Abstract
Vayssierea is an understudied nudibranch genus characterized by its orange colouration and small size (up to 5 mm in length). To date, there are four described species, distributed in the Indo-Pacific Ocean. Here, individuals of Vayssierea were recorded for the first time in the North Atlantic Ocean on the Canary Islands (Spain). This study aims to evaluate the systematic and taxonomic status and distribution of the genus through multilocus phylogenetic, morphological, and radular analyses. Phylogenetic results show the monophyly of Vayssierea and evidence indicating that the genus is included in the new subfamily Okadaiinae stat. nov. within Polyceridae. According to species delimitation tests, four different species have been sequenced from Russia to Australia, in addition to our new records in the Atlantic Ocean, but more information is needed to identify the species. Nevertheless, our specimens from the Canary Islands belong to two different species, one of which is identical to the Australian species. Bearing in mind that they lack a planktonic larval stage; we hypothesize that they arrived by shipping transportation or aquarium releases, becoming a non-indigenous species of the Atlantic Ocean.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studying marine ecosystem biodiversity is fundamental for conservation, particularly for a better understanding of the evolutionary history and role of species in the ecosystem. Biological diversity is defined as the variety of living organisms present within an ecosystem (Waldman and Shevah 2000). Mollusca is the second-most diverse existing phylum, just behind arthropods, and contains morphologically disparate classes such as bivalves, cephalopods, and gastropods. Nudibranch gastropods belong to the subclass Heterobranchia (commonly known as opisthobranchs) and are ecologically relevant in the ecosystem because of their great functional and species diversity. This makes them excellent candidates as environmental and complexity indicators of the ecosystem (Goddard and Pearse 2011). Species of nudibranchs have highly specific diets, consequently, a high diversity of nudibranchs in an ecosystem indicates a high variety of food sources and, thus, great biodiversity. The order Nudibranchia contains an estimated 3,000 species distributed in marine ecosystems worldwide, but its existing biodiversity has been underestimated (e.g., Korshunova et al. 2019; Araujo et al. 2022; Moles et al. 2021). Current phylogenetic studies show that there are many gaps in their systematics, and the description of new genera and species (e.g., Korshunova et al. 2020; Knutson and Gosliner 2022), resurrection, and synonymization (e.g., Moles and Riesgo 2019; Fernández-Vilert et al. 2021) has steadily increased our knowledge of systematics and diversity.
Vayssierea (Risbec, 1928) is the only nudibranch genus that belongs to the family Okadaiidae Baba, 1930 (Baba 1931). This family pertains to the infraorder Doridoidei; however, its systematic placement remains controversial. Okadaiidae is known as the senior synonym of Vayssiereidae Thiele, 1931 but the first prevails (see Bouchet et al. 2017). Recently, Okadaiidae was found to nest within the family Polyceridae (Korshunova et al. 2020), although the authors still considered it a valid family based on the morphological uniqueness of its species. The genus currently includes four species that are distributed mostly throughout the Indo-Pacific, reaching South Africa. The type species V. caledonica Risbec, 1928 was described from New Caledonia (Burn, 2006). Baba (1930) described the species Okadaia elegans Baba, 1930 from Akkeshi Bay, Japan, which was subsequently transferred to Vayssierea (Kantor and Sysoev 2006). Vayssierea cinnabarea (Ralph 1944) was described from Island Bay, Wellington (New Zealand) (Ralph, 1944) as Pellibranchus cinnabareus, and was transferred to Vayssierea. A fourth species, V. felis (Collingwood, 1881), from the South China Sea was described as Trevelyana felis (Collingwood, 1881). The most recent species described is V. tecticardia (Slavoshevskaya, 1971) from the Sea of Japan, which is likely to be a junior synonym of V. elegans (Martynov 2000).
The external morphological characteristics of Vayssierea species are very similar, making them difficult to diagnose. Although morphologically identical, some authors have revealed molecular diversity in this species complex (Gosliner et al. 2008). The descriptions of the four species are consistent with orange colouration, limaciform body, small size (approximately 5 mm), and reduced external features. They have two smooth rhinophores, lack gills and tentacles, and their anus opens dorsally halfway down the body (Rudman et al. 1998). In addition to this taxonomic conundrum, only V. cinnabarea and V. caledonica have detailed morphological descriptions. The only difference between V. cinnabarea and the rest of the species is that its rhinophores and the distal part of the foot have a paler vermilion colour (Ralph 1944). The developmental mode of all species appears to be intracapsular (Ralph 1944). All described species of this genus live in the intertidal zone, where they feed on serpulid annelids, generally Salmacina Claparède, 1870 and Spirorbis Daudin, 1800 (Burn 2006; Gosliner et al. 2008). Overall, accurate comparative descriptions are crude; thus, understanding genus diversity and establishing species boundaries remains fundamental.
The term ‘bioinvasion’, as defined by Carlton (2001), refers to species range expansions resulting from anthropogenic landscape alterations, human-assisted introductions, or unassisted secondary dispersal events. Introduced or non-indigenous species (NIS) may have significant impacts and are a major cause of biodiversity loss worldwide (Courchamp et al. 2017), thus becoming invasive. NIS have repeatedly altered species ranges throughout history (Vermeij and Sax 2005), but modern vectors have increased the frequency of such events (Carlton 2001). In this scenario, NIS dispersion could be facilitated by the intensification of human activity, such as ballast water (Bailey 2015), canal construction (Gollasch et al. 2006), aquaculture (Naylor et al. 2005), and maritime traffic (Castro et al. 2020), coupled with ongoing climate change (Pyšek et al. 2020; Bennett et al. 2021). In that sense, the early detection of the tempo and mode of NIS will aid in the monitoring and control of alien populations.
Several populations of Vayssierea were collected from the Canary Islands in the North Atlantic Ocean, very far away from the native distribution area of the genus. This study includes these specimens in a larger phylogenetic context, including samples from Australia, Japan, and additional material found in Spanish aquarium tanks (origin: Indo-Pacific). The main objectives are to: (1) evaluate the species diversity and systematic status of the genus Vayssierea in a broad phylogenetic context, and (2) provide an answer on how these specimens reached the Canary Islands, in the middle of the Atlantic Ocean, bearing in mind their limited dispersal ability due to their reproductive mode. Thus, whether these specimens belong to a NIS or represent undiscovered diversity.
Materials and methods
Sampling strategy
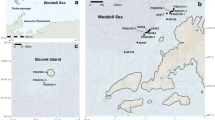
Samples were collected from Gran Canaria (Canary Islands) during August 2022, specifically, 18 individuals in tide pools in El Faro and 24 in El Confital, the North and the South of the island (Fig. 1). Specimens were fixed in 95% EtOH and kept at -20ºC for later analysis. Two specimens from each location were used for molecular analyses. Two additional specimens were sent from the Museum of Comparative Zoology (MCZ, Harvard University), one from Moffatt Beach (Queensland, Australia), collected from 0 to 6 m depth (https://mczbase.mcz.harvard.edu/guid/MCZ:Mala:385477), and the second from tide pools in Bisezaki Kaigan (Okinawa, Japan), (https://mczbase.mcz.harvard.edu/guid/MCZ:Mala:386352). Three more specimens were sent from the Murcia Aquarium (Spain), which were obtained from a tank containing Tridacna species belonging to the Indo-Pacific Ocean. All specimens were deposited at the MCZ and the sequences were uploaded to GenBank (Table S1).
(A) Vayssierea sp. (VayGC1N) from El Confital, North of Gran Canaria; (B) Vayssierea sp. (VayGC1S) from El Faro, South of Gran Canaria; (C) Vayssierea sp. (MCZ 385477) from Moffatt Beach, Queensland, Australia; (D) Vayssierea sp. (MCZ 386352) from Bisezaki Kaigan, Okinawa, Japan; (E) Vayssierea sp. from La Palma (sequenced); (F) Vayssierea sp. from Lanzarote (sequenced)
Two additional sightings from La Palma and one from Lanzarote are recorded here (Fig. S2); however, phylogenetic analyses were performed a posteriori, so only COI is analysed in species delimitation tests. The first record from La Palma was found under the rocks in a tide pool near the airport on the east coast of the island, and the second was found in Tazacorte’s harbour, on the west coast. A record from Lanzarote was found in the harbour of Puerto del Carmen, on the east coast of the island.
DNA extraction, amplification, and sequencing
Genomic DNA was extracted from tissue clips using the E.Z.N.A.® Mollusc DNA Kit (Omega Bio-Tek, USA) and following the manufacturer’s protocols. Five markers were amplified: the mitochondrial cytochrome c oxidase subunit I (COI) and the 16S rRNA (16S), the nuclear histone 3 (H3), 28S rRNA (28S), and 18S rRNA (18S). The specific primers and polymerase chain reaction (PCR) conditions are listed in Table S2. Amplifications were carried out in PCRs of 10 µL volume, with 3 µL Red DNA Polymerase REDExtract-N-AmpTMPCR ReadyMix (Sigma Aldrich, St. Louis, MO, USA), 0.3 µL of each primer, and 5.1 µL of purified water. Successful amplifications were sequenced by Macrogen, Inc. (Madrid, Spain) after purification with ExoSAP-IT™ Express PCR Product Cleanup Reagent.
Phylogenetic analyses
The BLAST algorithm (Altschul et al. 1997) from the GenBank nucleotide database (Benson et al. 2000) was used to check for contamination of the amplifications. All the sequences obtained were visualized, edited, and assembled using Geneious Pro v. 8.1.8. Additional sequences from GenBank and BOLD Systems (Ratnasingham et al. 2007) were downloaded for alignment. Sequences were aligned with MAFFT (Katoh et al. 2002) implemented in Geneious, using the G-INS-I algorithm for COI and H3, genes with global homology, and the L-INS-i algorithm for 16S, 28S, and 18S markers, which contain long gaps and conserved domains. Furthermore, the coding genes COI and H3 were translated into amino acids to check for possible sequencing errors.
The final dataset consisted of a total of 77 specimens that were included in the phylogenetic analysis (Table S1), all of them from the infraorder Doridoidei, particularly focusing on the families Gymnodorididae, Polyceridae, Chromodorididae, Cadlinellidae, Showajidaiidae, Hexabranchiidae, Phyllididae, Dendrodorididae, and Cadlinidae according to the latest trees by Korshunova and collaborators (2020) and Knutson and Gosliner (2022); including all available subfamilies from Polyceridae (i.e. Polycerinae, Nembrothinae, Triophinae, and Kalinginae) and families Phyllidiidae, Dendrodorididae, and Cadlinidae as outgroups.
Analyses were carried out for both single-gene alignments and concatenated genes. The CIPRES Science Gateway (Miller et al. 2010) was used for phylogenetic analysis. The maximum likelihood (ML) approach was performed using IQ-TREE v. 2.1.2 (Nguyen et al. 2015), accounting for partitions and codon positions. ModelFinder (Kalyaanamoorthy et al. 2017) was used to automatically select the best partition scheme and the corresponding evolutionary model using TESTMERGE with an edge-unlinked partition model to reduce overparameterization and increase model fit. Branch support was estimated using ultrafast bootstrap (bs) with 1500 replicates (Hoang et al. 2018).
Bayesian phylogenetic inference (BI) was performed using MrBayes 3.2.7a (Ronquist et al. 2012). The nucleotide substitution model selected for each partition was GTR + I + G (Tavaré 1986). The Markov chain Monte Carlo (MCMC) simulation technique was used to approximate the probability of a tree based on the observations and find the posterior probability (pp) distribution of trees. Four parallel runs of four coupled MCMC chains were run for 20 million generations with sampling and check frequencies of 1000 and 20,000 generations, respectively, discarding the first 25% of trees as burn-in. Trees were visualized with FigTree v. 1.4.4 (Rambaut 2010) and edited in Adobe Illustrator and Photoshop.
Species Delimitation Tests (SDT) were conducted on the COI and 16S alignments of the genus Vayssierea. The Assemble Species by Automatic Partitioning analysis (ASAP; Puillandre et al. 2021) was run using the web interface at https://bioinfo.mnhn.fr/abi/public/asap/asapweb.html with default parameters and Jukes-Cantor (JK69) as a substitution model to compute the distances. The Poisson Tree Processes (PTP, Zhang et al. 2013) was conducted only on the COI alignment of the genus Vayssierea using the web server https://species.h-its.org/, with 100,000 generations and a 10% burn-in. The Automatic Barcode Gap Discovery (ABGD, Puillandre et al. 2012) was also conducted only on the COI alignment of the genus Vayssierea using the web server https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html, to create a matrix of p-distances between species.
Radular analyses
To extract the radula of specimens from Gran Canaria, North (Fig. 1A) and South (Fig. 1B), three individuals from each species were immersed in a KaOH 10% solution for approximately 4 h followed by three rinses with distilled water, and a final rinse with 96% EtOH. Radulae were extracted with thin forceps using a stereomicroscope and photographed under an optical microscope (Figs. 2A and 3A). Posterior analyses of the radular teeth were performed using a Field Emission Scanning Electron Microscope JSM-7100 F (SEM). All the plates, including the photographs of the specimens, live, optic microscope, and SEM, were edited in Adobe Photoshop 2020.
Radular teeth of Vayssierea sp. (VayGC1N) from El Confital, North of Gran Canaria. (A) General view under the optical Microscope. (B) General view under the Scanning Electron Microscope (SEM). (C) Close-up of the central part of the radula. (D) Close-up of the lateral and marginal teeth. (E) Close-up of the outer lateral tooth. F: Close-up of the inner lateral tooth
Radular teeth of Vayssierea sp. (VayGC1S) from El Faro, South of Gran Canaria. (A) General view under the optical Microscope. (B) General view under the Scanning Electron Microscope (SEM). (C) Close-up of the central part of the radula. (D) Close-up of the inner lateral tooth. (E) Close-up of the outer lateral and marginal teeth. (F) Close-up of the inner lateral tooth from another point of view
Results
Systematics
The final dataset included 77 specimens and 4,516 base pairs (bp), i.e. 658 bp for COI (Fig. S1), 507 characters for 16S (Fig. S2), 328 bp for H3 (Fig. S3), 1,082 characters for 28S (Fig. S4), and 1,941 characters for 18S (Fig. S5). The substitution models selected according to ModelFinder were GTR + F + I + G4 (Tavaré, 1986), for the partitions and genes except for the 3rd codon position of H3, which was GTR + F. Both ML and BI analyses of the concatenated alignment yielded similar results (Figs. 4 and 5). The family Chromodorididae included the genus Tyrinna Bergh, 1898 (bs = 100, pp = 1), Mexichromis Bertsch, 1977 (bs = 92, pp = 0.99), Felimare Ev. Marcus & Er. Marcus, 1967 (bs = 100, pp = 1), Felimida Ev. Marcus, 1971 (bs = 100, pp = 0.98), Chromodoris Alder & Hancock, 1855 (bs = 100, pp = 1), Ardeadoris Rudman, 1984 (bs = 100, pp = 1), and Doriprismatica d’Orbigny, 1839 (bs = 100, pp = 1), most of them with full branch support. Despite this, the individual Chromodoris ambiguus SAMD 19260 from Australia is clustered with the genus Mexichromis with high branch support (bs = 90, pp = 0.87), which indicates a possible misidentification or a misplacement of the species, as Chromodoris ambiguous is deviating from other species of the genus (Johnson and Gosliner, 2012; Wilson and Healy, 2006).
Maximum likelihood phylogenetic tree of the nudibranch families Cadlinidae, Hexabranchidae, Cadlinellidae, Showajidiidae, Chromodorididae, and Polyceridae (including subfamilies Triophinae, Nembrothinae, Okadaiinae stat. nov., and Polycerinae) differentially colored, based on the concatenated alignment of COI, 16S, H3, 28S, and 18S markers. The outgroups used to root the tree were the families Dendrodorididae and Phyllidiidae. Red dots indicate nodes with 100% bootstrap support value. Stars indicate type species. Specimens in blue were sequenced in this study. Bootstrap support values are shown in the branches. The scalebar indicates substitutions per site
Bayesian phylogenetic tree of the nudibranch families Cadlinidae, Hexabranchidae, Cadlinellidae, Showajidiidae, Chromodorididae, and Polyceridae (including subfamilies Triophinae, Nembrothinae, Okadaiinae stat. nov., and Polycerinae) differentially colored, based on the concatenated alignment of COI, 16S, H3, 28S and 18S markers. The outgroups used to root the tree were the families Dendrodorididae and Phyllidiidae. Red dots indicate nodes with posterior probabilities of 1. Stars indicate type species. Specimens in blue were sequenced in this study. Posterior probability support values are shown in the branches. The scalebar indicates substitutions per site
The family Polyceridae Alder & Hancock, 1845 was found to be monophyletic (bs = 97, pp = 0.96). Triophinae Odhner 1941 (bs = 100, pp = 1) included Kalinga ornata ZMMU Op-83 (Kalinginae Pruvot-Fol, 1956) in the ML, while it appears to be a sister group to Polycerinae with low support in the BI. Nembrothinae Burn, 1967 (bs = 97, pp = 0.97) included a likely misidentification of K. ornata CASMBNB001 as sister to Nembrotha cristata. Polycerinae Alder & Hancock, 1845 was recovered as monophyletic in the BI (bs = 88, pp = 0.99), while Gymnodorididae (Lecithophorus and Gymnodoris) was included in the ML (bs = 88). Gymnodorididae was sister to Okadaiidae in the BI (pp = 0.99). Okadaiidae Baba 1930 was found to be monophyletic with maximum support in both analyses as sister to Gymnorididae (pp = 0.99) or sister to Polycerinae + Gymnodorididae (bs = 100).
The subfamily Triophinae embraces the following genera: Limacia O. F. Müller, 1781 (bs = 91, pp = 0.97), Triopha Bergh, 1880 (bs = 100, pp = 1), Kaloplocamus Bergh, 1880 (bs = 100, pp = 1), Plocamopherus Rüppell & Leuckart, 1828 (bs = 90, pp = 1), and Kalinga Alder & Hancock, 1864. This last one should not be included in this subfamily, as it forms another subfamily by itself called Kalinginae. The subfamily Nembrothinae includes the genera Nembrotha Bergh, 1877 (bs = 100, pp = 1) and Tambja Burn, 1962 (bs = 97, pp = 0.97). Despite this, the individual Kalinga ornata CASMBNB 001 from India was included within the genus Nembrotha (bs = 100, pp = 1). Maybe the individual is not well identified in the genus Kalinga, and it could belong to the species Nembrotha cristata Bergh, 1877, as stated in both trees (Figs. 4 and 5) using the same voucher number but changing the species. In the subfamily Polycerinae, there are some discrepancies between both analyses. In ML, it appears to include the genera Thecacera J. Fleming, 1828 (bs = 100), Lecithophorus Macnae, 1958 (bs = 95), Gymnodoris W. Stimpson, 1855 (bs = 100), Palio Gray, 1857 (bs = 67), Polycerella A. E. Verrill, 1880 (bs = 100), and Polycera Cuvier, 1816 (bs = 97), most of them with high node support (Fig. 6). In the BI analysis, the subfamily Polycerinae only includes the genera Thecacera, Palio, Polycerella, and Polycera, while Lecithophorus and Gymnodoris (Gymnodorididae) appeared to be included as the sister group Okadaiidae. Despite this, an unidentified individual of Thecacera CASIZ 182906 from the Philippines clustered with the genus Polycera with the highest branch support (bs = 100, pp = 1), another possible case of misidentification.
Distribution map of the genus Vayssierea including the locality of all sequenced individuals represented in the phylogenetic tree. Dots indicate individuals and colors indicate different species according to the ASAP analysis: green for specimens from Australia and South Gran Canaria, blue for specimens from Japan, orange for North Gran Canaria, La Palma and Lanzarote; and red for Russia.. Type species location represented with yellow stars (1) Vayssierea caledonica, (2) Vayssierea cinnabarea, (3) Vayssierea elegans, (4) Vayssierea felis. Generated with https://www.simplemappr.net and edited in Adobe Photoshop.
Regarding Okadaiidae, since it appears consistently deeply nested within the family Polyceridae with high branch support (bs = 88, pp = 0.96), we suggest the new status Okadaiinae stat. nov. At the species level, we seem to have four species of Vayssierea. The individual from Russia (ZSM Mol20071333) appeared to be the sister group to the rest of the species (bs = 100, pp = 1). The individual from the north of Gran Canaria (VayGC1N) appears to be the sister group to the individual from Japan (MCZ 386352) (bs = 100, pp = 1), which, at the same time, seems to be the sister group to the rest of the individuals (bs = 95, pp = 0.99). The latter group is composed of specimens from Australia (MCZ 385477 and CASIZ 190731), the South of Gran Canaria (VayGC1S), and the one from the Aquarium belonging to the Indo-Pacific region (ML6), which appear to be monophyletic species (bs = 100, pp = 0.99).
The SDT (Fig. S1) results from the ASAP, PTP, ABGD, and our previous data from the phylogenetic trees suggest that we have four species of Vayssierea (considering both COI and 16 S data). The second-best asap-score threshold distance for COI is coherent with significant differentiation in nucleotide position between species of about 5–10% and agrees with the PTP results.
Intra- and interspecific distances were given by the ABGD analyses. The intraspecific distance of Vayssierea between specimens from Australia, Southern Gran Canaria and the Indo-Pacific region ranges 0.3–1.6%; while the intraspecific distance between specimens from Northern Gran Canaria, La Palma and Lanzarote is 0–1.2%. The interspecific distance between species from Australia-Southern Gran Canaria-Indo-Pacific region and Northern Gran Canaria-La Palma-Lanzarote ranges 11.4–12.7%; while the interspecific distance between species from Australia-Southern Gran Canaria-Indo-Pacific region and Japan is 11.8–12.5%. Finally, the interspecific distance between species from Northern Gran Canaria-La Palma-Lanzarote and Japan is 13.3%.
Morphological analyses
The individuals from northern Gran Canaria (Fig. 1A) have a strong, homogeneous red-orange colouration, which is different from the other described species. Individuals from southern Gran Canaria are bright orange coloured and have a paler rhinophoral tip, with a whitish spot on the anterior dorsal part (Fig. 1B). Recently reported specimens from La Palma and Lanzarote (Fig. 1E and F) show the same external features as the southern ones.
The radular formula of the northern specimens is 39 × 1.2.1.2.1 (Fig. 2). The radular formula of the southern ones is 45 × 1.2.1.2.1 (Fig. 3). There are no noticeable differences in the shape of the teeth, but the southern specimens have more rows. However, Baba (1937) reported from 35 to 44 rows in the species V. elegans, and this is between the range of our two dissected species. The rachidian tooth is a subtle thickening of the cuticle and, therefore, likely vestigial. The inner lateral tooth bears two pairs of hook-shaped acute cusps, two long ones along the tooth and two smaller ones in the apex. The outer lateral tooth is hook-shaped, acute, and longer than the first inner tooth, extending almost double the total length. The marginal tooth is stick-shaped and presents a wide base.
Discussion
The phylogenetic position of Vayssierea
In this study, we sequenced five molecular markers from ten specimens representing three different species of the genus Vayssierea and placed them within a broader multilocus phylogenetic context. Our concatenated dataset included 75 specimens, 41 of which belonged to the family Polyceridae, and eight of them from the genus Vayssierea. Our molecular data suggest several systematic and taxonomic rearrangements within the family Polyceridae that will need to be revisited taxonomically in later studies. Nevertheless, we suggest suppressing the family Okadaiidae and incorporating the subfamily Okadaiinae stat. nov. within Polyceridae, which included the genus Vayssierea.
The systematics of the genus Vayssierea have been confounding since its inception. This confusion primarily arises from the scarcity of studies, many of which lack a systematics perspective (Ralph 1944; Mikhlina et al. 2019). Most of the studies where Vayssierea appears are checklists and biodiversity guides (Gosliner 1987; Burn 2006; Héros et al. 2007). The existing phylogenies of the family Polyceridae incorporated morphological and genetic data for only a few genera (Pola et al. 2007; Harris 2011; Palomar et al. 2014), and only Korshunova and collaborators (2020) included the genus Vayssierea. Through increased taxon sampling, expanded geographical range (see Fig. 3), and a higher number of sequenced genes, we provide evidence supporting the placement of the genus Vayssierea within the family Polyceridae, a proposition hinted by some authors based on morphological similarities (Baba 1930) and recently corroborated in a phylogenetic tree (Korshunova et al. 2020). Despite conflicting with molecular findings, the latter authors advocated for maintaining the family Okadaiidae due to its distinctive morphological features, such as the complete reduction of the rhinophoral sheath, notal margin, gills, and gill cavity. These characters may have arisen from paedomorphosis (Martynov and Korshunova 2011). Nonetheless, the reduction in external features likely reflects the miniaturization of the body plan to accommodate a specific diet. Consequently, the family should be reclassified as the subfamily Okadaiinae stat. nov., closely related to Polycerinae. The long branch observed in Vayssierea in both analyses indicated rapidly evolving lineages (Bergsten 2005), suggesting that it has undergone numerous mutations in a short period compared to its sister groups. Shifts in habitat towards highly specialized ecotopes and diets may have led to the reduction of external features to accommodate into the prey’s calcareous tube, consequently driving increased gene evolutionary rates. Interestingly, a similar trend is observed in the genus Gymnodoris within the other Polyceridae subfamilies, as both genera are active carnivorous predators. Indeed, some species of Gymnodoris exhibit radulae resembling those of Vayssierea species (Martynov 2000).
The taxonomy of the genus Gymnodoris has also been controversial in recent studies (Palomar et al. 2014). Some older studies included them in Polyceridae (e.g., Eliot 1903; Macnae 1958) due to minor differences in internal morphology, primarily the radula and buccal apparatus, which are characters susceptible to change with dietary shifts. Other studies supported Gymnodoridiidae Odhner 1941 (e.g., Korshunova et al. 2020), although it is currently recognized as a synonym of Polycerinae (Bouchet et al. 2017). The placement of the family Gymnodorididae in phylogenetic trees also varies, with our ML (Fig. 4) tree placing it within Polycerinae, consistent with some recent studies (e.g., Palomar et al. 2014), while our BI (Fig. 5) tree suggests it as a sister group to Okadaiinae stat. nov., albeit without support. Nevertheless, similar to Vayssierea, we found consensus regarding the inclusion of both Gymnodoris and Lecithophorus within Polyceridae. Therefore, we support the incorporation of Gymnodorididae within Polyceridae (Bouchet et al. 2017), although whether they belong to Polycerinae or constitute their own subfamily requires additional taxon sampling or genomic data for confirmation (JM work in progress), mirroring the situation with Okadaiinae stat. nov.
Currently, the family Polyceridae encompasses the subfamilies Triophinae, Nembrothinae, Polycerinae, Kalinginae, Kankelibranchinae Ortea, Espinosa & Caballer, 2005, and the newly proposed subfamily Okadaiinae stat. nov. The latter is diagnosed by a small limaciform body, approximately 5 mm in length, orange colouration, two short and smooth rhinophores, the absence of a notal margin, and a lack of gills and a gill pocket. A comparison of Okadaiinae stat. nov. with other Polyceridae subfamilies reveals few shared characters; unlike other subfamilies, Okadaiinae stat. nov. lacks external gills, oral tentacles, lamellate rhinophores, and exhibits a different colouration pattern (e.g., Gosliner and Vallès 2006; Pola et al. 2014; Palomar et al. 2014). The only genus with fewer differences is Gymnodoris, characterized by a limaciform body and the absence of external tentacles and papillae. As suggested here, the species within the genus Vayssierea may have adapted to an unusual intertidal habitat due to their unique dietary source, serpulid worms (Burn 2006; Gosliner et al. 2008). Consequently, their external characters may have undergone significant modification and differentiation from other family members, leading to their previous classification within the family Okadaiidae (Odhner 1941; Bouchet et al. 2017; Knutson and Gosliner 2022). Martynov and Korshunova (2011) hypothesize Okadaiidae may have evolved from Gymnodorididae by paedomorphosis, which may explain the simplification of body features.
Radulae analyses reveal similarities among species from Gran Canaria, but notable differences arise when comparing with other genera within the family Polyceridae. Various studies examining radulae of specimens of the subfamilies Nembrothinae, Polycerinae, and Triophinae (Pola et al. 2008, 2014; Jung et al. 2020; respectively) show flattened teeth with a rectangular shape, featuring numerous outer lateral teeth per row. However, when comparing our specimens with those of the genus Gymnodoris (Knutson and Gosliner 2022), more similarities emerge, including hook-shaped teeth and fewer lateral teeth per row. This discrepancy can be attributed to nutritional specialization. Subfamilies Triophinae, Nembrothinae, and Polycerinae primarily feed on sessile animals such as bryozoans and tunicates, employing their teeth to scrape them off (Pola et al. 2014). In contrast, Gymnodoris species actively prey on sea slugs, utilizing their hooked teeth to capture their prey. This specialization in feeding behaviour may also extend to the teeth of Vayssierea, adapted for a drilling feeding mode and subsequent extraction of tube worms (Mikhlina et al. 2019). These similarities suggest a close relationship between Gymnodoris and Vayssierea, as supported by our BI analysis.
Speciation and distribution of Vayssierea
Regarding the STD analyses, we have molecular data for four species of Vayssierea; however, the identities of these species remain unknown. The species to which individuals from Australia, southern Gran Canaria, and the ones from the Indo-Pacific could be either V. caledonica or V. cinnabarea, based on their geographical proximity. The type locality of the latter species is New Caledonia and New Zealand, respectively. Examining external morphology, V. cinnabarea appears to be a more suitable candidate, as specimens from southern Gran Canaria (Fig. 1B) exhibit a lighter rhinophoral tip and a paler colouration (Fig. 1C). The second species encompasses the individual from Japan (Fig. 1D), which may pertain to either V. elegans or V. felis, with type localities in the Yellow and South China seas, respectively. The third species includes the individual from northern Gran Canaria, La Palma, and Lanzarote, different from all other species. Their origin is uncertain, but they likely originated from the Indian or Pacific oceans as its sympatric counterpart. Finally, the fourth species includes the individual from Russia, which may also belong to V. elegans or V. felis based on its locality.
Our radular analyses yield results similar to those of Young’s (1969) pioneering study, the first examination of feeding mechanisms in the genus Vayssierea. However, our findings also diverge from another study on Vayssierea cf. elegans (Mikhlina et al. 2019), which demonstrates a distinct tooth shape (the outer lateral exhibiting two spikes instead of one). Without further taxon sampling from the type localities, species identification within the studied material remains challenging. External morphology, characterized by few distinguishing features, and radular shape alone are insufficient for species differentiation, especially given the limited knowledge regarding the radular morphology of the four already described species. Nonetheless, it is plausible that numerous species remain to be described.
Considering the available information on the distribution of this species and the findings of our study, it is possible to elucidate the global distribution of the genus Vayssierea and the geographical localization of its various species (Fig. 6). As illustrated, populations in Gran Canaria are significantly distant from the known distribution of this genus across the Indo-Pacific Ocean. Based on these observations, we propose that the genus Vayssierea represents a new NIS in the Atlantic Ocean, with the presence of not one but (at least) two distinct species. The malacofauna from the Canary Islands has been subject to intensive study in recent years, and it appears that only now is the widespread presence of Vayssierea across the archipelago becoming apparent, with increasing records reported by local divers.
A tiny introduction
These diminutive species have traversed from the Indo-Pacific Ocean to the Canary Islands situated in the middle of the Atlantic. Given that Vayssierea species undergo ‘direct’ (intracapsular) development (Ralph 1944; Burn 2006; Gosliner et al. 2008), larval dispersion appears improbable as a means of transportation. Instead, a potential driver for dispersion could be shipping transportation facilitated by adult individuals or egg masses being attached to hulls or being transported in ballast waters. This mode of introduction has been extensively studied worldwide due to its significant impacts and consequences on ecosystems. For instance, the ctenophore Mnemiopsis leidyi A. Agassiz, 1865, originating from coastal waters of the temperate and subtropical regions of North and South America, has now achieved broad distribution by ballast waters through the Black and Azov Seas (Bailey 2015). Toxic dinoflagellates belonging to the genus Alexandrium were transported in ballast tanks from Japan and Korea to Australia (Hallegraeff and Bolch 1992). Additionally, the butterflyfish Chaetodon sanctaehelenae Günther, 1868, native from St. Helena and Ascension Island, is now found in the Canary Islands due to shipping transportation (Brito et al. 2005).
Furthermore, the economy of the Canary Islands, heavily reliant on tourism, is deeply intertwined with sea transport. Given that accessibility and connectivity are crucial factors in international transport, harbours in the Canary Islands, particularly those in Gran Canaria and Tenerife, experience the highest influx of ships from various oceans (Tovar et al. 2015). Consequently, the likelihood of biological introductions of NIS via shipping transportation is elevated. As Vayssierea’s prey may attach to ships’ hulls, slugs could travel with them as well, possibly sheltered inside the annelid’s calcareous tube. The southern population of Gran Canaria is confirmed as an NIS introduced from Australia based on molecular identity. Yet, the origin of the most widespread species, from northern Gran Canaria, La Palma, and Lanzarote, remains uncertain, but it is plausible they too are an introduced species, given its absence from previous records. This suggests that an introduction with origins and characteristics akin to our Vayssierea populations is conceivable. Interestingly, sequenced populations from Gran Canaria and Lanzarote were discovered naturalized in the intertidal habitat, with numerous Spirorbis sp. specimens present. Only the recently found specimens from La Palma were observed in harbour or related intertidal pools. In summary, our observations validate the presence of Vayssierea across the Macaronesian archipelago.
Another plausible scenario to consider is the accidental escape from aquariums, as evidenced by genetically identical specimens from the Aquarium of Murcia (located south of the Iberian Peninsula) to those from Australia and southern Gran Canaria. These specimens were discovered alongside Tridacna giant clams from an unknown region in the Indo-Pacific. Unfortunately, such unwanted individuals have the potential to escape from aquariums and thrive in different seas if they encounter optimum habitats and conditions, as found in the Canary Islands. Studies of this mode of invasion (Padilla and Williams 2004) have focused on various aquarium organisms, including fishes in Florida (Semmens et al. 2004) and India (Krishnakumar et al. 2009), as well as anthozoans, scyphozoans, crustaceans, and molluscs (Lin et al. 2006), such as the flame scallop Ctenoides scaber (Born, 1778). More notably, there are documented cases of aquarium release invasions in the Canary Islands, such as the marine angelfish Pomacanthus maculosus (Forsskål, 1775) from the Red Sea and the Indian Ocean, which was discovered in Tenerife’s harbour (Brito et al. 2005). This underscores the potential for accidental introduction of NIS through this vector, which can subsequently become invasive. Instances of Indo-Pacific invasive species in the Atlantic Ocean further support this notion, such as the lionfish Pterois volitans (Linnaeus, 1758) (Whitfield et al. 2007), originating from the Red Sea but introduced to the Mediterranean Sea and the Atlantic Ocean through aquarium releases or ballast waters in shipping transportation (Whitfield et al. 2002). An analogous example of a gastropod with similar characteristics to Vayssierea is the muricid Bedeva paivae (Crosse, 1864). Originally restricted to New Zealand and southern Australia, this species has been accidentally introduced by shipping transport (Gofas et al. 2017) to South Africa, Madeira, and the Canary Islands (Barco et al. 2015). This reinforces the possibility of an introduction with similar characteristics and origins, thus supporting our primary hypothesis of the genus Vayssierea as a NIS in the Canary Islands.
Conclusions
Novel specimens and molecular markers were acquired from the genus Vayssierea to elucidate its systematics by situating them in a broader phylogenetic framework. Within the family Polyceridae, four subfamilies are recognized: Triophinae, Nembrothinae, Polycerinae, and the newly proposed Okadaiinae stat. nov. Owing to the diminished morphological characters, the latter taxon was originally classified as a distinct family without a definitive systematic position within the superfamily Doridoidei. The newly established subfamily Okadaiinae stat. nov. encompasses the aberrant genus Vayssierea, characterized by a rapid evolutionary rate that may account for the marked differences in external morphology observed in comparison to other polycerids. While the available molecular data for four species of Vayssierea help highlight discrete differences in external and radular characteristics, further investigation into internal anatomy is imperative to facilitate species identification.
The genus Vayssierea exhibits a distribution spanning the Indo-Pacific region, ranging from Japan to Australia, the Chinese Sea, and South Africa. Due to their limited dispersal, recent discoveries of individuals in the Canary Islands may have travelled long distances by attaching to ships’ hulls or escaping from aquaria. Among the two recognized non-indigenous species in the Atlantic Ocean, one belongs to a species present in Australia, while the other has not been previously sequenced. Therefore, it is paramount to intensify sampling efforts across a broader geographic range in the Indo-Pacific to elucidate their origin and identity. Anatomical analyses are required to differentiate between Vayssierea species, as comparative studies are hindered by the lack of precise descriptions. Recent expeditions in La Palma and Lanzarote have uncovered additional individuals of Vayssierea (Fig. 1E and F), exhibiting molecular similarities to those from northern Gran Canaria. This finding suggests a broader distribution of the species within the archipelago.
Data availability
All specimens were deposited at the MCZ and the sequences were uploaded to GenBank. The trees generated during and/or analysed during the current study are available in the supplementary material.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Araujo AK, Pola M, Malaquias MAE, Ballesteros M, Vitale F, Cervera JL (2022) Molecular phylogeny of European Runcinida (Gastropoda, Heterobranchia): the discover of an unexpected pool of complex species, with special reference to the case of Runcina coronata. Zool J Linn Soc 194:761–788
Baba K (1930) Studies on Japanese nudibranchs (2) A. Polyceridae, B. Okadaia, n. g. (preliminary report). Venus 2:43–50
Baba K (1931) A noteworthy gill-less holohepatic nudibranch, Okadaia Elegans Baba, with reference to its internal anatomy. Annot Zool Jpn 13:pls–5
Baba K (1937) Contribution to the knowledge of a nudibranch, Okadaia Elegans. Jpn J Zool 7:147–190
Bailey SA (2015) An overview of thirty years of research on ballast water as a vector for aquatic invasive species to freshwater and marine environments. Aquat Ecosyst Health Manag 18:261–268
Barco A, Marshall B, Houart R, Oliverio M (2015) Molecular phylogenetics of Haustrinae and Pagodulinae (Neogastropoda: Muricidae) with a focus on New Zealand species. J Molluscan Stud 81:476–488
Bennett S, Santana-Garcon J, Marbà N et al (2021) Climate-driven impacts of exotic species on marine ecosystems. Glob Ecol Biogeogr 30:1043–1055
Benson DA, Karsch-Mizrachi I, Lipman DJ, Ostell J, Rapp BA, Wheeler DL (2000) GenBank. Nucleic Acids Res 28:15–18
Bergsten J (2005) A review of long-branch attraction. Cladistics 21:163–193
Bouchet P, Rocroi JP, Hausdorf B et al (2017) Revised classification, nomenclator and typification of gastropod and monoplacophoran families. Malacologia 61:1–526
Brito A, Falcón JM, Herrera R (2005) Sobre La tropicalización reciente de la ictiofauna litoral de las islas canarias y su relación con cambios ambientales y actividades antrópicas. Vieraea 33:515–525
Burn R (2006) A checklist and bibliography of the Opisthobranchia (Mollusca: Gastropoda) of Victoria and the Bass Strait area, south-eastern Australia. Mus Vic Sci Rep 10:7–13
Carlton JT (2001) Introduced species in US coastal waters: environmental impacts and management priorities. Pew Oceans Commissions, Washington, DC
Castro N, Ramalhosa P, Jiménez J, Costa JL, Gestoso I, Canning-Clode J (2020) Exploring marine invasions connectivity in a NE Atlantic Island through the lens of historical maritime traffic patterns. Reg Stud Mar Sci 37:101333
Collingwood C (1881) II. On some New species of Nudibranchiate Mollusca from the Eastern seas. Trans Linn Soc Lond 2:123–140
Courchamp F, Fournier A, Bellard C, Bertelsmeier C, Bonnaud E, Jeschke JM, Russell JC (2017) Invasion biology: specific problems and possible solutions. Trends Ecol Evol 32:13–22
Eliot CN (1903) Nudibranchiata, with some remarks on the families and genera and description of a new genus. Doridomorpha Fauna Geogr Maldive Laccadive Archipelagoes 2:540–573
Fernández-Vilert R, Giribet G, Salvador X, Moles J (2021) Assessing the systematics of Tylodinidae in the Mediterranean Sea and Eastern Atlantic Ocean: resurrecting Tylodina Rafinesquii Philippi, 1836 (Heterobranchia: Umbraculida). J Molluscan Stud 87:eyaa031
Goddard J, Pearse J (2011) Long-term faunal changes in California nudibranchs: climate change and local ocean health. Research Final Reports, UC San Diego
Gofas S, Luque AA, Templado J, Salas C (2017) A national checklist of marine Mollusca in Spanish waters. Sci Mar 81:242–254
Gollasch S, Galil BS, Cohen AN (2006) Bridging divides: maritime canals as invasion corridors, vol 229. Dordrecht, Springer
Gosliner TM Nudibranchs of Southern Africa. A guide to opisthobranch molluscs of Southern Africa. Sea Challengers, Monterey, and, Hamann J (1987) EI Cajon, pp 136
Gosliner TM, Vallès Y (2006) Shedding light onto the genera (Mollusca: Nudibranchia) Kaloplocamus and Plocamopherus with description of new species belonging to these unique bioluminescent dorids. Veliger 48:178–205
Gosliner TM, Behrens DW, Valdés Á (2008) Indo-Pacific Nudibranchs and Sea Slugs. A field guide to the World’s most diverse fauna. San Francisco
Hallegraeff GM, Bolch CJ (1992) Transport of diatom and dinoflagellate resting spores in ships’ ballast water: implications for plankton biogeography and aquaculture. J Plankton Res 14:1067–1084
Harris JK (2011) A molecular phylogeny of Polyceridae (Nudibranchia) with comments on ‘Phanerobranchia’. Master Thesis. San Francisco State University
Héros V, Lozouet P, Maestrati P, von Cosel R, Brabant D, Bouchet P (2007) Mollusca of New Caledonia. Compendium of Marine species of New Caledonia. Doc. Sci Tech ed 2:199–254
Hoang DT, Chernomor O, Von Haeseler A, Minh BQ, Vinh LS (2018) UFBoot2: improving the ultrafast bootstrap approximation. Mol Biol Evol 35:518–522
Johnson RF, Gosliner TM (2012) Traditional taxonomic groupings mask evolutionary history: a molecular phylogeny and new classification of the chromodorid nudibranchs. PLoS ONE 7:e33479
Jung DW, Gosliner TM, Choi TJ, Kil HJ, Chichvarkhin A, Goddard JH, Valdés Á (2020) The return of the clown: pseudocryptic speciation in the North Pacific clown nudibranch, Triopha catalinae (Cooper, 1863) sensu lato identified by integrative taxonomic approaches. Mar Biodivers 50:1–17
Kalyaanamoorthy S, Minh BQ, Wong TK, Von Haeseler A, Jermiin LS (2017) ModelFinder: fast model selection for accurate phylogenetic estimates. Nat Methods 14:587–589
Kantor YI, Sysoev AV (2006) Marine and brackish water Gastropoda of Russia and adjacent countries: an illustrated catalogue. Moscow: KMK Scientific Press, 2018:16604
Katoh K, Misawa K, Kuma KI, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066
Knutson VL, Gosliner TM (2022) The first phylogenetic and species delimitation study of the nudibranch genus Gymnodoris reveals high species diversity (Gastropoda: Nudibranchia). Mol Phylogenetics Evol 171:107470
Korshunova T, Picton B, Furfaro G et al (2019) Multilevel fine-scale diversity challenges the ‘cryptic species’ concept. Sci Rep 9:6732
Korshunova T, Fletcher K, Picton B et al (2020) The Emperor’s Cadlina, hidden diversity and gill cavity evolution: new insights for the taxonomy and phylogeny of dorid nudibranchs (Mollusca: Gastropoda). Zool J Linn Soc 189:762–827
Krishnakumar K, Raghavan R, Prasad G, Bijukumar A, Sekharan M, Pereira B, Ali A (2009) When pets become pests–exotic aquarium fishes and biological invasions in Kerala, India. Curr Sci 97:474–476
Lin YH, Chang CH, Chen IH, Chiu YW, Wu SH, Chen JH (2006) The survey of the imported aquatic invertebrates via the live aquarium ornamental trade in Taiwan. Taiwania 51:99–107
Macnae W (1958) The families Polyceridae and Goniodorididae (Mollusca, Nudibranchiata) in southern Africa. Trans R Soc S Afr 35:341–372
Martynov A (2000) Vayssiereidae - further thoughts. [Message in] Sea Slug Forum. Australian Museum, Sydney. http://www.seaslugforum.net/find/2298
Martynov AV, Korshunova TA (2011) Opisthobranch molluscs of the seas of Russia. A colour guide to their taxonomy and biology. Fiton, Moscow
Mikhlina AL, Tzetlin AB, Ekimova IA, Vortsepneva EV (2019) Drilling in the dorid species Vayssierea cf. Elegans (Gastropoda: Nudibranchia): functional and comparative morphological aspects. J Morphol 280:119–132
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees in Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA pp 1–8
Moles J, Riesgo A (2019) A junior freckled nudibranch: chromatic variability in Felimida species from the Eastern Atlantic. Spixiana 42:193–202
Moles J, Berning MI, Hooker Y, Padula V, Wilson NG, Schrödl M (2021) Due South: the evolutionary history of Sub-antarctic and Antarctic Tritoniidae nudibranchs. Mol Phylogenetics Evol 162:107209
Naylor R, Hindar K, Fleming IA et al (2005) Fugitive salmon: assessing the risks of escaped fish from net-pen aquaculture. Bioscience 55:427–437
Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32:268–274. https://doi.org/10.1093/molbev/msu30
Odhner NH (1941) New Polycerid Nudibranchiate Mollusca and remarks on this family. Elander
Padilla DK, Williams SL (2004) Beyond ballast water: aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Front Ecol Environ 2:131–138
Palomar G, Pola M, Garcia Vazquez E (2014) First molecular phylogeny of the subfamily Polycerinae (Mollusca, Nudibranchia, Polyceridae). Helgol Mar Res 68:143–153
Pola M, Cervera JL, Gosliner TM (2007) Phylogenetic relationships of Nembrothinae (Mollusca: Doridacea: Polyceridae) inferred from morphology and mitochondrial DNA. Mol Phylogenetics Evol 43:726–742
Pola M, Cervera JL, Gosliner TM (2008) Revision of the Indo-Pacific Genus Nembrotha (Nudibranchia: Dorididae: Polyceridae), with a description of two new species. Sci Mar 72:145–183
Pola M, Padula V, Gosliner TM, Cervera JL (2014) Going further on an intricate and challenging group of nudibranchs: description of five novel species and a more complete molecular phylogeny of the subfamily Nembrothinae (Polyceridae). Cladistics 30:607–634
Puillandre N, Lambert A, Brouillet S, Achaz GJME (2012) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol Ecol 21:1864–1877
Puillandre N, Brouillet S, Achaz G (2021) ASAP: assemble species by automatic partitioning. Mol Ecol Resour 21:609–620
Pyšek P, Hulme PE, Simberloff D et al (2020) Scientists’ warning on invasive alien species. Biol Rev 95:1511–1534
Ralph P (1944) Pellibranchus Cinnabareus, a new genus and species of non-pelagic nudibranch mollusc of the family phyllirhoidae. Trans Proc Roy Soc New Z 74:24–31
Rambaut A (2010) FigTree v1.3.1. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh. http://tree.bio.ed.ac.uk/software/figtree/
Ratnasingham S, Hebert PD (2007) BOLD: the barcode of life data system. Mol Ecol Notes 7:355–364. http://www.barcodinglife.org
Risbec J (1928) Contribution a l’etude des nudibranches Neo-Caledoniens. Faune Colon Franc, 2.
Ronquist F, Teslenko M, Van Der Mark P et al (2012) MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Rudman WB, Beesley PL, Ross GJB, Wells A (1998) Suborder Doridina. Mollusca: the southern synthesis. Fauna Australia 5:990–1001
Semmens BX, Buhle ER, Salomon AK, Pattengill-Semmens CV (2004) A hotspot of non-native marine fishes: evidence for the aquarium trade as an invasion pathway. Mar Ecol Prog Ser 266:239–244
Slavoshevskaya LV (1971) A new aberrant nudibranchiate mollusc from the Sea of Japan. Explorations of the Fauna of the seas, VIII(XVI). Fauna and Flora of the Possiet Bay of the Sea of Japan (Hydrobiological investigations by diving method). Nauka, Leningrad. Zoological Institute of the Academy of Sciences of the USSR
Tavaré S (1986) Some probabilistic and statistical problems on the analysis of DNA sequence. Lect Math Life Sci 17:57
Thiele J (1931) Handbuch Der Systematischen Weichtierkunde (Vol. 1, No. 1). G. Fisher
Tovar B, Hernández R, Rodríguez-Déniz H (2015) Container port competitiveness and connectivity: the Canary Islands main ports case. Transp Policy 38:40–51
Vermeij GJ, Sax DF (2005) Invasion as expectation: a historical fact of life. In: Sax DF, Stachowicz JJ, Gaines SD (eds) Species invasions: insights into ecology, evolution, and biogeography, 315–339
Waldman M, Shevah Y (2000) Biological Diversity — an overview. In: Belkin S (ed) Environmental challenges. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-4369-1_24
Whitfield PE, Gardner T, Vives SP, Gilligan MR, Courtenay WR Jr, Ray GC, Hare JA (2002) Biological invasion of the Indo-Pacific lionfish Pterois volitans along the Atlantic coast of North America. Mar Ecol Prog Ser 235:289–297
Whitfield PE, Hare JA, David AW, Harter SL, Munoz RC, Addison CM (2007) Abundance estimates of the Indo-Pacific lionfish Pterois volitans/miles complex in the Western North Atlantic. Biol Invasions 9:53–64
Wilson NG, Healy JM (2006) Basal chromodorid sperm ultrastructure (Nudibranchia, Gastropoda, Mollusca). Zoomorphology 125:99–107
Young DK (1969) Okadaia Elegans, a tube-boring nudibranch mollusc from the central and West Pacific. Am Zool 9:903–907
Zhang J, Kapli P, Pavlidis P, Stamatakis A (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29:2869–2876. https://doi.org/10.1093/bioinformatics/btt499
Acknowledgements
We would like to thank the Slug Lab (@slug_lab) for their help in the molecular lab and the CCiT-UB for aiding during the SEM sessions. Special thanks are due to Rosa Canales-Cáceres (University of Alicante) for providing the specimens from the aquarium, Jennifer Trimble (MCZ, Harvard) for curatorial assistance, and Dennis Rabeling for providing the picture of the specimen from Lanzarote. We are grateful to four anonymous reviewers and the editor for their valuable comments.
Funding
The Spanish Government provided funding through the HETGEN1000 project (PID2021-127037NA-I00/MCIN/AEI/https://doi.org/10.13039/501100011033/ and by FEDER una manera de hacer Europa).
Author information
Authors and Affiliations
Contributions
JM contributed to the study conception and design, specimen collection, and funds. Both authors performed lab work, analysis, wrote, read, and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
All experimental procedures in this study were performed complying with Spanish legislation.
Additional information
Communicated by A. Zhan.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Canet-Miralda, C., Moles, J. Exploring the intriguing arrival of Vayssierea Risbec, 1928 slugs in the Atlantic Ocean from the Indo-Pacific (Mollusca, Nudibranchia). Mar Biol 171, 186 (2024). https://doi.org/10.1007/s00227-024-04505-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-024-04505-3