as a marker for blood vessels and activated fibroblasts (Erkan et al. 2012), both of which could be expressing Cx43. In Fig. 3e, SMA clearly defines a blood vessel (arrow); we see little to no association with Cx43 with vessels while we do clearly see Cx43 circumscribing the adjacent duct. In Fig. 3f, SMA is likely marking activated fibroblasts surrounding the duct (arrowhead). Here Cx43 is found in stroma, seemingly independent of SMA staining. Further labeling and histology is required to more fully characterize these cells. One interesting question is whether these apparent gap junctions are being utilized for homocellular communication in the stroma or whether they are used for heterocellular communication, thus allowing interaction between the potential tumor cells and their surrounding environment.
Gap junctions become increasingly associated with the stroma during PDA progression. Serial paraffin-embedded sections from pancreas taken from K* mice at 6 months (a, c) and 16 months (b, d) were labeled with antibody to Cx43 (green) and DAPI (blue) (a, b) or hematoxylin and eosin (c, d). Arrowheads point to specific areas of Cx43 expression discussed in Results. e, f Pancreas tissue from a 16 month mouse was stained with antibodies to Cx43 (green) or SMA (red) (DAPI is in blue). The arrow in e points to a blood vessel and the arrowhead in f to a duct. Bars = 50 μm
Another question that arises is whether these Cx43 expressing stromal cells are the same cell type found adjacent to ductal cells in younger animals but that have proliferated and migrated or whether this represents a separate communication compartment active during this later stage of tumorigenesis. It is also possible that these cells are not engaged in gap junction communication; the immunoblot data indicates that much of Cx43 at this time point is a slow migrating isoform, which can be associated with closed channels. Further experiments are underway to examine these events.
Discussion
Progression of PDA results in a dramatic change in the cellular makeup of the pancreas. There is a loss of acinar cells with a concomitant increase in glandular epithelial cells and infiltration of connective and immune components, or stroma, which is a distinct feature of PDA. We show here that this shift is accompanied by an increase in expression of Cx43. Initially this increase in Cx43 expression is associated with ductal structures. Changes in the ducts have been well characterized through elaboration of histological (Cubilla and Fitzgerald 1976; Hruban et al. 2001; Klimstra and Longnecker 1994) and genetic mouse models (Hingorani et al. 2003). These models provide evidence that pancreas cancer evolves from definable precursor lesions (Hruban et al. 2000). These preinvasive lesions, collectively termed pancreatic intraepithelial neoplasias, or PanINs, were codified by an assembled working group of pathologists (Kern et al. 2001) into a system of classification originally proposed by Klimstra and Longnecker (1994). In this scheme, the lesions are divided into three discrete stages (PanIN-1, -2, and -3) characterized by increasing degrees of cellular and architectural atypia. It may be that the changes we see in Cx43 expression over time are associated with specific lesions or stages of PDA; to examine this idea a rigorous histological analysis of Cx43 localization is presently underway. Previous studies have indicated that Cx26 is overexpressed in human pancreatic cancer (Garcia-Rodriguez et al. 2011; Kyo et al. 2008). It will be interesting to confirm that this occurs in the mouse model and to compare the Cx26 expression profile to what we see with Cx43.
It is notable that PanIN lesions overexpress ERBB2/HER2, a member of the family of epidermal growth factor receptors and exhibit increased levels of phosphorylated extracellular signal-related kinase (ERK) (Hingorani et al. 2005). We know that gap junction communication and connexin localization can be modulated via activation of ERK through growth factor stimulation (Kanemitsu and Lau 1993). We also know that a slow migrating isoform of Cx43, similar to that seen in the K* animals at 16 months, is associated with ERK activation. Given the localization of Cx43 at the apparent interface between PanINs and the stroma (Figs. 1 and 3) it is plausible that gap junctions may be regulated or even play a regulatory role in ERK activation.
At the later time points Cx43 expression increases both around the PanINs, where it seems to be expressed through multiple cell layers circumscribing these lesions and is also found increasingly in the stroma, seemingly independent of PanINs. This shift in expression also parallels the progression of PanINs to carcinoma and invasive stages suggesting that gap junctions may play a role in invasion and metastasis. It is possible that these patterns correspond to an epithelial cell-associated communication compartment that is permissive to invasion possibly through Cx43 mediated interaction with stromal cells. The lack of localization with SMA could support this. However, it is also possible that the stromal and PanIN-associated Cx43 expression represent two distinct and independent communication compartments. Several markers used to distinguish different cell types in PDA including those for epithelial cells (cytokeratin 19), immune cells (CD45), and pancreatic stellate cells (smooth muscle actin and glial fibrillary acidic protein) (Erkan et al. 2012) will be useful to distinguish between these possibilities.
PDA, like other cancers, requires cells to alter not only cell autonomous features but also interactions with neighboring cells and tissue components—functions that connexins fulfill (Laird 2006). If Cx43 is indeed acting to regulate invasion and metastasis through interactions between epithelial tumor cells and the stroma, modulating these interactions could represent a viable therapeutic strategy.
Reference
Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, Redston MS, DePinho RA (2003) Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev 17:3112–3126
Allison DC, Piantadosi S, Hruban RH, Dooley WC, Fishman EK, Yeo CJ, Lillemoe KD, Pitt HA, Lin P, Cameron JL (1998) DNA content and other factors associated with ten-year survival after resection of pancreatic carcinoma. J Surg Oncol 67:151–159
Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M (1988) Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53:549–554
Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA (2008) Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res 14:5995–6004
Brentnall TA, Lai LA, Coleman J, Bronner MP, Pan S, Chen R (2012) Arousal of cancer-associated stroma: overexpression of palladin activates fibroblasts to promote tumor invasion. PLoS One 7:e30219
Chen S-C, Pelletier DB, Ao P, Boynton AL (1995) Connexin43 reverses the phenotype of transformed cells and alters their expression of cyclin/cyclin dependent kinases. Cell Growth Differ 6:681–690
Cronier L, Crespin S, Strale PO, Defamie N, Mesnil M (2009) Gap junctions and cancer: new functions for an old story. Antioxid Redox Signal 11:323–338
Cubilla AL, Fitzgerald PJ (1976) Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res 36:2690–2698
Erkan M, Reiser-Erkan C, Michalski CW, Kleeff J (2010) Tumor microenvironment and progression of pancreatic cancer. Exp Oncol 32:128–131
Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, Esposito I, Friess H, Gress TM, Habisch HJ, Hwang RF, Jaster R, Kleeff J, Kloppel G, Kordes C, Logsdon CD, Masamune A, Michalski CW, Oh J, Phillips PA, Pinzani M, Reiser-Erkan C, Tsukamoto H, Wilson J (2012) StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut 61:172–178
Fitzgerald DJ, Yamasaki H (1990) Tumor promotion: models and assay systems. Teratog Carcinog Mutagen 10:89–102
Garcia-Rodriguez L, Perez-Torras S, Carrio M, Cascante A, Garcia-Ribas I, Mazo A, Fillat C (2011) Connexin-26 is a key factor mediating gemcitabine bystander effect. Mol Cancer Ther 10:505–517
Hanna EA, Umhauer S, Roshong SL, Piechocki MP, Fernstrom MJ, Fanning JD, Ruch RJ (1999) Gap junctional intercellular communication and connexin43 expression in human ovarian surface epithelial cells and ovarian carcinomas in vivo and in vitro. Carcinogenesis 20:1369–1373
Hansel DE, Kern SE, Hruban RH (2003) Molecular pathogenesis of pancreatic cancer. Annu Rev Genomics Hum Genet 4:237–256
Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA (2003) Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4:437–450
Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA (2005) Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7:469–483
Hossain MZ, Wilkens LR, Mehta PP, Loewenstein WR, Bertram JS (1989) Enhancement of gap junctional communication by retinoids correlates with their ability to inhibit neoplastic transformation. Carcinogenesis 10:1743–1748
Hruban RH (2007) Tumors of the pancreas. In: Hruban RH, Pitman MB, Klimstra DS (eds) Atlas of tumor pathology. Armed Forces Institute of Pathology, Washington DC
Hruban RH, Wilentz RE, Kern SE (2000) Genetic progression in the pancreatic ducts. Am J Pathol 156:1821–1825
Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Kloppel G, Longnecker DS, Luttges J, Offerhaus GJ (2001) Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol 25:579–586
Hruban RH, Adsay NV, Albores-Saavedra J, Anver MR, Biankin AV, Boivin GP, Furth EE, Furukawa T, Klein A, Klimstra DS, Kloppel G, Lauwers GY, Longnecker DS, Luttges J, Maitra A, Offerhaus GJ, Perez-Gallego L, Redston M, Tuveson DA (2006) Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res 66:95–106
Jou Y-S, Layhe B, Matesic DF, Chang C-C, de Feijter AW, Lockwood L, Welsch CW, Klaunig JE, Trosko JE (1995) Inhibition of gap junctional intercellular communication and malignant transformation of rat liver epithelial cells by neu oncogene. Carcinogenesis 16:311–317
Kanemitsu MY, Lau AF (1993) Epidermal growth factor stimulates the disruption of gap junctional communication and connexin43 phosphorylation independent of 12-O-tetradecanoyl 13-acetate-sensitive protein kinase C: the possible involvement of mitogen-activated protein kinase. Mol Biol Cell 4:837–848
Kern S, Hruban R, Hollingsworth MA, Brand R, Adrian TE, Jaffee E, Tempero MA (2001) A white paper: the product of a pancreas cancer think tank. Cancer Res 61:4923–4932
Kihara K, Fukui I, Higashi Y, Oshima H (1990) Inhibitory effect of testosterone on gap junctional intercellular communication of human transitional cell carcinoma cell lines. Cancer Res 50:2848–2852
King TJ, Lampe PD (2004) Mice deficient for the gap junction protein Connexin32 exhibit increased radiation-induced tumorigenesis associated with elevated mitogen-activated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis 25:669–680
King TJ, Fukushima LH, Hieber AD, Shimabukuro KA, Sakr WA, Bertram JS (2000) Reduced levels of connexin43 in cervical dysplasia: inducible expression in a cervical carcinoma cell line decreases neoplastic potential with implications for tumor progression. Carcinogenesis 21:1097–1109
King TJ, Fukushima LH, Yasui Y, Lampe PD, Bertram JS (2002) Inducible expression of the gap junction protein connexin43 decreases the neoplastic potential of HT-1080 human fibrosarcoma cells in vitro and in vivo. Mol Carcinog 35:29–41
Klimstra DS, Longnecker DS (1994) K-ras mutations in pancreatic ductal proliferative lesions. Am J Pathol 145:1547–1550
Krutovskikh V, Mazzoleni G, Mironov N, Omori Y, Aguelon AM, Mesnil M, Berger F, Partensky C, Yamasaki H (1994) Altered homologous and heterologous gap-junctional intercellular communication in primary liver tumors associated with aberrant protein localization but not gene mutation of connexin 32. Int J Cancer 56:87–94
Kyo N, Yamamoto H, Takeda Y, Ezumi K, Ngan CY, Terayama M, Miyake M, Takemasa I, Ikeda M, Doki Y, Dono K, Sekimoto M, Nojima H, Monden M (2008) Overexpression of connexin 26 in carcinoma of the pancreas. Oncol Rep 19:627–631
Laird DW (2006) Life cycle of connexins in health and disease. Biochem J 394:527–543
Laird DW, Fistouris P, Batist G, Alpert L, Huynh HT, Carystinos GD, Alaoui-Jamali MA (1999) Deficiency of connexin43 gap junctions is an independent marker for breast tumors. Cancer Res 59:4104–4110
Lau AF, Kurata WE, Kanemitsu MY, Loo LWM, Warn-Cramer BJ, Eckhart W, Lampe PD (1996) Regulation of connexin43 by activated tyrosine protein kinases. J Bioenerg Biomembr 28:357–365
Loewenstein WR (1981) Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev 61:829–913
Meda P, Pepper MS, Traub O, Willecke K, Gros D, Beyer E, Nicholson B, Paul D, Orci L (1993) Differential expression of gap junction connexins in endocrine and exocrine glands. Endocrinology 133:2371–2378
Moennikes O, Buchmann A, Ott T, Willecke K, Schwarz M (1999) The effect of connexin32 null mutation on hepatocarcinogenesis in different mouse strains. Carcinogenesis 20:1379–1382
Musil LS, Beyer EC, Goodenough DA (1990) Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol 116:163–175
Naus CC, Laird DW (2010) Implications and challenges of connexin connections to cancer. Nat Rev Cancer 10:435–441
Piechocki MP, Burk RD, Ruch RJ (1999) Regulation of connexin32 and connexin43 gene expression by DNA methylation in rat liver cells. Carcinogenesis 20:401–406
Pistorio AL, Ehrlich HP (2011) Modulatory effects of connexin-43 expression on gap junction intercellular communications with mast cells and fibroblasts. J Cell Biochem 112:1441–1449
Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC (2003) Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83:1359–1400
Serre-Beinier V, Le Gurun S, Belluardo N, Trovato-Salinaro A, Charollais A, Haefliger JA, Condorelli DF, Meda P (2000) Cx36 preferentially connects beta-cells within pancreatic islets. Diabetes 49:727–734
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Temme A, Buchman A, Babriel HC, Nelles E, Scharz M, Willecke K (1997) High incidence of spontaneous and chemically-induced liver tumors in mice deficient for connexin 32. Curr Biol 7:713–718
Theis M, Mas C, Doring B, Degen J, Brink C, Caille D, Charollais A, Kruger O, Plum A, Nepote V, Herrera P, Meda P, Willecke K (2004) Replacement by a lacZ reporter gene assigns mouse connexin36, 45 and 43 to distinct cell types in pancreatic islets. Exp Cell Res 294:18–29
Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, de Sauvage FJ (2009) Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA 106:4254–4259
Trosko JE, Chang CC, Madhukar BV, Klaunig JE (1990) Chemical, oncogene, and growth factor inhibition of gap junctional intercellular communication: an integrative hypothesis of carcinogenesis. Pathobiology 58:265–278
Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G (2002) Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383:725–737
Yamasaki H, Omori Y, Krutovskikh V, Zhu W, Mironov N, Yamakage K, Mesnil M (1999) Connexins in tumour suppression and cancer therapy. Novartis Found Symp 219:241–260
Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ (2008) A paracrine requirement for hedgehog signalling in cancer. Nature 455:406–410
Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, Coleman J, Abrams RA, Hruban RH (2002) Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg 236:355–366
Zhang W, Couldwell WT, Simard MF, Song H, Lin JH, Nedergaard M (1999) Direct gap junction communication between malignant glioma cells and astrocytes. Cancer Res 59:1994–2003
Acknowledgments
Supported in part by grants CA149554 and GM55632 from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solan, J.L., Hingorani, S.R. & Lampe, P.D. Changes in Connexin43 Expression and Localization During Pancreatic Cancer Progression. J Membrane Biol 245, 255–262 (2012). https://doi.org/10.1007/s00232-012-9446-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-012-9446-2