Abstract
Purpose
Adjusting the antibiotic dose based on an estimation of the glomerular filtration rate (eGFR) may result in subdosing, which may actually be significantly more problematic for intensive care unit (ICU) patients than not adjusting the dose. The aim of this study was to assess the outcomes of antibiotic dose adjustment in ICU patients with renal impairment.
Methods
A retrospective cohort study was conducted in adult patients admitted to an ICU of a Brazilian hospital from January 2014 to December 2015. The eGFR was determined using Cockcroft–Gault and Modified Diet in Renal Disease equations for each day of hospitalization. Treatment failure was defined based on the clinical, laboratory, and radiological criteria.
Results
A total of 126 patients were assessed to meet the inclusion criteria and subsequently enrolled in the study (19.9% of patients admitted to the ICU during the study period). Of the 168 opportunities for dose adjustment, 99 (58.9%) adjustments were made. The mean eGFR in the group with dose adjustment was lower than that in the group without dose adjustment (38.5 vs. 40.7 mL/min/1.73 m2, respectively). The treatment failure rate among patients with dose adjustment and those treated with the usual dose was 59.3 and 38.9%, respectively (p = 0.023), and the mortality rates in the respective groups were 74.1 and 55.5% (p = 0.033). An association between dose adjustment and treatment failure/mortality rates was also observed in the multivariate analysis including the prognostic score.
Conclusions
In ICU patients with renal impairment, adjustments in antibiotic dose based on eGFR, significantly increased the risk of treatment failure and death.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infections are the leading cause of death in intensive care units (ICU) worldwide. Despite the development of new drugs, diagnostic tests, and monitoring tests, the rate of mortality associated with infections in patients admitted to the ICU has increased in recent years [1, 2]. This increase is primarily associated with the rapid emergence of antibiotic-resistant bacteria, delays in starting treatment, and the prescribing of incorrect antimicrobial drugs or the incorrect dose [1, 3, 4].
Prescribing the wrong dose of antibiotic is a common medical error in some groups of patients, including those of extreme age and/or with multiple comorbidities, or critically ill patients admitted to the ICU [5], since these conditions produce physiological disturbances that result in important pharmacokinetic changes. However, data on antibiotic dose adequacy in these specific patient populations are limited [6, 7]. Patients with renal impairment (RI) are commonly exposed to incorrect medication dosing and are, consequently, at higher risk of adverse drug events caused by drug overdosing or subdosing [8].
Many drugs are excreted by the kidneys. When the glomerular filtration barrier is compromised, some substances may accumulate in the body, resulting in toxicity. Thus, in several situations, it is necessary to reduce the dose to avoid harming the patients [9, 10]. This dose adjustment is usually conducted based on the glomerular filtration rate (GFR), which can be estimated using equations derived from patient data and some constants. However, several limitations are associated with these equations [11]. Moreover, the dose indicated for patients with RI differs between databases, which raises doubts when a dose adjustment is being considered. Consequently, suboptimal doses are frequently used, resulting in treatment failure [12, 13].
Most of the studies that have defined the doses and dose adjustments of antimicrobials to be used for patients with RI were performed in healthy patients, patients in non-critical conditions, or even in patients with restricted clinical profiles, such as adults without other associated comorbidities [3]. While dose adjustment is associated with an increased risk of treatment failure or adverse drug reactions, data on the clinical impact of antimicrobial dose adjustment in patients with RI remain unclear. Hence, many doubts still persist regarding the risks and benefits of dose adjustment for different groups of patients, such as those in the ICU, the elderly, newborns, or those with multiple conditions [14,15,16]. The choice is between adjusting the dose and risking negative outcomes for the patients, such as treatment failure or death, and not adjusting the dose and exposing the patients to the risk of severe adverse drug reactions. This is the dilemma frequently faced by ICU healthcare teams when defining the treatment of infections [17]. Thus, the aim of this study was to assess the outcomes of antimicrobial dose adjustment in ICU patients with RI.
Methods
This cohort study was conducted with retrospective data from ICU patients admitted in a tertiary hospital in the northeast region of Brazil. The hospital primarily handles urgent and emergency cases and covers 134 municipalities.
Data were collected from patients’ medical records using a digital form developed in the KoBoToolbox for Android (KoBoToolbox, Harvard Humanitarian Initiative, Cambridge, USA, available at: https://www.kobotoolbox.org/). The data extracted from the patients’ medical records were analyzed, beginning on the day before ICU admission until the last day in the unit. The following data were obtained: cause of admission, comorbidities, medications, laboratory test results, infection sites, cultures, weight, height, daily urine output, relevant medical history, and clinical outcomes. In addition, the severity of each patient’s clinical status upon admission was assessed according to the Simplified Acute Physiology Score (SAPS 3).
All patients admitted between January 2014 and December 2015, aged ≥18 years, who stayed in the ICU for > 24 h were considered eligible for this study. Patients whose missing data did not allow the calculation of the eGFR or the evaluation of treatment results (success/failure) or those patients whose prescribed dose did not match the recommendations of the used guidelines were subsequently excluded from the study.
The Cockcroft–Gault (CG) and the four-variable Modified Diet in Renal Disease (MRD-4) equations were used to calculate the eGFR [18, 19]. The eGFR was calculated daily, and the need for dose adjustment was assessed based on the following sources of information: Micromedex Healthcare Series® and the American Hospital Formulary Service (AHFS) Drug Information Handbook 2015 [20, 21]. Therapeutic drug monitoring through measurement of the serum drug levels is not performed in ICU under study. Thus, the dose adjustment of antibiotics is usually performed as follows: for the first 24 h, the usual dose is prescribed for individuals with normal renal function; from the second day onwards, an adjusted dose is prescribed according to the daily updated eGFR.
After entry into the study, patients were divided into two groups. The first group consisted of patients with a range of eGFR values that indicated dose adjustment but who were treated with the usual dose of the antibiotic (not adjusted) for the whole treatment. The second group consisted of patients with a range of eGFR values that indicated dose adjustment and whose dose was reduced based on this eGFR range (adjusted).
Two outcomes were compared between the groups: treatment failure and death. Treatment failure was assessed by physical examination, organic dysfunction, comorbidities, broadening antimicrobial spectrum, laboratory tests, and examination of microscopic images. Successful treatment was defined by an improvement in the signs and symptoms of infection, while failure was defined by the persistence of infection, clinical deterioration, or death [22, 23].
In the statistical analyses, continuous variables were reported as the mean with the standard deviation for variables with a normal distribution or as the median with the interquartile range for variables that did not present a normal distribution, and comparisons were made using Student’s t test or the Mann–Whitney U test, respectively. Proportions were compared using Pearson’s chi-squared test. The bivariate analysis was conducted to examine the association between the outcomes and antibiotic dose adjustment and clinical features, including sex, age, diagnosis at admission to the ICU, SAPS 3, admission source, and antibiotic class. The SAPS 3 results was categorized into scores of ≤ 57 or > 57. This cutoff point was previously defined as a better prediction of higher mortality in ICU patients in a previous Brazilian study [24]. All significant factors in the bivariate analysis were included in the multiple regression model (Poisson with robust variance). Data were analyzed using STATA 14.2 (Stata Corp., College Station, TX, USA).
Results
During the period covered by this study, a total of 632 patients were admitted in the ICU, among whom 279 (46.9%) presented with RI. Of these 279 patients with RI, 126 (45.2%) met the inclusion criteria and had been prescribed at least one antimicrobial agent that needed dose adjustment during their time in the ICU. The general characteristics of the study population are given in Table 1.
Mortality rate in the studied patient population was 69% (87 patients). The most frequent infection sites were the pulmonary, abdominal, and skin systems, with 47 (37%), 36 (29%), and 10 (7.9%) patients affected, respectively. The site of infection was not identified in 22 (17.5%) patients. Approximately 69 patients (54.8%) had sepsis or septic shock and 93 (73.8%) presented higher probabilities of death (SAPS 3 > 57).
From the 126 patients included in this study, we identified a total of 168 opportunities for dose adjustment of prescribed antimicrobial agents. In all of these cases, antibiotics were used at the standard dose on the first day of treatment. The group of patients with dose adjustment following recommendations based on eGFR values had higher rates of treatment failure (Table 2) and mortality (Table 3) than the group without dose adjustment. Among all patients, 60 (47.6%) showed treatment failure. The mortality rate was higher among patients with therapeutic failure than among those whose treatment was effective (44 [73%] vs. 36 [52%], respectively; p = 0.029). When mortality was assessed only in patients with therapeutic failure, the mortality rate was lower in the dose adjustment group than in the group with no adjustment (7 [43.8%] vs. 9 [56.2%], respectively; p = 0.370).
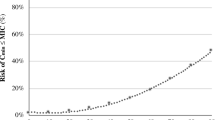
The multivariate analysis with those variables significantly associated with mortality in the bivariate analysis are shown in Fig. 1. With the exception of the use of glycopeptide antibiotics, no other variable was significant in the bivariate analysis for therapeutic failure. Notwithstanding, glycopeptides were not included in the multivariate analysis because only four patients had used glycopeptides and in all cases their doses were adjusted.
Discussion
Th results of this study demonstrate that continuing the practice of adjusting antimicrobial dose based on eGFR values may significantly increase therapeutic failure and mortality rates in ICU patients with RI. The association with higher mortality remained even when the outcome was pooled in the multivariate analysis with the SAPS 3 score, categorized as higher or minor risk of death.
The uncertainties around the adjustment of medication dose in patients with RI have led to a broad discussion on this issue. Nevertheless, a slight change in the recommendations for dose adjustment has occurred, although these are still implemented in a non-individualized manner, independent of the patient’s clinical status. These recommendations are general for a wide range of GFR values, which are often estimated. In several cases, the estimates seemed to be unreliable, mainly because the patient’s serum creatinine concentration was used for all equations and because the level may vary with a patient’s muscle mass, diet, hydration status, ethnic characteristics, among others [13]. Moreover, these equations were obtained from a specific group of individuals, and their use in critically ill patients could be inadequate [14, 25]. Under these conditions, the GFR must be determined directly with laboratory tests instead of being estimated. However, the methods currently available for the direct measure of GFR are so laborious and expensive that it is impractical to implement them on a routine basis [11, 13].
The inappropriate use of antibiotics has been identified more frequently in ICU patients than in other patient groups [26]. Errors involving the use of anti-infective agents even include the wrong choice of antimicrobial agent(s) and the administration of inappropriate doses that do not achieve therapeutic levels at the site of infection. Difficulties in establishing correct doses are caused by several factors that produce constant changes in GFR, such as metabolic and physiologic variations, use of nephrotoxic drugs, invasive procedures or devices, and various comorbidities that affect ICU patients [27]. Additionally, the rapid increase in minimal inhibitory concentrations (MICs) that has been observed in recent years and the unavailability of technologies or knowledge that can support the choice of the correct dose have made the prescribing of antimicrobial agents for ICU patients a real challenge.
Most studies suggest that, when possible, the use of agents that are poorly excreted by the kidneys should be considered in patients with renal failure. However, most often, there are no alternatives that can fulfill this criteria. Hence, the risks and benefits of prescribing antimicrobials without dose adjustment in the first 24 h must be evaluated [28]. The application of this recommendation was observed in the patients included in this study when doses were adjusted in the presence of RI. In these cases, the doses were administered without adjustment during the first 24 h and were only adjusted after this period. However, this intervention was not sufficient to avoid the high rate of therapeutic failure.
During the treatment of an infection, therapeutic failure is strongly associated with death. However, treatment response is not due solely to the antimicrobial agent, but also to other factors, such as age, site and severity of the infection, and comorbidities. Thus, an improvement in the patient’s condition, based on both clinical assessment and laboratory tests, may be observed despite the administration of an inaccurate dose of the antimicrobial agent. Moreover, in the specific case of infections, underdosing of antimicrobials by dose adjustment can result in other problems that are more difficult to be measured but which can also increase the risk of death. Low antibiotic levels at the site of infection, for example, may retard the patient’s response, which in turn may extend the length of stay in the ICU and promote antimicrobial resistance. Consequently, the patient will be exposed to several other risks, and if a subsequent infection occurs, it may be caused by a multiresistant strain, which significantly increases the risk of death [1].
In view of the lack of studies conducted in specific groups with the aim to clearly define the appropriate medication doses, one of the more used measures to avoid negative outcomes is to provide therapeutic drug monitoring by measuring the serum level of the drug [4]. Several studies have suggested this practice for the treatment of patients with RI. However, only a few hospitals have adopted the use of serum drug measurements, as the tests are expensive. Moreover, these tests are available only for a few antimicrobial drugs [29]. Thus, the use of this tool in clinical practice is limited and has not been sufficiently efficacious in optimizing antimicrobial use.
There are inherent difficulties with retrospective chart reviews, including the possible absence of relevant information, primarily regarding the prescriber’s impression of the patient’s clinical status at the time of prescribing the antimicrobial agent; this may be a limitation of this study. In addition, the unavailability of local data on MICs could prompt doctors to prescribe amounts that are lower than the recommended dose, thereby increasing the risk of treatment failure. However, this study was performed in an ICU with high rates of infections caused by multiresistant microorganisms, where the vast majority of treatments are started with maximum doses, reducing the risk of possible bias. In the same way, appropriate choice of the antimicrobial agent could have influenced treatment failure and mortality rates. However, with rare exceptions, both groups included in this study were treated in the same ICU by the same healthcare team, which reduces the chance of high differences in decision-making.
Finally, the lack of data or tools supporting the decision-making process for antimicrobial dose adjustment has been a source of uncertainty in the care of patients with RI, particularly under clinical conditions that significantly change the pharmacokinetics. Therefore, the prescribers constantly face the following dilemma: not to perform the adjustment and expose patients to the risk of overdose, which in general are known, monitorable, and controllable, or to prescribe the antimicrobial agents with a dose adjustment, perhaps at a subdose, and possibly reduce the chance of microbiological cure, which may have a more significant impact on the patient’s clinical outcomes, especially in the current scenario with the shortage of therapeutic alternatives. In this way, even if the answer to that doubt is reasonably foreseeable, in daily clinical practice, the conduct of healthcare providers has been conflicting among the different clinical settings or even within a same team. The data from our study should reduce the uncertainty surrounding this decision and reinforce confidence when prescribing an antibiotic for an ICU patient with RI.
Conclusion
In our study of ICU patients with RI, antibiotic dose adjustments based on eGFR were seen to significantly increase the risk of treatment failure and death. These data suggest that when the only strategy available for adjustment is based on the eGFR, the use of the full dose of the antibiotic should be considered.
References
Barrasa-Villar JI, Aibar-Remon C, Prieto-Andres P, Mareca-Donate R, Moliner-Lahoz J (2017) Impact on morbidity, mortality, and length of stay of hospital-acquired infections by resistant microorganisms. Clin Infect Dis 65(4):644–652. https://doi.org/10.1093/cid/cix411
Garcia-Lamberechts EJ, Gonzalez-Del Castillo J, Hormigo-Sanchez AI, Nunez-Orantos MJ, Candel FJ, Martin-Sanchez FJ (2017) Factors predicting failure in empirical antibiotic treatment. An Sist Sanit Navar 40(1):119–130. https://doi.org/10.23938/ASSN.0011
Denny KJ, Cotta MO, Parker SL, Roberts JA, Lipman J (2016) The use and risks of antibiotics in critically ill patients. Expert Opin Drug Saf 15(5):667–678. https://doi.org/10.1517/14740338.2016.1164690
Jager NG, van Hest RM, Lipman J, Taccone FS, Roberts JA (2016) Therapeutic drug monitoring of anti-infective agents in critically ill patients. Expert Rev Clin Pharmacol 9 (7):961-979. https://doi.org/10.1586/17512433.2016.1172209
Tasa T, Metsvaht T, Kalamees R, Vilo J, Lutsar I (2017) DosOpt: a tool for personalized Bayesian dose adjustment of vancomycin in neonates. Ther Drug Monit 39(6):604–613. https://doi.org/10.1097/FTD.0000000000000456
Mouton JW, Muller AE, Canton R, Giske CG, Kahlmeter G, Turnidge J (2018) MIC-based dose adjustment: facts and fables. J Antimicrob Chemother 73(3):564–568. https://doi.org/10.1093/jac/dkx427
van Hasselt JG, Schellens JH, Beijnen JH, Huitema AD (2014) Design of informative renal impairment studies: evaluation of the impact of design stratification on bias, precision and dose adjustment error. Investig New Drugs 32(5):913–927. https://doi.org/10.1007/s10637-014-0103-8
Sharif-Askari FS, Syed Sulaiman SA, Saheb Sharif-Askari N, Al Sayed Hussain A (2014) Development of an adverse drug reaction risk assessment score among hospitalized patients with chronic kidney disease. PLoS One 9(4):e95991. https://doi.org/10.1371/journal.pone.0095991
Elinder CG, Barany P, Heimburger O (2014) The use of estimated glomerular filtration rate for dose adjustment of medications in the elderly. Drugs Aging 31(7):493–499. https://doi.org/10.1007/s40266-014-0187-z
Surana S, Kumar N, Vasudeva A, Shaikh G, Jhaveri KD, Shah H, Malieckal D, Fogel J, Sidhu G, Rubinstein S (2017) Awareness and knowledge among internal medicine house-staff for dose adjustment of commonly used medications in patients with CKD. BMC Nephrol 18(1):26. https://doi.org/10.1186/s12882-017-0443-7
Martin JH, Fay MF, Udy A, Roberts J, Kirkpatrick C, Ungerer J, Lipman J (2011) Pitfalls of using estimations of glomerular filtration rate in an intensive care population. Intern Med J 41(7):537–543. https://doi.org/10.1111/j.1445-5994.2009.02160.x
Bicalho MD, Soares DB, Botoni FA, Reis AM, Martins MA (2015) Drug-induced nephrotoxicity and dose adjustment recommendations: agreement among four drug information sources. Int J Environ Res Public Health 12(9):11227–11240. https://doi.org/10.3390/ijerph120911227
Kumar BV, Mohan T (2017) Retrospective comparison of estimated GFR using 2006 MDRD, 2009 CKD-EPI and Cockcroft–Gault with 24 hour urine creatinine clearance. J Clin Diagn Res 11(5):BC09–BC12. https://doi.org/10.7860/JCDR/2017/25124.9889
Delanaye P, Guerber F, Scheen A, Ellam T, Bouquegneau A, Guergour D, Mariat C, Pottel H (2017) Discrepancies between the Cockcroft-gault and chronic kidney disease epidemiology (CKD-EPI) equations: implications for refining drug dosage adjustment strategies. Clin Pharmacokinet 56(2):193–205. https://doi.org/10.1007/s40262-016-0434-z
Karsch-Volk M, Schmid E, Wagenpfeil S, Linde K, Heemann U, Schneider A (2013) Kidney function and clinical recommendations of drug dose adjustment in geriatric patients. BMC Geriatr 13:92. https://doi.org/10.1186/1471-2318-13-92
Khanal A, Peterson GM, Castelino RL, Jose MD (2014) Renal drug dosing recommendations: evaluation of product information for brands of the same drug. Intern Med J 44(6):591–596. https://doi.org/10.1111/imj.12446
Brown DL, Masselink AJ, Lalla CD (2013) Functional range of creatinine clearance for renal drug dosing: a practical solution to the controversy of which weight to use in the Cockcroft–Gault equation. Ann Pharmacother 47(7-8):1039–1044. https://doi.org/10.1345/aph.1S176
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology C (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145(4):247–254
Truven Health Analytics, Inc. (2016) Micromedex® 2.0. Truven Health Analytics, Inc. Greenwood Village http://www.micromedexsolutions.com
American Pharmacists Association (2015). Drug information handbook, 24th edn. Lexi-Comp, Ohio State University, Columbus
Ismail B, Shafei MN, Harun A, Ali S, Omar M, Deris ZZ (2017) Predictors of polymyxin B treatment failure in Gram-negative healthcare-associated infections among critically ill patients. J Microbiol Immunol Infect. https://doi.org/10.1016/j.jmii.2017.03.007
Sanchez Garcia M (2009) Early antibiotic treatment failure. Int J Antimicrob Agents 34[Suppl 3]:S14–S19. https://doi.org/10.1016/S0924-8579(09)70552-7
Silva Junior JM, Malbouisson LM, Nuevo HL, Barbosa LG, Marubayashi LY, Teixeira IC, Nassar Junior AP, Carmona MJ, Silva IF, Auler Junior JO, Rezende E (2010) Applicability of the simplified acute physiology score (SAPS 3) in Brazilian hospitals. Rev Bras Anestesiol 60(1):20–31
Sunder S, Jayaraman R, Mahapatra HS, Sathi S, Ramanan V, Kanchi P, Gupta A, Daksh SK, Ram P (2014) Estimation of renal function in the intensive care unit: the covert concepts brought to light. J Intensive Care 2(1):31. https://doi.org/10.1186/2052-0492-2-31
Zander J, Dobbeler G, Nagel D, Maier B, Scharf C, Huseyn-Zada M, Jung J, Frey L, Vogeser M, Zoller M (2016) Piperacillin concentration in relation to therapeutic range in critically ill patients—a prospective observational study. Crit Care 20:79. https://doi.org/10.1186/s13054-016-1255-z
Roberts JA, Lipman J (2009) Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 37(3):840–851; quiz 859. https://doi.org/10.1097/CCM.0b013e3181961bff
Eppenga WL, Kramers C, Derijks HJ, Wensing M, Wetzels JF, De Smet PA (2016) Drug therapy management in patients with renal impairment: how to use creatinine-based formulas in clinical practice. Eur J Clin Pharmacol 72(12):1433–1439. https://doi.org/10.1007/s00228-016-2113-2
Charmillon A, Novy E, Agrinier N, Leone M, Kimmoun A, Levy B, Demore B, Dellamonica J, Pulcini C (2016) The ANTIBIOPERF study: a nationwide cross-sectional survey about practices for beta-lactam administration and therapeutic drug monitoring among critically ill patients in France. Clin Microbiol Infect 22(7):625–631. https://doi.org/10.1016/j.cmi.2016.04.019
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
MS Camargo was responsible for the study design, data analysis, and manuscript writing. S Mistro and MG Oliveira were responsible for the manuscript writing and data analysis. LC Passos supervised the study and was responsible for writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. The study was approved by the Research Ethics Committee at Multidisciplinary Institute of Health, Federal University of Bahia, Vitória da Conquista, Brazil, with number (CAAE): 52721616.6.0000.5556.
Datasets
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Rights and permissions
About this article
Cite this article
Camargo, M.S., Mistro, S., Oliveira, M.G. et al. Association between increased mortality rate and antibiotic dose adjustment in intensive care unit patients with renal impairment. Eur J Clin Pharmacol 75, 119–126 (2019). https://doi.org/10.1007/s00228-018-2565-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2565-7