Abstract
Purpose
The long-term efficacy of tolvaptan, a vasopressin V2 receptor antagonist, has been reported. However, the safety of long-term treatment remains to be fully elucidated. We assessed the safety profile of tolvaptan with respect to hypernatremia.
Methods
This retrospective study included 371 patients treated with tolvaptan. Risk factors for hypernatremia (serum sodium concentration ≥147 mEq/L) were determined.
Results
Hypernatremia occurred in 95 patients (25.6 %), of whom 71 (19.1 %) developed hypernatremia within 7 days of tolvaptan treatment (early onset). Stepwise logistic regression analysis demonstrated that baseline serum sodium ≥140 mEq/L, an initial tolvaptan dosage >7.5 mg, and a BUN/serum creatinine ratio ≥20 were independent risk factors for early onset of hypernatremia. Tolvaptan was prescribed for more than 7 days to 233 patients, of whom 123 were administrated tolvaptan for more than 1 month. Hypernatremia occurred in 24 of these patients (10.3 %) (late onset). Predictive factors for late onset of hypernatremia were an average daily dosage of tolvaptan >7.5 mg and age ≥75 years.
Conclusions
A daily dosage of 7.5 mg or less was recommended to prevent hypernatremia in short- as well as long-term tolvaptan treatment, and mainly elderly patients were at risk for hypernatremia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tolvaptan is an orally active selective vasopressin V2 receptor antagonist that increases free-water clearance without electrolyte secretion [1–3]. Short-term tolvaptan treatment has been applied to decrease body weight and edema in patients with heart failure who have insufficient response to other diuretics [4–6]. While tolvaptan treatment also benefits hyponatremia patients by increasing serum sodium concentrations [7], it can lead to hypernatremia, which was reported as a significant adverse reaction of this drug in heart failure patients in Japan [8]. However, the evidence regarding the efficacy and safety of tolvaptan is limited to short-term treatment.
Tolvaptan decreases body weight and improves hepatic edema in patients with liver cirrhosis who had an insufficient response to conventional diuretics [9–11]. Moreover, the largest clinical study targeting patients with autosomal dominant polycystic kidney disease (ADPKD)—the tolvaptan efficacy and safety in management of autosomal dominant polycystic kidney disease and its outcomes (TEMPO) 3:4 trial—demonstrated that 3 years of tolvaptan treatment decreased the rate of growth in total kidney volume and the speed of kidney function decline [12]. Thus, tolvaptan was recently approved for the treatment of liver cirrhosis in Japan and for ADPKD in Europe, Canada, and Japan. Therefore, we expect that the number of patients prescribed tolvaptan will increase for both short- and long-term use.
The clinical benefits of long-term tolvaptan treatment are still unclear [13–18]. A large-scale clinical trial—the efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan (EVEREST) trial—demonstrated that tolvaptan has no impact on mortality or morbidity in heart failure patients with long-term treatment [14], whereas a few studies with a small sample size indicated its long-term benefit in decreasing the mortality of heart failure patients [13, 15]. Thus, although several studies have attempted to evaluate long-term efficacy, few have focused on the safety profile of long-term tolvaptan treatment.
We therefore aimed to determine the safety and risk factors associated with long-term tolvaptan treatment with regard to hypernatremia.
Methods
Study design and subjects
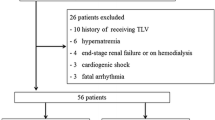
This single-center, observational study was performed using clinical data from patients who had received tolvaptan treatment between January 2013 and December 2015 at the Shizuoka General Hospital. This study was approved by the ethics committee of the Shizuoka General Hospital. Exclusion criteria included patients under 18 years of age and those undergoing dialysis.
Demographic data, including age, sex, height, weight, serum creatinine, serum potassium, serum albumin, and BUN at the start of treatment, were collected. The dosage of tolvaptan, concomitant drugs, and serum sodium was recorded during tolvaptan treatment. Hypernatremia was defined as a serum sodium concentration ≥147 mEq/L. The estimated glomerular filtration rate (eGFR) was calculated using the Japanese Society of Nephrology formula [19]. Serum sodium was measured almost every day for at least 7 days following tolvaptan treatment and every 2–3 days during in-patient treatment. The out-patients with long-term tolvaptan treatment received blood tests every 1–2 weeks.
Statistics analysis
Categorical variables are summarized as frequencies and proportions, and continuous variables as the median and interquartile range, as the data were not normally distributed. All continuous variables were checked for normality using the Shapiro–Wilk test. In univariate analysis, the chi-squared test or Fisher’s exact test was used for comparison of categorical variables. Continuous variables were compared using the Mann–Whitney U-test. Risk factors associated with hypernatremia were examined using univariate analysis followed by logistic regression analysis with stepwise variable selection. The covariates with P < 0.20 determined by univariate analysis were included in the stepwise logistic regression analysis. For logistic regression analysis, continuous variables were converted into categorical variables according to optimal cut-off values defined by receiver operating characteristic (ROC) curve analysis. Logistic regression analysis results are shown as the odds ratio (OR) with 95 % confidence interval (CI). All analyses were performed using R software (version 3.1.0, R Foundation for Statistical Computing). A two-tailed P < 0.05 was considered to be statistically significant.
Results
Patient characteristics
Patient characteristics are summarized in Table 1. A total of 371 patients who received tolvaptan treatment were included (median age, 76.7 years; 60.1 % male), and all subjects were Japanese. The primary target disease for tolvaptan treatment was heart failure with volume overload (91.1 %). The median duration of tolvaptan treatment was 13 days, and 33.2 and 18.1 % patients were prescribed tolvaptan for more than 1 and 3 months, respectively. The median initial and average daily dosage during tolvaptan treatment were both 7.5 mg. At the point of departure from tolvaptan treatment, 83 patients (22.3 %) manifested hyponatremia (Na <135 mEq/L), and its frequency was high in patients with liver cirrhosis (16/33 patients, 48.5 %).
Emergence of hypernatremia
During tolvaptan treatment, 95 patients (25.6 %) had hypernatremia (Table 2). Of them, 71 (19.1 %) experienced hypernatremia within 7 days of beginning tolvaptan treatment. In contrast, hypernatremia occurred beyond 7 days after initiation of tolvaptan treatment in 24 patients (10.3 %).Thus, we hypothesized that the risk factors for hypernatremia with tolvaptan treatment differed between early and late onset of hypernatremia. Among 71 patients with early onset of hypernatremia, 30 patients (42.3 %) showed serum sodium ≥150 mEq/L, 46 patients (64.8 %) required a dose reduction or withdrawal of tolvaptan, and 11 patients (15.5 %) showed a transient increase of serum sodium concentration. Correspondingly, among 24 patients with late onset of hypernatremia, 11 patients (45.8 %) showed serum sodium ≥150 mEq/L, 16 patients (66.7 %) required a dose reduction or withdrawal of tolvaptan, and 5 patients (20.8 %) showed a transient increase of serum sodium concentration.
Risk factors for early onset of hypernatremia
Univariate analysis demonstrated that early onset of hypernatremia was significantly influenced by serum sodium concentration (P < 0.0001), the initial dosage of tolvaptan (P < 0.0001), carperitide use (P = 0.0122), and body mass index (P = 0.0064) (Table 2). In patients with hypernatremia, the frequency of liver cirrhosis was significantly low (P = 0.0137) and the BUN/serum creatinine ratio tended to be higher (P = 0.0713). Stepwise logistic regression analysis revealed that baseline serum sodium concentration ≥140 mEq/L (OR, 5.94; 95 % CI, 3.29–11.21), initial dosage of tolvaptan >7.5 mg (OR, 3.09; 95 % CI, 1.64–5.83), and BUN/serum creatinine ratio ≥20 (OR, 2.39; 95 % CI, 1.33–4.43) were independent risk factors for early onset of hypernatremia (Table 3).
Risk factors for late onset of hypernatremia
Tolvaptan was prescribed for more than 7 days in 233 patients, 24 (10.3 %) of whom experienced hypernatremia after the first 7 days. Univariate analysis determined that the average daily dosage of tolvaptan (P = 0.0051), heart failure (P = 0.0511), and age (P = 0.1798) was positively associated with an increased risk for the occurrence of late hypernatremia (Table 2). Stepwise logistic regression analysis revealed that an average daily dosage of tolvaptan >7.5 mg (OR, 4.85; 95 % CI, 1.85–15.19) and age ≥75 years (OR, 3.62; 95 % CI, 1.37–11.39) was independent risk factors for the late onset of hypernatremia with tolvaptan treatment (Table 3).
Discussion
The present study investigated the incidence of hypernatremia with tolvaptan treatment, including long-term administration. Hypernatremia occurred in 95 patients (25.6 %), three-quarters of whom developed hypernatremia early in treatment (within 7 days). Of the patients administered tolvaptan for more than 7 days, 10.3 % developed hypernatremia in the late phase of treatment (8 days to over 1 month). These observations underscore the importance of frequent monitoring of serum sodium concentrations during both the early and late phases of tolvaptan treatment. In real-world clinical setting, physicians regularly monitor electrolytes and attempt to decrease the dose or withdraw tolvaptan to avoid increases in serum sodium concentration >150 mEq/L. Thus, we defined hypernatremia as a serum sodium concentration ≥147 mEq/L in this study. A previous study that used an observation period of 2 weeks observed that hypernatremia (serum sodium concentration ≥150 mEq/L) occurred in 35 of the 1057 patients (3.31 %) within 14 days of treatment; most cases occurred within 3 days of beginning tolvaptan administration [8]. The safety profile of short-term use of tolvaptan indicated that hypernatremia frequently emerged in the early phase, similar to this study. The present study is the first to describe the safety of long-term tolvaptan treatment regarding hypernatremia.
The frequency of hypernatremia differed between the early and late phases of tolvaptan treatment; therefore, we evaluated the risk factors for hypernatremia according to treatment period. The significant independent factors for early onset of hypernatremia which occurred within 7 days of tolvaptan administration were serum sodium concentration ≥140 mEq/L, an initial dosage of tolvaptan >7.5 mg, and BUN/serum creatinine ratio ≥20. These three factors will be useful for predicting the occurrence of hypernatremia, especially in the early phase of tolvaptan treatment. Patients with a higher baseline serum sodium concentration likely reached the upper limit of the normal range more readily, even with a smaller rise in serum sodium. Thus, frequent electrolyte monitoring is particularly necessary when tolvaptan is prescribed to patients with a higher baseline serum sodium level. Attention should also be paid to the initial dosage. We recommended a lower daily dosage of 3.75 or 7.5 mg to prevent adverse drug reactions. The risk factors for early onset of hypernatremia indicated by our results are similar to those previously reported, where serum sodium ≥142.0 mEq/L, a starting dosage of tolvaptan of 15 mg/day, and serum potassium <3.8 mEq/L were suggested to be independent predictive factors for the occurrence of hypernatremia emerging within 14 days of tolvaptan treatment [8]. Although we did not observe a significant difference in baseline serum potassium concentration in univariate analysis, patients with elevated BUN/serum creatinine ratio were shown to have a higher risk for hypernatremia with tolvaptan. An elevated BUN/creatinine ratio is used as a clinical indicator of intravascular volume depression. Such hypovolemia is a common condition in hospitalized patients with hypernatremia [20, 21].
Our analysis of risk factors for the late onset of hypernatremia showed that an average daily dose of tolvaptan >7.5 mg was an independent predictive variable for hypernatremia occurring after 7 days of tolvaptan treatment. The dosage of tolvaptan was demonstrated to influence both early and late onset of hypernatremia. Single oral doses of tolvaptan at 15–120 mg have been shown to dose-dependently increase urine volume and area under the plasma concentration–time curve (AUC) [22]. A Japanese phase III clinical study has shown that tolvaptan administrated at 7.5 or 15 mg in combination with furosemide for 7 days increased urine volume and weight loss dose-dependently in heart failure patients [23]. The efficacy of tolvaptan might differ between doses of 7.5 and >7.5 mg according to the variability of pharmacokinetics; however, the safety of tolvaptan treatment has been insufficiently evaluated with respect to prospective and pharmacokinetic–pharmacodynamic studies. The pharmacokinetics of tolvaptan are thought to be associated with renal function, and increased AUC was observed in patients with kidney failure [24, 25]. However, our results showed that eGFR was not associated with the incidence of hypernatremia with tolvaptan.
We also observed that older age (≥75 years) was a risk factor for the late onset of hypernatremia. A previous study that compared the effectiveness and safety profiles of tolvaptan between aged patients with those <80 years reported that the incidence of hypernatremia occurring within 14 days did not differ significantly between these patients; however, the incidence of thirst was significantly lower in aged patients [26]. As in our study, they observed that age was not associated with the incidence of hypernatremia in patients during the early phase of tolvaptan treatment. However, on the late phase of therapy, aged patients who had a lower threshold for thirst become relatively depleted of fluid ingestion, which can lead to hypernatremia.
Conclusion
The present study investigated the risk factors for hypernatremia with short- and long-term tolvaptan treatment. When starting tolvaptan treatment, it is important to check the status of electrolytes and blood volume. A low dose of tolvaptan is recommended to prevent hypernatremia, regardless of the treatment period. Elderly patients, especially those on long-term tolvaptan treatment, have a higher risk of hypernatremia.
References
Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, Yamada Y, Tsujimae K, Aoyama M, Kotosai K, Ogawa H, Yamashita H, Kondo K, Tominaga M, Tsujimoto G, Mori T (1998) OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther 287:860–867
Decaux G, Soupart A, Vassart G (2008) Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet 371:1624–1632. doi:10.1016/S0140-6736(08)60695-9
Dohi K, Ito M (2014) Novel diuretic strategies for the treatment of heart failure in Japan. Circ J 78:1816–1823. doi:10.1253/circj.CJ-14-0592
Gheorghiade M, Konstam MA, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators (2007) Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 297:1332–1343. doi:10.1001/jama.297.12.1332
Matsuzaki M, Hori M, Izumi T, Fukunami M, Tolvaptan Investigators (2011) Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study). Cardiovasc Drugs Ther 25:S33–S45. doi:10.1007/s10557-011-6304-x
Watanabe K, Dohi K, Sugimoto T, Yamada T, Sato Y, Ichikawa K, Sugiura E, Kumagai N, Nakamori S, Nakajima H, Hoshino K, Machida H, Okamoto S, Onishi K, Nakamura M, Nobori T, Ito M (2012) Short-term effects of low-dose tolvaptan on hemodynamic parameters in patients with chronic heart failure. J Cardiol 60:462–469. doi:10.1016/j.jjcc.2012.09.002
Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C (2006) Tolvaptan, a selective oral vasopressin V 2-receptor antagonist, for hyponatremia. N Engl J Med 355:2099–2112. doi:10.1056/NEJMoa065181
Kinugawa K, Sato N, Inomata T, Shimakawa T, Iwatake N, Mizuguchi K (2014) Efficacy and safety of tolvaptan in heart failure patients with volume overload. Circ J 78:844–852. doi:10.1253/circj.CJ-14-0126
Sakaida I, Yanase M, Kobayashi Y, Yasutake T, Okada M (2012) The pharmacokinetics and pharmacodynamics of tolvaptan in patients with liver cirrhosis with insufficient response to conventional diuretics: a multicentre, double-blind, parallel-group, phase III study. J Int Med Res 40:2381–2393. doi:10.1177/030006051204000637
Okita K, Kawazoe S, Hasebe C, Kajimura K, Kaneko A, Okada M, Sakaida I (2014) Dose-finding trial of tolvaptan in liver cirrhosis patients with hepatic edema: a randomized, double-blind, placebo-controlled trial. Hepatol Res 44:83–91. doi:10.1111/hepr.12099
Sakaida I, Kawazoe S, Kajimura K, Saito T, Okuse C, Takaguchi K, Okada M, Okita K (2014) Tolvaptan for improvement of hepatic edema: a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res 44:73–82. doi:10.1111/hepr.12098
Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang JJ, Czerwiec FS (2011) Rationale and design of the TEMPO (tolvaptan efficacy and safety in management of autosomal dominant polycystic kidney disease and its outcomes) 3-4 study. Am J Kidney Dis 57:692–699. doi:10.1053/j.ajkd.2010.11.029
Gheorghiade M, Gattis WA, O’Connor CM, Adams KF, Elkayam U, Barbagelata A, Ghali JK, Benza RL, McGrew FA, Klapholz M, Ouyang J, Orlandi C, Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure (ACTIV in CHF) Investigators (2004) Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA 291:1963–1971. doi:10.1001/jama.291.16.1963
Konstam MA, Gheorghiade M, Burnett JC, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, Zimmer C, Orlandi C, Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators (2007) Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 297:1319–1331. doi:10.1001/jama.297.12.1319
Udelson JE, McGrew FA, Flores E, Ibrahim H, Katz S, Koshkarian G, O’Brien T, Kronenberg MW, Zimmer C, Orlandi C, Konstam MA (2007) Multicenter, randomized, double-blind, placebo-controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J Am Coll Cardiol 49:2151–2159. doi:10.1016/j.jacc.2007.01.091
Suzuki S, Yoshihisa A, Yamaki T, et al. (2013) Acute heart failure volume control multicenter randomized (AVCMA) trial: comparison of tolvaptan and carperitide. J Clin Pharmacol 53:1277–1285. doi:10.1002/jcph.197
Imamura T, Kinugawa K, Ohtani T, Sakata Y, Higo T, Kinugawa S, Tsutsui H, Sunagawa K, Komuro I (2014) Assessment of quality of life during long-term treatment of tolvaptan in refractory heart failure: design and rationale of the AQUA-TLV study. Int Heart J 55:264–267. doi:10.1536/ihj.13-326
Xiong B, Huang Y, Tan J, Yao Y, Wang C, Qian J, Rong S, Deng S, Cao Y, Zou Y, Huang J (2015) The short-term and long-term effects of tolvaptan in patients with heart failure: a meta-analysis of randomized controlled trials. Heart Fail Rev 20:633–642. doi:10.1007/s10741-015-9503-x
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53:982–992. doi:10.1053/j.ajkd.2008.12.034
Liamis G, Tsimihodimos V, Doumas M, Spyrou A, Bairaktari E, Elisaf M (2008) Clinical and laboratory characteristics of hypernatraemia in an internal medicine clinic. Nephrol Dial Transplant 23:136–143. doi:10.1093/ndt/gfm376
Overgaard-Steensen C, Ring T (2013) Clinical review: practical approach to hyponatraemia and hypernatraemia in critically ill patients. Crit Care 17:206. doi:10.1186/cc11805
Kim SR, Hasunuma T, Sato O, Okada T, Kondo M, Azuma J (2011) Pharmacokinetics, pharmacodynamics and safety of tolvaptan, a novel, oral, selective nonpeptide AVP V2-receptor antagonist: results of single- and multiple-dose studies in healthy Japanese male volunteers. Cardiovasc Drugs Ther 25:S5–17. doi:10.1007/s10557-011-6299-3
Inomata T, Izumi T, Matsuzaki M, Hori M, Hirayama A, Tolvaptan Investigators (2011) Phase III clinical pharmacology study of tolvaptan. Cardiovasc Drugs Ther 25:S57–S65. doi:10.1007/s10557-011-6349-x
Shoaf SE, Bricmont P, Mallikaarjun S (2013) Pharmacokinetics and pharmacodynamics of oral tolvaptan in patients with varying degrees of renal function. Kidney Int 85:953–961. doi:10.1038/ki.2013.350
Kida K, Shibagaki Y, Tominaga N, Matsumoto N, Akashi YJ, Miyake F, Kimura K (2015) Efficacy of tolvaptan added to furosemide in heart failure patients with advanced kidney dysfunction: a pharmacokinetic and pharmacodynamic study. Clin Pharmacokinet 54:273–284. doi:10.1007/s40262-014-0194-6
Kinugawa K, Inomata T, Sato N, Yasuda M, Shimakawa T, Bando K, Mizuguchi K (2015) Effectiveness and adverse events of tolvaptan in octogenarians with heart failure. Int Heart J 56:137–143. doi:10.1536/ihj.14-332
Authors’ contributions
Hirai K, Moriwaki H, Tsuji D, Inoue K, Kadoiri T and Itoh K designed and managed this study. Hirai K, Shimomura T, Moriwaki H, Ishii H, and Shimoshikiryo T collected the clinical data. Hirai K analyzed all data and wrote the manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they had no support from any organization for the submitted work, no financial relationship with any organizations that might have an interest in the submitted work, and no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval
This study was approved by the ethics committee of the Shizuoka General Hospital (Shizuoka, Japan).
Rights and permissions
About this article
Cite this article
Hirai, K., Shimomura, T., Moriwaki, H. et al. Risk factors for hypernatremia in patients with short- and long-term tolvaptan treatment. Eur J Clin Pharmacol 72, 1177–1183 (2016). https://doi.org/10.1007/s00228-016-2091-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-016-2091-4