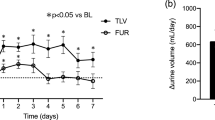
Abstract
Background and Objectives
The pharmacokinetics and pharmacodynamics of tolvaptan (7.5 or 15 mg/day) in combination with furosemide have been investigated in heart failure (HF) patients with normal kidney function but not in HF patients with advanced kidney dysfunction. This study evaluated the efficacy of tolvaptan in HF patients with advanced kidney dysfunction (estimated glomerular filtration rate <45 mL/min/1.73 m2) by conducting a pharmacokinetic and pharmacodynamic study in these patients.
Methods
Tolvaptan (15 mg once daily) was administered orally for 7 days in combination with furosemide (40–200 mg).
Results
The peak plasma tolvaptan concentration and area under the plasma concentration–time curve were 379.41 ± 149.69 ng/mL and 4,657.38 ± 2,741.79 ng·h/mL, respectively, in HF patients with advanced kidney dysfunction. These values were greater in HF patients with advanced kidney dysfunction than values reported in the literature for healthy subjects and HF patients with normal kidney function. Urine volume increased and body weight decreased significantly compared with those before tolvaptan administration in HF patients with advanced kidney dysfunction.
Conclusion
This study showed that adding tolvaptan to furosemide was effective in HF patients with advanced kidney dysfunction. This study also suggests that in these patients 15 mg/day of tolvaptan should be sufficient, and increasing the dose or the frequency of dosing to overcome diuretic resistance should not be necessary, and consideration should be given to using a lower dose and/or prolonging the dosing interval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A pharmacokinetic and pharmacodynamic study of tolvaptan (15 mg/day) plus furosemide was conducted in heart failure patients with advanced kidney dysfunction. |
Urine volume increased and body weight decreased upon administration of tolvaptan (15 mg/day) in these patients. |
The 15 mg/day dose of tolvaptan was effective, but lower doses or greater dosing intervals could be needed. |
1 Introduction
A wide variety of pharmacological agents, including β-blockers (β-adrenoceptor antagonists), ACE inhibitors, angiotensin receptor blockers, aldosterone blockers, and diuretics are prescribed for the treatment of heart failure (HF). In particular, diuretics, especially loop diuretics, are effective pharmacological agents for treating fluid retention and its sequelae such as jugular venous distention, hepatomegaly, peripheral edema, and pulmonary congestion [1]. However, treatment with loop diuretics is less effective in patients with kidney dysfunction, a common co-morbidity and one of the strongest predictors of mortality in HF [2]. Diuretic resistance is also a predictor of poor outcomes [3]. To overcome diuretic resistance, it is a common practice to give high doses of loop diuretics. However, higher doses of loop diuretics have been associated with worse renal, cardiac, and patient outcomes [4]. Loop diuretics can also trigger numerous adverse events including hyponatremia, hypokalemia, metabolic alkalosis, and hypotension in patients with HF [5].
Tolvaptan competitively binds to the vasopressin V2 receptors and inhibits vasopressin-mediated water reabsorption in the renal collecting ducts [6], resulting in increased free water clearance (water diuresis) [7]. Tolvaptan has not been shown to cause kidney dysfunction, hypotension, or electrolyte/acid-base disturbances in major clinical trials [8, 9], making this diuretic an attractive treatment option in patients with HF and kidney dysfunction.
In a phase III clinical pharmacology study of tolvaptan conducted in Japan, tolvaptan was administered for 7 days at a dose of 7.5 or 15 mg/day in combination with furosemide in HF patients with volume overload that had not resolved with furosemide alone [10]. Although a high prevalence of kidney dysfunction in patients hospitalized with decompensated HF has been reported [11], this clinical pharmacology study of tolvaptan excluded patients with serum creatinine >3.0 mg/dL [10]. The efficacy of tolvaptan has not been fully investigated in HF patients with advanced kidney dysfunction. The aim of the present study is to assess the efficacy of tolvaptan in Japanese HF patients with an estimated glomerular filtration rate (eGFR) of <45 mL/min/1.73 m2 by conducting a pharmacokinetic and pharmacodynamic study.
2 Methods
This investigation was conducted as an open-label, non-randomized, uncontrolled, single-dose study for evaluating the efficacy or the pharmacokinetics and pharmacodynamics of tolvaptan in HF patients with advanced kidney dysfunction.
2.1 Patients
The primary inclusion criteria were (1) a diagnosis of HF [12]; (2) age between 20 and 85 years; (3) ability to tolerate furosemide at doses of 40–200 mg/day throughout the study period (from the run-in to post-treatment periods); (4) signs or symptoms of venous congestion, such as peripheral edema, pulmonary congestion, or jugular venous distention because of excessive volume overload; and (5) chronic phase of HF during hospitalization. Patients who met the inclusion criteria were divided into the following three groups according to eGFR by using classification of chronic kidney disease (CKD): group G3b, eGFR ≥30 and <45 mL/min/1.73 m2; group G4, eGFR ≥15 and <30 mL/min/1.73 m2; and group G5, eGFR <15 mL/min/1.73 m2 [13]. The eGFR was calculated using the revised equation for the Japanese population [14].
The exclusion criteria were as follows: (1) hypersensitivity to tolvaptan or its components and similar compounds (vasopressin V2 antagonists, e.g., mozavaptan hydrochloride); (2) adipsia and difficulty in swallowing liquids; (3) inability to take drugs orally; (4) patients with acute decompensated HF, marked serious and clinically unstable HF (e.g., orthopnea); (5) use of assisted circulation apparatus; (6) suspected hypovolemia; (7) systolic blood pressure in the recumbent position <90 mmHg; (8) hypertrophic cardiomyopathy (excluding the condition in the dilated phase); (9) active myocarditis or amyloid cardiomyopathy; (10) valvular diseases with dominant stenosis; (11) poorly controlled diabetes mellitus; (12) morbid obesity with a body mass index of >35 kg/m2; (13) anuria or oliguria due to stricture, stones, or urinary tract tumors; (14) hepatic coma; (15) pregnancy or suspected pregnancy; (16) a history of sustained ventricular tachycardia or ventricular fibrillation within 30 days before the screening test; (17) a history of cerebrovascular disorder within 6 months before screening; (18) onset of acute myocardial infarction within 30 days before screening; (19) biochemistry findings including total bilirubin level of >3.0 mg/dL, serum sodium level of >147 mEq/L, or serum potassium level of >5.5 mEq/L; or (20) a judgment of being inappropriate for inclusion in the study population by physicians in charge.
2.2 Study Protocol
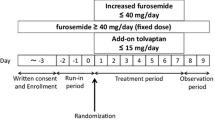
Figure 1a shows the study protocol outlines. The screening test was performed on eligible patients who gave informed consent to participate in the study. The dose of furosemide was determined during the 3-day run-in period (Days −3 to −1). After the run-in period, tolvaptan was administered orally for 7 days at 15 mg once daily after breakfast. The study patients were hospitalized from the run-in period until the first follow-up observation period (Day 9 and Day 10). All medications were fixed throughout the study course. Fluid intake was not restricted during the study period.
The measurements obtained during the run-in period were used as baseline values. The pharmacokinetic and pharmacodynamic measurements were performed on Day 1 and Day 7 in the 7-day treatment. Plasma concentrations were measured at 2, 4, 6, 8, 12, and 24 h after tolvaptan administration on Day 1 and at 0, 4, 8, and 12 h after tolvaptan administration on Day 7. The maximum (peak) plasma drug concentration (C max) and area under the plasma concentration–time curve (AUC) from time zero to infinity (AUC∞) of tolvaptan were determined from the plasma concentration data. Three cumulative urine collections (4 h collections) were made during the first 12 h after tolvaptan administration, whereas one cumulative urine collection (12 h collection) was made during the subsequent 12 h.
The following drugs were prohibited throughout the study period: injectable drugs, including human atrial natriuretic peptides, phosphodiesterase III inhibitors, catecholamines, colforsin; injectable diuretics; loop diuretics other than furosemide, and cytochrome P450 (CYP) 3A4 inhibitors or inducers. Foods that interfere with the CYP3A4 system, e.g., grapefruit, were also prohibited. Concomitant drugs not on the exclusion list and therapy affecting patients’ volume status were allowed throughout the study period.
2.3 Pharmacodynamic Efficacy Variables
For the pharmacodynamic analysis, the following endpoints were measured: urine volume, urine osmolality, urine biochemistry (sodium, potassium, uric acid, and creatinine concentrations), fluid intake, serum osmolality, and serum biochemistry (concentrations of sodium, potassium, and creatinine). Body weight and blood pressure were measured before breakfast each morning and signs of volume overload were assessed as part of the efficacy analysis.
2.4 Statistical Analysis
Demographic and other baseline characteristics were analyzed according to the eGFR categories. Descriptive statistics of plasma concentrations and pharmacokinetic parameters of tolvaptan at each timepoint were calculated using the eGFR categories on Day 1 and Day 7. Pharmacokinetic parameters were calculated using the pharmacokinetic analysis software, WinNonlin® (Pharsight, St. Louis, MO, USA) and SAS® version 9.3 (SAS Institute Inc., Cary, NC, USA). The descriptive statistics were performed in each eGFR category for the assessment of obtained values and changes in pharmacodynamic parameters and body weight, whereas 95 % confidence interval for the differences between the treatment groups were calculated in daily urine volume, water intake, and urine volume per unit time.
2.5 Ethics
This study was performed in accordance with the ethical principles set forth in the Declaration of Helsinki. The study protocol was approved by the St. Marianna University School of Medicine Institutional Committee on Human Resource, Kawasaki, Japan (No. 1886). The study patients were informed about the details of the study prior to the screening examination. Written informed consent was obtained before enrollment (clinical registration with the University Hospital Medical Information Network: UMIN 000006859).
3 Results
3.1 Patient Characteristics
The present study included 23 patients who met the inclusion criteria between July 2011 and August 2012. One patient (in the G5 group) was dropped from the study because of a protocol deviation (furosemide was not administered); therefore, 22 patients were treated with tolvaptan. Of the patients treated with tolvaptan, two patients (G4 n = 1; G5 n = 1) were dropped before completing the study for safety reasons; one discontinued the study due to worsening of the primary disease and the patient was offered to discontinuation of the study and the other discontinued due to increasing furosemide dosages for intractable pleural effusion. The concentration of tolvaptan on Day 7 was not measured in one patient in the G3b group, and one patient in the G4 group withdrew from the study because of urinary incontinence. Six patients in the G3b group, seven patients in the G4 group, and five patients in the G5 group completed the study (Fig. 1b). The baseline characteristics of the patients in each group are summarized in Table 1. The mean dose of furosemide was 56 ± 39 mg, resulting in no significant differences between the three groups. The levels of serum albumin, AST, and ALT did not significantly differ between the three groups. None of the study patients had a history of hepatitis C or non-alcoholic steatohepatitis.
3.2 Pharmacokinetics
The time courses for plasma tolvaptan concentrations on Day 1 and Day 7 are presented in Fig. 2a. The plasma concentrations increased at the first timepoint after tolvaptan administration (2 h on Day 1), reaching C max values at 4 h on Day 1, and Day 7 in HF patients with advanced kidney dysfunction. When these data were analyzed based on the eGFR groups, the data in the G3b and G4 groups were similar to the pooled data. However, the data in the G5 group showed a C max at 2 h on Day 1 (Fig. 2b, c). The tolvaptan plasma concentration before administration on Day 7 was higher than that at 24 h after administration on Day 1 (91 ± 92 ng/mL vs. 67 ± 58 ng/mL), but this difference was not statistically significant (p = 0.07).
a Time course changes in plasma tolvaptan concentration up to 24 h after administration. Comparison between Day 1 and Day 7 (n = 18). b Time course changes in plasma tolvaptan concentration up to 24 h after administration. Comparisons between Day 1 and Day 7 and between the G3b, G4, and G5 groups (n = 18). c Time course changes in plasma tolvaptan concentration up to 24 h after administration. Comparisons between Day 1 (n = 22) and Day 7 (n = 18) and between the G3b, G4, and G5 groups
Table 2 shows the pharmacokinetic parameters after tolvaptan administration on Day 1 in HF patients with advanced kidney dysfunction. For all patients, the C max and AUC∞ on Day 1 were 379 ± 150 ng/mL and 4,657 ± 2,742 ng·h/mL, respectively, in HF patients with advanced kidney dysfunction. When the data for Day 1 were divided per the eGFR groups, C max became higher whereas AUC∞ became lower as kidney function worsened.
Table 2 also shows the comparisons in pharmacokinetic parameters of tolvaptan between Day 1 and Day 7. C max on Day 7 was higher than that on Day 1 in the G3b group, while the G4 and G5 groups exhibited a lower C max on Day 7 than on Day 1.
3.3 Pharmacodynamics
The changes in daily urine volume and daily water intake during the treatment period are shown in Fig. 3a, b. Daily urine volume increased above baseline levels in the three study groups. The G3b group showed the remarkable increase on Day 1 (total 1,279 ± 431 to 2,201 ± 937 mL; G3b 1,140 ± 259 to 2,749 ± 1,073 mL; G4 1,400 ± 614 to 1,930 ± 879 mL; G5 1,277 ± 245 to 1,786 ± 201 mL). The differences in 24-h urine volume between the three groups were small except Day 1. The rate of urine excretion consistently peaked at 4–8 h after the administration of tolvaptan in the G3b and G4 groups, and at 0–4 h in the G5 group on Day 1 (Fig. 4a).
The G3b group tended to drink less water than the other groups, but water intake in each group was stable throughout the study period. Urine osmolality rapidly decreased at 0–4 h from baseline and peaked at 4–8 h after tolvaptan administration on Day 1. Urine osmolality remained low at approximately 200 mOsm/kg H2O 24 h after tolvaptan administration, although it rebounded slightly in the G3b group. Urine osmolality was already low before tolvaptan administration on Day 7 and remained the same throughout Day 7 (Fig. 4b).
Serum sodium concentrations increased during the study period (Fig. 5a), particularly in patients with a serum sodium concentration under 135 mEq/L (131 ± 4 to 138 ± 2 mEq/L, p = 0.01). Meanwhile, patients with an initial serum sodium concentration over 135 mEq/L showed no significant changes during the study period (138 ± 2 to 138 ± 5 mEq/L, p = 0.78). Serum creatinine showed no significant changes during the study period (Fig. 5b).
3.4 Efficacy
When the data for all patients were analyzed together, body weight significantly decreased on Day 2 (57.4 ± 11.0 kg) compared with Day 1 (57.8 ± 10.9 kg, p = 0.002). Body weight was 56.9 ± 11.1 kg on Day 8, showing a weight reduction of 1.1 ± 1.5 kg after 7-day tolvaptan administration. The reduction in body weight consistently tended to be greater in the G3b group; however, no significant differences among the groups were found (Fig. 3c).
No significant changes in systolic or diastolic blood pressure were observed during the study period (systolic blood pressure 115 ± 18 to 113 ± 24 mmHg; diastolic blood pressure 61 ± 13 to 60 ± 10 mmHg).
Edema in the lower extremities and S3 heart sounds of patients in the G4 group improved on Day 2, Day 8, and Day 9 compared with the baseline assessments. In addition, distension of the jugular veins of patients in the G3b and G4 groups lessened on Day 2, Day 8, and Day 9 compared with the baseline assessment.
4 Discussion
In the present study, we investigated the pharmacokinetic and pharmacodynamic properties of oral tolvaptan 15 mg/day and verified its efficacy profile in Japanese HF patients with volume overload and advanced kidney dysfunction. Since the pharmacokinetics and pharmacodynamics of tolvaptan are known not to be clinically or significantly affected by race [15], we assume that our study results can be extrapolated to non-Japanese patients.
4.1 Pharmacokinetics
Tolvaptan is metabolized in the liver and eliminated in the feces. Tolvaptan is not excreted in urine; therefore, we hypothesized that the concentration of tolvaptan would not be increased in HF patients with advanced kidney dysfunction. However, even in these patients, the C max of tolvaptan on Day 1 was 2.8-fold higher than that in healthy subjects [16] and 1.5-fold higher than that in HF patients with normal kidney function [10]. In our study, the patients with worse renal function had higher C max, suggesting that C max may be affected by renal function. Tolvaptan is primarily metabolized via CYP3A4 [17]. Since high levels of parathyroid hormone, cytokines, and uremic toxins have been shown to reduce CYP activity, non-renal clearance by CYP decreased from 54 to 87 % in patients with kidney dysfunction [18]. These factors may have had an effect on the high plasma concentrations of tolvaptan observed in our study. Inomata et al. [10] speculated that this trend might be due to the decreased capacity for drug elimination and reduced volume of distribution in HF patients.
The median time to C max (t max) in HF patients with advanced kidney dysfunction was 4 h after tolvaptan 15 mg/day administration, which was similar to the t max in HF patients with normal kidney function [10]. t max in HF patients with advanced kidney dysfunction was obtained 2 h later than the t max in healthy subjects [16]. The reason for this delay may be due to compromised drug absorption in HF patients with volume overload due to congestion or reduced function of the intestine and other organs [10].
In our study, the elimination half-life (t ½ ) calculated from the data on Day 1 was 2.5-fold higher in HF patients with advanced kidney dysfunction than in healthy subjects [16] and 1.3-fold higher than HF patients with normal kidney function [10]. This observation may suggest that drug elimination is also impaired in patients with advanced kidney dysfunction. This hypothesis is supported by the observation that the plasma concentration of tolvaptan 24 h after the first dose was higher in our study patients than in healthy subjects or patients with HF without kidney dysfunction.
The plasma concentration of tolvaptan was higher before tolvaptan administration on Day 7 than 24 h after administration on Day 1, although no statistical significance was found between these two values. This study result demonstrated the tendency that tolvaptan in plasma was accumulative; however, the plasma concentration of tolvaptan was not accumulative at all in HF patients with normal kidney function [10]. Accordingly, we believe that the accumulation of tolvaptan should be focused in HF patients with advanced kidney dysfunction. The reason for accumulation could be associated with reduced elimination and prolongation of t ½ , although prolongation of t ½ due to depressed kidney function has not fully elucidated. These results suggest that, in patients with HF and advanced kidney dysfunction, a dose of tolvaptan 15 mg daily may be too high and the dose should be reduced or the dosing interval extended.
4.2 Pharmacodynamics
The Japanese phase III study has demonstrated that tolvaptan exerts diuretic effects and causes body weight loss at a low dose of 7.5 mg/day; however, these effects are less than those elicited by 15 mg/day [10]. The present study showed that tolvaptan 15 mg once daily was effective; urine volume moderately but significantly increased (approximately 500 mL on average) and body weight significantly decreased compared with those before administration, even in HF patients with advanced kidney dysfunction.
Of note, urine output in the study patients was moderate even though the plasma concentration of tolvaptan was higher than that of healthy subjects receiving tolvaptan 45–90 mg/day [16]. Therefore, the 24-h cumulative urine volume was correlated with plasma AUC from time zero to 24 h (AUC24) on Day 1 in healthy Japanese subjects [16]; however, the present study did not demonstrate these correlations in HF patients with advanced kidney dysfunction. These results did not support our theory that the optimal dose of tolvaptan should not exceed 15 mg/day in HF patients with advanced kidney dysfunction. In the present study, the increase in urine output and the decrease in urine osmolality, both of which indicate effects of tolvaptan, lasted 12–24 h after the administration of a single dose of tolvaptan; we concluded that it was unnecessary to administer tolvaptan more than once a day.
One recent study suggested that patients who respond well to tolvaptan (responders) could be identified by urine osmolality 4–6 h after tolvaptan administration in HF patients with CKD [19]. The present study demonstrated that the median t max was 4 h and urine osmolality rapidly decreased at 0–4 h from baseline and peaked at 4–8 h after tolvaptan administration on Day 1. Thus, the new criteria for determining the responder of tolvaptan judged by the percentage decrease in urine osmolality at 4–6 h after its administration are reasonable. Kinugawa et al. [20] reported an interim result of a post-marketing surveillance study of tolvaptan, in which the mean dose of furosemide used for 1,057 HF patients with insufficient response to loop diuretics was 69.2 ± 66.2 mg/day.
One of the intriguing results in the present study is that tolvaptan can be used effectively without causing worsening renal function in HF patients with advanced kidney dysfunction. Earlier studies are in agreement with our study results [21, 22]. Increasing doses of furosemide often lead to worsening renal function and increased mortality in HF patients with advanced kidney dysfunction. These poor outcomes have been linked to activation of the renin–angiotensin–aldosterone system and the sympathetic nervous system [4, 23]. However, previous studies have shown that tolvaptan does not activate these systems [7, 24]. The present study showed that tolvaptan could help resolve excessive body fluid without worsening renal function. Worsening of renal function is a big disadvantage in the use of high doses of loop diuretics.
Tolvaptan has been used around the world to treat hyponatremia in HF patients. In Japan, tolvaptan can also be used in normonatremic HF patients for the treatment of congestion [25]. One study reported that changes in serum sodium concentrations after treatment with tolvaptan 7.5 mg/day were inversely correlated with baseline values in HF patients with serum creatinine 1.2 ± 0.3 mg/dL (r = −0.58, p < 0.05) [26]. The present study showed improvement in serum sodium concentrations in patients with hyponatremia (serum sodium concentration under 135 mEq/L) at baseline. Meanwhile, treatment of patients without hyponatremia (serum sodium concentration over 135 mEq/L) at baseline showed no increase in serum sodium concentrations. Our study results demonstrated that tolvaptan 15 mg/day could be effectively administered to normonatremic patients because it did not trigger excessive free-water excretion resulting in an excessive and rapid increase in serum sodium concentration.
4.3 Limitations
There are several limitations to this study. First, this study included HF patients with advanced kidney dysfunction and their urinary protein levels were not measured. The detailed etiology of renal dysfunction was not fully identified either. Second, tolvaptan is primarily metabolized via CYP3A4. As some patients used warfarin for atrial fibrillation or after heart valve surgery, we monitored prothrombin time–international normalized ratio (PT-INR) values for these patients. However, we did not measure it in all patients. We considered accurate assessment of PT-INR values to be difficult whether subjects were on a warfarin or not. Third, we did not use the mineralocorticoid receptor antagonist in G5 group to prevent the hyperkalemia. We speculated that the response in patients in receipt of a mineralocorticoid receptor antagonist would be different/improved compared with those not on one. However, the rate of patients treated with mineralocorticoid receptor antagonists did not significantly differ between the three groups. Moreover, a small dose of furosemide improved the response in this study. Finally, this study did not evaluate the differences in the etiology of advanced kidney dysfunction and HF.
5 Conclusion
This study showed that add-on tolvaptan to furosemide was effective even in HF patients with advanced kidney dysfunction. This study also suggested that in these HF patients with advanced kidney dysfunction, a 15 mg daily dose of tolvaptan should be adequate. Increasing the dose or the frequency of dosing to overcome resistance to a standard dose of loop diuretics may not be effective. Lowering the dose and/or prolonging the dosing interval should be considered.
References
von Lueder TG, Atar D, Krum H. Diuretic use in heart failure and outcomes. Clin Pharmacol Ther. 2013;94(4):490–8.
Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Yokota T, Ide T, Takeshita A, et al. Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J. 2009;73(8):1442–7.
Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97(12):1759–64.
Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O’Connor CM, Califf RM, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. 2007;9(10):1064–9.
Cooper HA, Dries DL, Davis CE, Shen YL, Domanski MJ. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation. 1999;100(12):1311–5.
Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, Yamashita T, et al. OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther. 1998;287(3):860–7.
Miyazaki T, Fujiki H, Yamamura Y, Nakamura S, Mori T. Tolvaptan, an orally active vasopressin V(2)-receptor antagonist—pharmacology and clinical trials. Cardiovasc Drug Rev. 2007;25(1):1–13.
Gheorghiade M, Gattis WA, O’Connor CM, Adams KF Jr, Elkayam U, Barbagelata A, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. 2004;291(16):1963–71.
Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319–31.
Inomata T, Izumi T, Matsuzaki M, Hori M, Hirayama A. Phase III clinical pharmacology study of tolvaptan. Cardiovasc Drugs Ther. 2011;25(Suppl 1):S57–65.
Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13(6):422–30.
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–847.
Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53(6):982–92.
Shoaf SE, Kim SR, Bricmont P, Mallikaarjun S. Pharmacokinetics and pharmacodynamics of single-dose oral tolvaptan in fasted and non-fasted states in healthy Caucasian and Japanese male subjects. Eur J Clin Pharmacol. 2012;68(12):1595–603.
Kim SR, Hasunuma T, Sato O, Okada T, Kondo M, Azuma J. Pharmacokinetics, pharmacodynamics and safety of tolvaptan, a novel, oral, selective nonpeptide AVP V2-receptor antagonist: results of single- and multiple-dose studies in healthy Japanese male volunteers. Cardiovasc Drugs Ther. 2011;25(Suppl 1):S5–17.
Shoaf SE, Elizari MV, Wang Z, Sekar K, Grinfeld LR, Barbagelata NA, et al. Tolvaptan administration does not affect steady state amiodarone concentrations in patients with cardiac arrhythmias. J Cardiovasc Pharmacol Ther. 2005;10(3):165–71.
Dreisbach AW, Lertora JJ. The effect of chronic renal failure on drug metabolism and transport. Expert Opin Drug Metab Toxicol. 2008;4(8):1065–74.
Imamura T, Kinugawa K, Shiga T, Kato N, Muraoka H, Minatsuki S, et al. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients–association between non-responders and chronic kidney disease. Circ J. 2013;77(2):397–404.
Kinugawa K, Sato N, Inomata T, Shimakawa T, Iwatake N, Mizuguchi K. Efficacy and safety of tolvaptan in heart failure patients with volume overload. Circ J. 2014;78(4):844–52.
Matsue Y, Suzuki M, Seya M, Iwatsuka R, Mizukami A, Nagahori W, et al. Tolvaptan reduces the risk of worsening renal function in patients with acute decompensated heart failure in high-risk population. J Cardiol. 2013;61(2):169–74.
Vaduganathan M, Gheorghiade M, Pang PS, Konstam MA, Zannad F, Swedberg K, et al. Efficacy of oral tolvaptan in acute heart failure patients with hypotension and renal impairment. J Cardiovasc Med (Hagerstown). 2012;13(7):415–22.
Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the studies of left ventricular dysfunction (SOLVD). Circulation. 1990;82(5):1724–9.
Costello-Boerrigter LC, Smith WB, Boerrigter G, Ouyang J, Zimmer CA, Orlandi C, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol. 2006;290(2):F273–8.
Kinugawa K, Imamura T, Komuro I. Experience of a vasopressin receptor antagonist, tolvaptan under the unique indication in Japanese heart failure patients. Clin Pharmacol Ther. 2013;94(4):449–51.
Watanabe K, Dohi K, Sugimoto T, Yamada T, Sato Y, Ichikawa K, et al. Short-term effects of low-dose tolvaptan on hemodynamic parameters in patients with chronic heart failure. J Cardiol. 2012;60(6):462–9.
Acknowledgments
We would like to thank the staff of the Heart Center, Kidney Center, and the Heart Failure Team at the St. Marianna University School of Medicine Hospital for their help in patient recruitment and data acquisition.
Conflict of interest/Disclosure
Payment for lectures: Keisuke Kida (Otsuka Pharmaceutical Co., Ltd.), Yugo Shibagaki (Otsuka Pharmaceutical Co., Ltd.), Naoto Tominaga (Otsuka Pharmaceutical Co., Ltd.). Research funding: Yoshihiro J. Akashi (Otsuka Pharmaceutical Co., Ltd.), Fumihiko Miyake (Otsuka Pharmaceutical Co., Ltd.), Kenjiro Kimura (Otsuka Pharmaceutical Co., Ltd.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kida, K., Shibagaki, Y., Tominaga, N. et al. Efficacy of Tolvaptan Added to Furosemide in Heart Failure Patients with Advanced Kidney Dysfunction: A Pharmacokinetic and Pharmacodynamic Study. Clin Pharmacokinet 54, 273–284 (2015). https://doi.org/10.1007/s40262-014-0194-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-014-0194-6