Abstract
Infaunal invertebrate communities are structured by various factors, including predation, resource availability, and environmental conditions. Given that these invertebrates live within sediment, it is not surprising that sediment properties play a critical role in many infaunal behaviours. When models explaining spatial and temporal variation in infaunal community composition are constructed using physical, biophysical, environmental, and sediment properties (salinity, detrital cover, elevation, particle size distribution, organic and water content, redox conditions, and penetrability), a considerable portion of the variation in the data is typically unaccounted for. This suggests that we do not fully understand all the variables that influence infaunal invertebrate communities. One suite of under-explored variables is the elemental composition/concentration of the sediments themselves. As such, we evaluated if sediment geochemistry improved model performance of the spatial variation in infaunal invertebrate communities on three intertidal mudflats in northern British Columbia, Canada. We observed that models including geochemistry data outperformed models that only included physical, biophysical, and environmental properties. Our results, therefore, suggest that some of the observed, and previously unaccounted for spatial variation in infaunal community composition may be a product of variation in sediment geochemistry. As such, sediment geochemistry should be accounted for when studying infaunal communities and assessing human impacts upon intertidal systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Located at the interface between the land and the sea, intertidal ecosystems exist within a world of extremes. Oscillating between exposure and inundation, hot and cold, safety and danger, the intertidal is home to resilient and dynamic biological communities (Ferraro and Cole 2012; Musetta-Lambert et al. 2015; Delgado et al. 2018). Far from a uniform ecosystem, the intertidal is a mosaic of marshes (Virgin et al. 2020), rocky shores (Menge et al. 1997), and expanses of soft sediment, such as sandy beaches and mudflats (Barbeau et al. 2009). In coastal ecosystems, infaunal (animals that live within the sediment) are often used to study or identify disturbances (Fukuyama et al. 2014; Drylie et al. 2020; Gerwing et al. 2022a). For instance, biodiversity and total abundance of infauna (Sherman and Coull 1980; Campbell et al. 2019b) can be used to identify and study disturbances; however, infauna can respond to a disturbance in a variety of ways. For instance, amphipods, cumacea, and small bivalves are sensitive to disturbances, decreasing in abundances with disturbances (Sánchez-Moyano and García-Gómez 1998; Gerwing et al. 2022b). Conversely, Oligochaeta (Cowie et al. 2000), Nematoda (Mazzola et al. 2000), and Capitella species complex (Pearson and Rosenberg 1978; Gerwing et al. 2022b) are tolerant of disturbances, and can survive, or thrive in disturbed systems. Therefore, studying the population and community dynamics of these intertidal invertebrates is useful to advance the development of general ecological theories (Underwood and Fairweather 1989; Hamilton et al. 2006) and to undertake applied research, such as elucidating human impacts or the effectiveness of restoration strategies (Pearson and Rosenberg 1978; Borja et al. 2008).
Various factors influence the structure of infaunal invertebrate communities, including predation (Floyd and Williams 2004), resource availability (Hamilton et al. 2006), and environmental factors such as salinity, weather, and climate (Ghasemi et al. 2014; Gerwing et al. 2015b). While all of these variables are important, given the intimate nature of the relationship between infauna and the sediment they inhabit, it is not surprising that sediment properties have been observed to play a critical role in most infauna behaviours, including locomotion, foraging, reproduction, and larval settlement (Ólafsson et al. 1994; Lu and Grant 2008; Gerwing et al. 2020a). More specifically, sediment properties such as particle size distribution, water content, organic matter content, redox state, and sediment penetrability/consistency have all been observed to play important roles in shaping the composition of infaunal communities, by influencing infauna behaviour, survival, and physiology (Diaz and Trefry 2006; Valdemarsen et al. 2010; Pilditch et al. 2015a; Gerwing et al. 2018b, 2020a). These relationships are far from unidirectional, as infauna can greatly modify their sedimentary environment (De Backer et al. 2011; Quintana et al. 2013; Sizmur et al. 2013; Gerwing et al. 2017b).
Despite a wealth of information on the relationships between sediment properties/environmental conditions and infaunal communities, models constructed from the variables listed in the previous paragraph that predict the spatiotemporal variation in an infaunal community often leave a portion of the variation in the data (20–97%) unaccounted for (Chapman et al. 1987; Thrush et al. 2003; Dashtgard et al. 2014; Gerwing et al. 2015b, 2016, 2020a; Campbell et al. 2020). While no model is perfectly representative of the process it is simulating, this unaccounted variation suggests that we do not fully understand all the factors that influence the structure of infaunal invertebrate communities. One suite of potentially important, but under-explored, variables are those related to sediment geochemistry (concentration of elements such as Hg, Mn, Ti, etc.). Especially, since the abundance/concentration of some elements in intertidal sediments have been observed to influence infaunal invertebrate community composition and population size (Chapman et al. 1987; Waldock et al. 1999; Sizmur et al. 2019).
Elements that can negatively affect intertidal invertebrate communities are referred to as potentially toxic elements (PTEs). While PTEs are naturally occurring, human activities may elevate concentrations to levels that can induce deleterious impacts upon the physiology, behaviour, and survival of flora and fauna (Martinez-Colon et al. 2009; Pourret and Bollinger 2018; Sizmur et al. 2019). The concentration and availability of PTEs have generated substantial ecological insight in theoretical and applied settings (Chapman et al. 1987; Mermillod-Blondin and Rosenberg 2006; Spencer and Harvey 2012). However, interactions between invertebrates and sediment geochemistry are often studied in the context of contamination or pollution when the concentrations present are many times greater than ambient background values (Chapman et al. 1987; Yunker et al. 2011; Amoozadeh et al. 2014).
Another important group of elements found in intertidal sediments (which overlap with PTEs) are essential elements. Essential elements are those that are required in specific stochiometric ratios for organisms to complete their life cycle (Karimi and Folt 2006; Bradshaw et al. 2012). Previous investigations have focused upon the interaction between specific infaunal species and the presence or availability of a few, albeit important, elements (Christensen et al. 2000; Teal et al. 2013; Kalman et al. 2014). What is currently lacking is a holistic understanding of how sediment geochemistry, influences entire infaunal communities (Sizmur et al. 2019; Eccles et al. 2020), and if the geochemical composition of sediments plays a greater role than other sediment parameters in structuring infaunal communities.
To better understand how sediment geochemistry influences infaunal communities, we quantified whether adding sediment geochemistry data (elemental concentrations) to models containing traditionally studied sediment and environmental variables (salinity, detritus cover, distance from shore, particle size distribution, organic and water content, redox conditions, and penetrability) improved their performance when modelling infaunal community composition and population abundances. We then explored in more detail how specific elements were associated with the observed variation in specific members of an infaunal invertebrate community. A better understanding of how sediment geochemistry influences infaunal community species composition and abundances will expand our theoretical knowledge of the processes that structure these communities. Such information will also inform interpretations of human impacts on intertidal systems.
Methods
Study sites
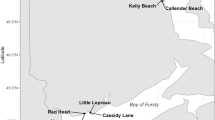
Our study focused on three intertidal mudflats surrounding the Skeena River estuary in northern British Columbia, Canada (Fig. 1; ~ 3 m tidal amplitude): Cassiar Cannery (CC), Wolfe Cove (WC), and Tyee Banks (TB). Cassiar Cannery (N54° 10′ 40.4, W130° 10′ 40.4) is a mudflat adjacent to a former salmon cannery that closed in 1983 and is now an ecotourism lodge. Wolfe Cove (N54° 14′ 33.0, W130° 17′ 34.5) is a mudflat located approximately 1 km from a decommissioned papermill. The papermill was closed in 2001, ceasing all operations and discharge (Yunker et al. 2011; Sizmur et al. 2019). Tyee Banks (N54° 11′ 59.1, W129° 57′ 36.7) is a large intertidal mudflat 20 km upstream of the mouth of the Skeena River, that previously had a small-scale sawmill operating and accumulations of sawdust and woodchips are still present in the upper intertidal sediment. All three sites have a diverse and abundant infaunal community (Campbell et al. 2020), with the infaunal community dominated by Cumacea (primarily Nippoleucon hinumensis with Cumella vulgaris observed less frequently), Polychaetes (Families Phyllodocidae [Eteone californica], Capitellidae [Capitella species complex], and Spionidae [Pygospio elegans]), Oligochaetes (Paranais litoralis), Nematodes, Copepods (order Harpacticoida), Amphipods (Americorophium salmonis), and the bivalve Macoma balthica (Gerwing et al. 2017a; Campbell et al. 2020) (Supplemental Table S1). Average volume-weighted sediment particle size varied from 60 to 180 µm, organic matter content varied from 2.5 to 4.5%, and sediment water content from 28 to 37% (Campbell et al. 2020). No evidence of PTE concentrations above naturally occurring levels was observed in the top 20 cm of sediment (Sizmur et al. 2019). Observed elemental concentrations and all abiotic variables are detailed in Supplemental Table S2. More details on these sites are available in Sizmur et al. (2019) and Campbell et al. (2020).
Sampling scheme
At each mudflat, transects were established running from the landward start of the mudflat to the low water line (five transects per site, separated by ~ 25 m and 60–200 m long). Transects were stratified into zones based on distance from shore (near, middle, and far). Within each zone, one sampling location was randomly selected from which all data types, detailed below, were collected (n = 3 per transect, 15 per site; 45 overall). Samples were collected July 13–25, 2017, on one of the lowest low tides of the year. More details of the sampling scheme can be found in Campbell et al. (2020).
Infauna, sediment, and environmental parameter sampling
At each sampling location, a 1 m2 plot was established and infauna were collected with a corer 10 cm in length, and 7 cm in diameter. (Campbell et al. 2020). A pit (20 cm long, by 20 cm wide, by 20 cm deep) was also dug in the plot where the core was taken to sample and identify larger or more mobile specimens in situ (Campbell et al. 2019b) that may have been missed by the infaunal core. Sediment from the core was passed through a 250 µm sieve, and the infauna retained by the sieve were stored in vials of 95% ethanol (Campbell et al. 2020). Specimens were identified to the lowest possible taxonomic unit, as follows: cumaceans, amphipods, tanaids, polychaetes, nemerteans and bivalves were identified to species; chironomids (larvae) to family; copepods to order; ostracods to class; and nematodes to phylum (Campbell et al. 2020; Gerwing et al. 2020b). Observed abundances from the pit and cores were combined, making note of the processed sediment from each method (Gerwing et al. 2022a), and converted to density per m2.
At each sampling location, sediment penetrability was assessed by dropping a metal weight (15 cm long, 1.9 cm diameter, 330 g) from a height of 0.75 m above the sediment and measuring how far it penetrated into the sediment (Meadows et al. 1998; Gerwing et al. 2020a). Penetrability is an integrative variable that reflects the overall in situ conditions experienced by biota. Increased penetrability indicates finer grained sediment with high water content, and fewer rocks or shell hash present in or on the sediment. Sediment characterised by low penetrability is indicative of larger-grained sediment with low water content and with more rocks or shell hash present (Gerwing et al. 2020a). A sediment core (4.5 cm diameter, 5 cm depth) was collected from each sampling location. From this core, the top 1 cm was processed to determine sediment water content (mass lost by drying at 110 °C for 12 h), organic matter content (mass lost by ashing at 550 °C for 4 h) and volume-weighted average particle size (Malvern Mastersizer). More details of these processes can be found in Campbell et al. (2020). While in the field, the void created in the sediment from the collection of the infaunal core was used to determine the depth to the apparent redox potential discontinuity, aRPD (Gerwing et al. 2013). aRPD depth is a relative measure of sediment porewater dissolved oxygen and redox conditions. Sediment with a deeper aRPD has more available dissolved oxygen, and the sediment is more oxidized, or less reduced, than sediment with a shallower aRPD depth (Gerwing et al. 2015a, 2018b). We refer to these variables as physical variables.
The proportion of each 1 m2 plots covered in woody debris, as well as deposited algae and eelgrass (Zostera spp.) debris was also quantified visually, as this debris can create hypoxic conditions and smother infauna. Such debris are commonly observed in the area. No eelgrass detritus was observed at these sites during this period; therefore, eelgrass cover was not used in any analyses. These variables are referred to as biophysical variables. Salinity was measured at each transect in each tidal flat on each sampling trip with a YSI multimeter in the water approximately 1 cm above the sediment surface at high tide (n = 5). Finally, relative distance of each plot from the start of the mudflat (transition of saltmarsh, bedrock, or sandy beaches to mud (see Gerwing et al. (2016)) was included for each plot, as a proxy for intertidal elevation or duration of inundation. These variables are referred to as environmental variables.
Sediment geochemistry sampling
At each sample location, sediment samples of the top 5 cm were collected from each site using polycarbonate cores (5 cm diameter, 20 cm length). Samples were dried at 40 °C, digested in reverse aqua regia following US EPA Method 3051A, and the concentration of elements (Al, As, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, P, Pb, S, Sr, Ti, and Zn; a mixture of elements that are broadly representative of the lithophile, siderophile, and chalcophile geochemical groups (Goldschmidt 1937) determined by ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy) or ICP-MS (Inductively Coupled Plasma Mass Spectrometry). The total concentration of Hg in sediments was determined using thermal degradation–gold amalgamation atomic absorbance spectroscopy as outlined in EPA Method 7473 using a Nippon MA-3000 analyser (Sizmur et al. 2019). These elemental variables are then referred to as geochemical variables.
Data analysis
Prior to analysis, we assessed possible correlations between all pairs of covariates by calculating univariate Pearson’s correlation coefficients. We used a threshold of 0.95 (Clarke and Ainsworth 1993) for variables too correlated to be considered independent. Since the highest correlation coefficient observed was 0.76, all variables were included in our models.
Data visualization
Statistical analyses (sequential analysis procedures are detailed in Supplemental Figure S1) were conducted in PRIMER with the PERMANOVA add-on (Anderson et al. 2008; Clarke and Gorley 2015). First, non-metric multidimensional scaling (nMDS) plots were used to visualize variation in infaunal communities (Supplemental Table S1) and sediment geochemistry as well as environmental parameters (Supplemental Table S2) between sites (100 restarts; infaunal community resemblance matrix calculated using Brays-Curtis similarities on fourth root transformed density data, and geochemical or environmental resemblance matrix calculated using Euclidean distances after square root transforming Sr to correct for a skewed distribution). Vector overlays were used to identify which infauna and elements had a Pearson’s univariate correlation coefficient of 0.2 or greater (Clarke 1993).
Assessing model performance with inclusion of sediment geochemistry
To determine if models of the infaunal community (all species) that included sediment geochemistry performed better than models that included other traditionally measured physical, biophysical, and environmental parameters (distance from shore, salinity, algae, eelgrass, and woody debris cover, sediment water content, organic matter content, mean particle size, aRPD depth, and penetrability) a distance based linear model (DISTLM) was created using the BEST routine (9999 permutations) was used. Infaunal data were fourth root transformed, while Sr was square root transformed, and aRPD depth, water content, and mean particle size were fourth root transformed to correct for skewed distributions. All physical, biophysical, and environmental variables were normalized; however, variation exists in the precision of our variables. For instance, the precision of visual estimates of detrital cover or organic matter content analyzed via combustion is lower than that obtained from the spectrometry used to elucidate elemental concentrations. We evaluated candidate models using the Information Theoretic Model Selection Approach (Burnham and Anderson 2001, 2002). This approach utilizes multiple lines of evidence to select the “top” performing or “best” model from amongst a series of candidate models designed a priori. All possible permutations of physical, biophysical, and environmental variables were assessed. Candidate top-ranked models were selected by calculating Akaike Information Criterion, corrected for small sample sizes (AICc) values. Models within 2 AICc units of each other were considered to be equivalent (Burnham and Anderson 2001, 2002). Model performance was further assessed using the R2 value, which denotes the proportion of explained variation for the model (Anderson et al. 2008). R2 values were contrasted between models only containing traditional physical, biophysical, and environmental parameters, those only containing elements, and those containing all variables.
Exploring relationships between elements and the infaunal community
While DISTLMs are effective at comparing models, they are not able to determine the relative importance of variables included in a model (Supplemental Figure S1). As such, a permutational multivariate analysis of covariance (PERMANCOVA; 9999 permutations) was also used to quantify the relationship between the infaunal community and physical, biophysical, environmental, and geochemical variables (Gerwing et al. 2016). The multivariate response was a resemblance matrix of the densities of the infaunal community, calculated using Bray–Curtis similarity. Taxa densities were fourth root transformed to better equalize the influence of abundant and rare species on the outcome of the analysis. Within the PERMANCOVA, site (three levels; fixed factor), and transect nested within site (transect (site); five per site; random factor) were included. Geochemical, as well as physical, biophysical, and environmental variables were included as covariates. As part of the PERMANCOVA, we quantified components of variation, the proportion of the multivariate variation accounted for by each independent variable (Searle et al. 1992; Anderson et al. 2008; Gerwing et al. 2016). An α of 0.05 was used to determine statistical significance for all analyses (Beninger et al. 2012). Elements identified as accounting for a statistically significant proportion of the variation in the infaunal community were retained for downstream analysis (Pearson’s univariate correlation assessment).
As we were primarily interested in differences between sites, to reduce the number of taxa included in downstream analyses (Supplemental Figure S1), Similarity Percentages Analyses (SIMPER) were then used to determine which infaunal species were responsible for the observed variation between sites (Clarke 1993). Only species that accounted for ≥ 5% of the dissimilarity between sites were retained for the next analytical step, as these taxa are the drivers of the observed variation in the infaunal community between sites. Finally, Pearson’s univariate correlation coefficient was used to assess if the elements and infaunal species identified above had a positive or negative relationship (Gerwing et al. 2016; Campbell et al. 2020).
Results
Infaunal community composition and sediment parameters varied between sites, with all variables tending to cluster by site (Figs. 2, 3; Supplemental Tables S1, 2 and 3). More details on the biotic and abiotic parameters can be found in Sizmur et al. (2019) and Campbell et al. (2020).
Non-metric multidimensional scaling (nMDS) plots exploring the variation in the infaunal community (a) and sediment geochemistry (b) at three intertidal mudflats near the Skeena Estuary in northern British Columbia, Canada. Vector overlays are overlain, and longer lines indicate greater values associated with that species or element along that axis. CC Cassiar Cannery, TB Tyee Banks, WC Wolfe Cove
Non-metric multidimensional scaling (nMDS) plots exploring the variation in environmental parameters at three intertidal mudflats near the Skeena Estuary in northern British Columbia, Canada. Vector overlays are overlain, and longer lines indicate greater values associated with that parameter along that axis. CC Cassiar Cannery, TB Tyee Banks, WC Wolfe Cove
Assessing model performance with inclusion of sediment geochemistry data
Models containing only physical (penetrability, aRPD depth, water/organic matter content, and mean particle size), environmental variables (distance from shore, salinity), or biophysical variables (detrital cover), performed the worst, explaining only 0.01–0.50 (Supplemental Table S4) of the variation in the model (R2). Models that included only sediment geochemistry performed moderately well (R2: 0.38–0.55), while models including all variables (R2: 0.40–0.60) performed the best (Supplemental Table S4).
Exploring relationships between sediment properties and the infaunal community
The PERMANCOVA (Table 1) identified that a mixture of environmental variables (distance from shore, and salinity) and elements (K, Mn, P, SR, and Ti) were significantly correlated to the observed variation in the infaunal community composition. No physical or biophysical variables were statistically significant in this model. Environmental variables accounted for 5% of this variation, while geochemistry accounted for 65%. The strongest relationship was observed with Ti, with this single element accounting for 33% of the variation in the infaunal community composition. Differences in the infaunal community between sites and transects were not statistically significant, but the infaunal community varied between plots (6%).
With regards to differences between sites, taxa that accounted for ≥ 5% of the dissimilarity in the SIMPER analysis (Supplemental Table S3), and were thus retained for downstream analyses, included: Americorophium salmonis, Cumella vulgaris, Nippoleucon hinumensis, Eteone californica, Capitella Species Complex, Fabricia stellaris, Pygospio elegans, Macoma balthica, Harpacticoida, Ostracoda, and Nematoda. Table 2 summarizes the Pearson’s correlation coefficients between the concentrations of individual elements measured in sediments and the infaunal community composition. In general, the infaunal community had a complex relationship with sediment geochemistry, with some taxa having a positive correlation (increasing population density with increasing element concentration) with some elements, and a negative correlation with others (decreasing population density with increasing element concentration). However, Ti and K had a strong negative correlation with most taxa.
Discussion
To create models explaining infaunal community composition, the relationship between the intertidal invertebrate communities of three intertidal mudflats in the Skeena River estuary and sediment/environmental properties was explored.
Assessing model performance with inclusion of sediment geochemistry
Inclusion of sediment geochemistry into models explaining the infaunal community composition produced models that preformed the best. These models were either comprised exclusively of geochemical data, or a combination of geochemistry, physical, biophysical, and environmental variables. Previous studies reporting models to explain the observed spatiotemporal variation in the infaunal community using physical, biophysical, and environmental variables, often leave a portion of the variation (20–97%) unaccounted for (Thrush et al. 2003; Gerwing et al. 2016, 2020a; Campbell et al. 2020; Norris et al. 2022). However, our results indicate that including sediment geochemistry into such models, alongside sediment and environmental variables, will produce better preforming models. Further research exploring these relationships, as well as the interaction between sediment geochemistry and other physical/environmental properties, will offer greater insight into how the elemental composition of sediments influences infaunal community composition and dynamics. Note, R2 values can be sensitive to overfitting, with models with a higher number of terms performing better, in some situations (Anderson et al. 2008). However, this does not seem to be the case in our data, as models with more terms do not consistently perform better than more parsimonious models (Supplemental Table S4). Further, AICc values were also used to evaluate model performance, and AICc values penalize for every term included. As such, AICc values also suggest that our models do not suffer from overfitting.
Exploring relationships between individual variables and the infaunal community
The infaunal community composition was statistically correlated (Table 1) with environmental variables (5%; distance from shore, and salinity) as well as sediment geochemistry (65%). Physical, and biophysical variables were not statistically correlated to observed variation within the infaunal community. Interestingly, when similar analyses, excluding sediment geochemistry, were conducted upon similar communities along Canada’s Pacific and Atlantic coasts (Gerwing et al. 2016; Campbell et al. 2020; Gerwing et al. Submitted), physical, and biophysical parameters were significantly related to the infaunal community and accounted for 9–11% of the observed variation. As discussed above, our findings here may suggest that when sediment geochemistry is included in models, traditionally studied physical and biophysical parameters (particle size, organic matter and water content penetrability, and aRPD depth) may not be as important in explaining the structure of an infaunal community. However, sediment variables exert a known influence upon infaunal communities, (Diaz and Trefry 2006; Valdemarsen et al. 2010; Pilditch et al. 2015a; Gerwing et al. 2018b, 2020a), and it seems likely that sediment biophysical and geochemical properties both influence infaunal community composition and population dynamics. More research is required to better understand the relative importance of each variable, as well as how and why this varies spatially and temporally.
In our analysis, the site term was non-significant and accounted for none of the infaunal community variation, while the transect term was also not significant, and the plot term only accounted for 8% of the variation (Table 1). The plot and transect terms are likely a product of local hydrology and delivery of larvae, intra and interspecific interactions, as well as post-settlement dispersal and mortality (Flach and Beukema 1995; Bringloe et al. 2013; Drolet et al. 2013; Pilditch et al. 2015b; Gerwing et al. 2016; Sizmur et al. 2019; Norris et al. 2022). The lack of statistical differences observed between sites, is in stark contrast to the apparent site level clustering of infaunal communities observed between sites in Fig. 2 (nMDS plot). The results of similar investigations, that did not include elemental data, reported that the site term accounted for 32–35% of the infaunal community variation, and the plot term 33–37% (Gerwing et al. 2016; Campbell et al. 2020; Gerwing et al. Submitted). These authors hypothesized that the observed variation in the infaunal community associated with this site term was likely a product of processes such as larval supply (Weersing and Toonen 2009; Einfeldt and Addison 2013), post-settlement dispersal and mortality (Pilditch et al. 2015b), unmeasured site variables such as hydrology, elemental concentrations (Sizmur et al. 2019), and exposure to waves/tides (Williams et al. 2013; Gerwing et al. 2015b; Rubin et al. 2017), or disturbance and human development history (Gerwing et al. 2017c, 2018a; Campbell et al. 2019a; Cox et al. 2019). However, in our study, when sediment geochemistry was included in models of the infaunal community, the variation attributed to the site and plot terms decreased and in the case of our site term, became statistically insignificant. When taken together, these results suggest that some, or even most, of the previously observed variation between sites and plots in infaunal communities (as seen in Fig. 2) may not be purely spatial in nature, nor the product of unmeasured or unknown variables that vary at these spatial scales. Instead, this infaunal community variation previously accounted for by spatial terms such as site or plot, may be the product of variation in sediment geochemistry.
Of the elements identified as of interest in the PERMANCOVA (Table 1), only K (17%), Mn (2%), P (4%), Sr (6%), and Ti (36%) accounted for a significant portion of the variation in the infaunal community. Of these elements, K, Mn, and P are essential elements for life whereas Sr and Ti are not known to play any biological role. While K and P are elements that are essential for survival, excess concentrations can have deleterious effects (Yool et al. 1986; Tyrrell 1999; Nath et al. 2011; Chiarelli and Roccheri 2014). Mn and Sr are common contaminants in marine ecosystems, with known deleterious effects on many species (Mejía‐Saavedra et al. 2005; Blaise et al. 2008; Pinsino et al. 2012; Whiteway et al. 2014).
Sediment geochemistry can also be used to identify the provenance of sediments (Zhang et al. 2014) and may indicate that the provenance of the sediment in an intertidal mudflat influences the composition of the infaunal community. Whereas K, P, and to a certain extent Mn, may be applied as fertilisers within the Skeena watershed and their enrichment representative of sediments eroded from farmland, Ti (the element responsible for the greatest variation in infaunal community composition) is a lithogenic element that has previously been found to correlate positively with grain size (Bábek et al. 2015). It may, therefore, be the case that Ti concentrations are a sensitive indicator of sediment grain size, which is already known to be a property that shapes the composition of benthic infaunal communities (Coblentz et al. 2015). Interestingly, when Ti and particle size were included in our analyses (Table 1) Ti was significantly correlated with the infaunal community, while particle size was not. Nor was Ti correlated with sediment particle size in our dataset (Pearson univariate correlation; n = 45, p = 0.13). Conversely, Ti can also have acute and chronic deleterious effects upon invertebrates (Das et al. 2013). Finally, all of our measured elements and environmental variables could be correlated with another, unmeasured variable, that is driving the observed infaunal change. For instance, TB was characterized by lower infaunal abundances and higher concentrations of Ti, K and S, lower concentrations of Mn, as well as lower salinity than the other sites. It is possible that an unmeasured variable resulted in this variation, and more research is required to better understand these relationships. Specifically, future studies should not only include more study sites, spanning a broader range of environmental conditions, but also include more samples per site to increase analytical resolution.
At the level of the infaunal invertebrate populations and individual elements, a complex relationship was detected between sediment geochemistry and infaunal species, with a mixture of positive and negative correlations observed (Table 2). Many of these elements are known to have positive and negative impacts upon infaunal species (Smith 1984; Vitousek and Howarth 1991; Tyrrell 1999; Little et al. 2017), but these impacts can vary between species and at different concentrations (Pourret and Bollinger 2018; Sizmur et al. 2019; Eccles et al. 2020). Given the correlational nature of the data presented in Table 2, we will not attempt to postulate causal mechanisms. Especially as in situ context, such as information regarding nuanced ecological interactions between species and covariates, is lacking. For instance, an observed positive correlation could suggest that increasing concentrations of a given element induces immigration into an area, enhances reproductive output, or has positive impacts upon survival and physiological processes. Conversely, the element itself may have negative impacts upon a species, however, it may have greater effects on a competitor, leading to competitive release. As such, instead of postulating causal relationships, we suggest that more research incorporating more study sites and more samples per site is required to untangle the complicated positive and negative relationships observed in our study, between infaunal invertebrate community composition and sediment geochemistry.
Conclusions
Focusing on three intertidal mudflats in the Skeena estuary, we observed complex relationships between sediment geochemistry and the infaunal community composition. Despite these complicated relationships, combining sediment geochemistry data with traditionally studied physical, biophysical and environmental variables (salinity, distance from shore, detrital cover, penetrability, aRPD depth, organic matter/water content, mean particle size) produced models that outperformed models that contained only traditional sediment and environmental parameters. Our results suggest that some of the observed, and previously unaccounted for, spatial variation (between sites and plots) in infaunal community composition may be a product of variation in sediment geochemistry. As such, in situ sediment chemical composition should be integrated into how we study infaunal communities, as well as how we interpret human impacts upon intertidal systems, and assess the success of restoration and conservation projects.
Data availability
Data will be shared upon reasonable request.
References
Amoozadeh E, Malek M, Rashidinejad R, Nabavi S, Karbassi M, Ghayoumi R, Ghorbanzadeh-Zafarani G, Salehi H, Sures B (2014) Marine organisms as heavy metal bioindicators in the Persian Gulf and the Gulf of Oman. Environ Sci Pollut Res 21:2386–2395
Anderson M, Gorley RN, Clarke RK (2008) Permanova+ for Primer: Guide to software and statistical methods. PRIMER-E Ltd Plymouth, United Kingdom
Bábek O, Grygar TM, Faměra M, Hron K, Nováková T, Sedláček J (2015) Geochemical background in polluted river sediments: how to separate the effects of sediment provenance and grain size with statistical rigour? CATENA 135:240–253
Barbeau M, Grecian L, Arnold E, Sheahan D, Hamilton D (2009) Spatial and temporal variation in the population dynamics of the intertidal amphipod Corophium volutator in the upper Bay of Fundy, Canada. J Crustac Biol 29:491–506
Beninger PG, Boldina I, Katsanevakis S (2012) Strengthening statistical usage in marine ecology. J Exp Mar Biol Ecol 426:97–108
Blaise C, Gagné F, Ferard J, Eullaffroy P (2008) Ecotoxicity of selected nano-materials to aquatic organisms. Enviro Toxicol 23:591–598
Borja A, Dauer DM, Diaz RJ, Llansó RJ, Muxika I, Rodriguez JG, Schaffner L (2008) Assessing estuarine benthic quality conditions in Chesapeake Bay: a comparison of three indices. Ecol Ind 8:395–403
Bradshaw C, Kautsky U, Kumblad L (2012) Ecological stoichiometry and multi-element transfer in a coastal ecosystem. Ecosystems 15:591–603
Bringloe TT, Drolet D, Barbeau MA, Forbes MR, Gerwing TG (2013) Spatial variation in population structure and its relation to movement and the potential for dispersal in a model intertidal invertebrate. PLoS ONE 8:e69091
Burnham KP, Anderson DR (2001) Kullback-Leibler information as a basis for strong inference in ecological studies. Wildl Res 28:111–119
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer Verlag, New York
Campbell L, Sizmur T, Juanes F, Gerwing TG (2019a) Passive reclamation of soft-sediment ecosystems on the North Coast of British Columbia. Canada J Sea Res 155:101796
Campbell L, Wood L, Allen Gerwing AM, Allen S, Sizmur T, Rogers M, Gray O, Drewes M, Juanes F, Gerwing TG (2019b) A rapid, non-invasive population assessment technique for marine burrowing macrofauna inhabiting soft sediments. Estuar Coast Shelf Sci 227:106343
Campbell L, Dudas SE, Juanes F, Allen Gerwing AM, Gerwing TG (2020) Invertebrate communities, sediment parameters, and food availability of intertidal soft-sediment ecosystems on the North Coast of British Columbia, Canada. J Nat Hist 54:919–945
Chapman P, Dexter R, Long uE, (1987) Synoptic measures of sediment contamination, toxicity and infaunal community composition (the sediment quality triad) in San Francisco Bay. Mar Ecol Prog Ser 37:75–96
Chiarelli R, Roccheri MC (2014) Marine invertebrates as bioindicators of heavy metal pollution. Open J Metal. https://doi.org/10.4236/ojmetal.2014.44011
Christensen B, Vedel A, Kristensen E (2000) Carbon and nitrogen fluxes in sediment inhabited by suspension-feeding (Nereis diversicolor) and non-suspension-feeding (N. virens) polychaetes. Mar Ecol Prog Ser 192:203–217
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Ainsworth M (1993) A method of linking multivariate community structure to environmental variables. Mar Ecol Prog Ser 92:205–219
Clarke KR, Gorley RN (2015) PRIMER v7: user manual/tutorial Primer-E Ltd, Plymouth, UK
Coblentz KE, Henkel JR, Sigel BJ, Taylor CM (2015) The use of laser diffraction particle size analyzers for inference on infauna-sediment relationships. Estuaries Coasts 38:699–702
Cowie PR, Widdicombe S, Austen MC (2000) Effects of physical disturbance on an estuarine intertidal community: field and mesocosm results compared. Mar Biol 136:485–495
Cox KD, Gerwing TG, Macdonald T, Hessing-Lewis M, Millard-Martin B, Command RJ, Juanes F, Dudas SE (2019) Infaunal community responses to ancient clam gardens. ICES J Mar Sci 76:2362–2373
Das P, Xenopoulos MA, Metcalfe CD (2013) Toxicity of silver and titanium dioxide nanoparticle suspensions to the aquatic invertebrate, Daphnia magna. Bull Environ Contam Toxicol 91:76–82
Dashtgard SE, Pearson NJ, Gingras MK (2014) Sedimentology, ichnology, ecology and anthropogenic modification of muddy tidal flats in a cold-temperate environment: Chignecto Bay, Canada. Geol Soc, London, Spec Publ 388:229–245
De Backer A, Van Coillie F, Montserrat F, Provoost P, Van Colen C, Vincx M, Degraer S (2011) Bioturbation effects of Corophium volutator: importance of density and behavioural activity. Estuar Coast Shelf Sci 91:306–313
Delgado P, Hensel P, Baldwin A (2018) Understanding the impacts of climate change: an analysis of inundation, marsh elevation, and plant communities in a tidal freshwater marsh. Estuaries Coasts 41:25–35
Diaz RJ, Trefry JH (2006) Comparison of sediment profile image data with profiles of oxygen and Eh from sediment cores. J Mar Syst 62:164–172
Drolet D, Coffin MRS, Barbeau MA, Hamilton DJ (2013) Influence of intra-and interspecific interactions on short-term movement of the amphipod Corophium volutator in varying environmental conditions. Estuaries Coasts 36:1–11
Drylie TP, Lohrer AM, Needham HR, Pilditch CA (2020) Taxonomic and functional response of estuarine benthic communities to experimental organic enrichment: consequences for ecosystem function. J Exp Mar Biol Ecol 532:151455
Eccles KM, Pauli B, Chan HM (2020) Geospatial analysis of the patterns of chemical exposures among biota in the Canadian Oil Sands Region. PLoS ONE 15:e0239086
Einfeldt AJ, Addison JA (2013) Hydrology influences population genetic structure and connectivity of the intertidal amphipod Corophium volutator in the northwest Atlantic. Mar Biol 160:1015–1027
Ferraro SP, Cole FA (2012) Ecological periodic tables for benthic macrofaunal usage of estuarine habitats: insights from a case study in Tillamook Bay, Oregon, USA. Estuar Coast Shelf Sci 102:70–83
Flach EC, Beukema JJ (1995) Density-governing mechanisms in populations of the lugworm Arenicola marina on tidal flats. Oceanogr Lit Rev 42:139–149
Floyd T, Williams J (2004) Impact of green crab (Carcinus maenas L.) predation on a population of soft-shell clams (Mya arenaria L.) in the southern Gulf of St. Lawrence J Shellfish Res 23:457–463
Fukuyama AK, Shigenaka G, Coats DA (2014) Status of intertidal infaunal communities following the Exxon Valdez oil spill in Prince William Sound, Alaska. Mar Pollut Bull 84:56–69
Gerwing TG, Allen Gerwing AM, Drolet D, Hamilton DJ, Barbeau MA (2013) Comparison of two methods of measuring the depth of the redox potential discontinuity in intertidal mudflat sediments. Mar Ecol Prog Ser 487:7–13
Gerwing TG, Allen Gerwing AM, Hamilton DJ, Barbeau MA (2015a) Apparent redox potential discontinuity (aRPD) depth as a relative measure of sediment oxygen content and habitat quality. Int J Sedim Res 30:74–80. https://doi.org/10.1016/S1001-6279(15)60008-7
Gerwing TG, Drolet D, Barbeau MA, Hamilton DJ, Allen Gerwing AM (2015b) Resilience of an intertidal infaunal community to winter stressors. J Sea Res 97:40–49. https://doi.org/10.1016/j.seares.2015.01.001
Gerwing TG, Drolet D, Hamilton DJ, Barbeau MA (2016) Relative importance of biotic and abiotic forces on the composition and dynamics of a soft-sediment intertidal community. PLoS ONE 11(11):e0147098
Gerwing TG, Allen Gerwing AM, Macdonald T, Cox K, Juanes F, Dudas SE (2017a) Intertidal soft-sediment community does not respond to disturbance as postulated by the intermediate disturbance hypothesis. J Sea Res 129:22–28
Gerwing TG, Gerwing AMA, Cox K, Juanes F, Dudas SE (2017b) Relationship between apparent redox potential discontinuity (aRPD) depth and environmental variables in soft-sediment habitats. Int J Sedim Res 32:472–480
Gerwing TG, Hamilton DJ, Barbeau MA, Haralampides KA, Yamazaki G (2017c) Short-term response of a downstream marine system to the partial opening of a tidal-river causeway. Estuaries Coasts 40:717–725. https://doi.org/10.1007/s12237-016-0173-2
Gerwing TG, Allen Gerwing AM, Allen S, Campbell L, Rogers M, Gray O, Drewes M, Juanes F (2018a) Potential impacts of logging on intertidal infaunal communities within the Kitimat river estuary. J Nat Hist 52:2833–2855
Gerwing TG, Cox K, Allen Gerwing AM, Carr-Harris CN, Dudas SE, Juanes F (2018b) Depth to the apparent redox potential discontinuity (aRPD) as a parameter of interest in marine benthic habitat quality models. Int J Sedim Res 33:472–480
Gerwing TG, Barbeau MA, Hamilton DJ, Allen Gerwing AM, Sinclair J, Campbell L, Davies MM, Harvey B, Juanes F, Dudas SE (2020a) Assessment of sediment penetrability as an integrated in situ measure of intertidal soft-sediment conditions. Mar Ecol Prog Ser 648:67–78
Gerwing TG, Cox K, Allen Gerwing AM, Campbell L, Macdonald T, Dudas SE, Juanes F (2020b) Varying intertidal invertebrate taxonomic resolution does not influence ecological findings. Estuar Coast Shelf Sci 232:106516
Gerwing TG, Campbell L, Hamilton DJ, Barbeau MA, Dudas SE, Juanes F (Submitted) Structuring factors of an invertebrate community: assessing the relative importance of top down, bottom up, middle out, and abiotic variables Marine Biology
Gerwing TG, Allen Gerwing AM, Davies MM, Juanes F, Dudas SE (2022a) Required sampling intensity for community analyses of intertidal infauna to detect a mechanical disturbance. Environ Monit Assess 194:1–6
Gerwing TG, Dudas SE, Juanes F (2022b) Increasing misalignment of spatial resolution between investigative and disturbance scales alters observed responses of an infaunal community to varying disturbance severities. Estuar Coast Shelf Sci 265:107718
Ghasemi AF, Clements JC, Taheri M, Rostami A (2014) Changes in the quantitative distribution of Caspian Sea polychaetes: prolific fauna formed by non-indigenous species. J Great Lakes Res 40:692–698
Goldschmidt VM (1937) The principles of distribution of chemical elements in minerals and rocks. The seventh Hugo Müller Lecture, delivered before the Chemical Society on March 17th, 1937. Journal of the Chemical Society (Resumed): 655–673
Hamilton DJ, Diamond AW, Wells PG (2006) Shorebirds, snails, and the amphipod Corophium volutator in the upper Bay of Fundy: top-down vs. bottom-up factors, and the influence of compensatory interactions on mudflat ecology. Hydrobiologia 567:285–306
Kalman J, Smith B, Bury N, Rainbow P (2014) Biodynamic modelling of the bioaccumulation of trace metals (Ag, As and Zn) by an infaunal estuarine invertebrate, the clam Scrobicularia plana. Aquat Toxicol 154:121–130
Karimi R, Folt CL (2006) Beyond macronutrients: element variability and multielement stoichiometry in freshwater invertebrates. Ecol Lett 9:1273–1283
Little S, Wood P, Elliott M (2017) Quantifying salinity-induced changes on estuarine benthic fauna: the potential implications of climate change. Estuar Coast Shelf Sci 198:610–625
Lu L, Grant J (2008) Recolonization of intertidal infauna in relation to organic deposition at an oyster farm in Atlantic Canada—a field experiment. Estuaries Coasts 31:767–775
Martinez-Colon M, Hallock P, Green-Ruiz C (2009) Strategies for using shallow-water benthic foraminifers as bioindicators of potentially toxic elements: a review. J Foramin Res 39:278–299
Mazzola A, Mirto S, La Rosa T, Fabiano M, Danovaro R (2000) Fish-farming effects on benthic community structure in coastal sediments: analysis of meiofaunal recovery. ICES J Marine Sci 57:1454–1461
Meadows PS, Murray JM, Meadows A, Wood DM, West FJ (1998) Microscale biogeotechnical differences in intertidal sedimentary ecosystems. Geol Soc, London, Spec Publ 139:349–366
Mejía-Saavedra J, Sánchez-Armass S, Santos-Medrano GE, Gonzáaaalez-Amaro R, Razo-Soto I, Rico-Martínez R, Díaz-Barriga F (2005) Effect of coexposure to DDT and manganese on freshwater invertebrates: pore water from contaminated rivers and laboratory studies. Environ Toxicol Chem 24:2037–2044
Menge B, Daley B, Wheeler P, Dahlhoff E, Sanford E, Strub P (1997) Benthic–pelagic links and rocky intertidal communities: bottom-up effects on top-down control? Proc Natl Acad Sci USA 94:14530
Mermillod-Blondin F, Rosenberg R (2006) Ecosystem engineering: the impact of bioturbation on biogeochemical processes in marine and freshwater benthic habitats. Aquat Sci 68:434–442
Musetta-Lambert JL, Scrosati RA, Keppel EA, Barbeau MA, Skinner MA, Courtenay SC (2015) Intertidal communities differ between breakwaters and natural rocky areas on ice-scoured Northwest Atlantic coasts. Mar Ecol Prog Ser 539:19–31
Nath NS, Bhattacharya I, Tuck AG, Schlipalius DI, Ebert PR (2011) Mechanisms of phosphine toxicity. J Toxicol 2011:494168
Norris GS, Gerwing TG, Barbeau MA, Hamilton DJ (2022) Using successional drivers to understand spatiotemporal dynamics in intertidal mudflat communities. Ecosphere 13(10):e4268. https://doi.org/10.1002/ecs2.4268
Ólafsson EB, Peterson CH, Ambrose WG Jr (1994) Does recruitment limitation structure populations and communities of macro-invertebrates in marine soft sediments: the relative significance of pre-and post-settlement processes. Oceanogr Mar Biol Annu Rev 32:65–109
Pearson TH, Rosenberg R (1978) Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr Mar Biol Annu Rev 16:229–331
Pilditch CA, Leduc D, Nodder SD, Probert PK, Bowden DA (2015a) Spatial patterns and environmental drivers of benthic infaunal community structure and ecosystem function on the New Zealand continental margin. NZ J Mar Freshwat Res. https://doi.org/10.1080/00288330.2014.995678
Pilditch CA, Valanko S, Norkko J, Norkko A (2015b) Post-settlement dispersal: the neglected link in maintenance of soft-sediment biodiversity. Biol Let 11:20140795
Pinsino A, Matranga V, Roccheri MC (2012) Manganese: a new emerging contaminant in the environment. In: Environmental contamination, pp 17–36
Pourret O, Bollinger J-C (2018) ‘Heavy metals’-what to do now: to use or not to use? Sci Total Environ 610–611:419–420
Quintana CO, Kristensen E, Valdemarsen T (2013) Impact of the invasive polychaete Marenzelleria viridis on the biogeochemistry of sandy marine sediments. Biogeochemistry 115:95–109
Rubin SP, Miller IM, Foley MM, Berry HD, Duda JJ, Hudson B, Elder NE, Beirne MM, Warrick JA, McHenry ML (2017) Increased sediment load during a large-scale dam removal changes nearshore subtidal communities. PLoS ONE 12:e0187742
Sánchez-Moyano JE, García-Gómez JC (1998) The arthropod community, especially Crustacea, as a bioindicator in Algeciras Bay (Southern Spain) based on a spatial distribution. J Coastal Res 14:1119–1133
Searle SR, Casella G, McCulloch CE (1992) Variance Components. John Wiley & Sons, New York
Sherman KM, Coull BC (1980) The response of meiofauna to sediment disturbance. J Exp Mar Biol Ecol 46:59–71
Sizmur T, Canário J, Edmonds S, Godfrey A, O’Driscoll NJ (2013) The polychaete worm Nereis diversicolor increases mercury lability and methylation in intertidal mudflats. Environ Toxicol Chem 32:1888–1895
Sizmur T, Campbell L, Dracott K, Jones M, O’Driscoll NJ, Gerwing TG (2019) Relationships between potentially toxic elements in intertidal sediments and their bioaccumulation by benthic invertebrates. PLoS ONE 14:e0216767
Smith SV (1984) Phosphorus versus nitrogen limitation in the marine environment. Limnol Oceanogr 29:1149–1160
Spencer K, Harvey G (2012) Understanding system disturbance and ecosystem services in restored saltmarshes: integrating physical and biogeochemical processes. Estuar Coast Shelf Sci 106:23–32
Teal L, Parker E, Solan M (2013) Coupling bioturbation activity to metal (Fe and Mn) profiles in situ. Biogeosciences 10:2365–2378
Thrush SF, Hewitt JE, Norkko A, Nicholls PE, Funnell GA, Ellis JI (2003) Habitat change in estuaries: predicting broad-scale responses of intertidal macrofauna to sediment mud content. Mar Ecol Prog Ser 263:101–112
Tyrrell T (1999) The relative influences of nitrogen and phosphorus on oceanic primary production. Nature 400:525–531
Underwood AJ, Fairweather PG (1989) Supply-side ecology and benthic marine assemblages. Trends Ecol Evol 4:16–20
Valdemarsen T, Kristensen E, Holmer M (2010) Sulfur, carbon, and nitrogen cycling in faunated marine sediments impacted by repeated organic enrichment. Mar Ecol Prog Ser 400:37–53
Virgin SDS, Beck AD, Boone LK, Dykstra AK, Ollerhead J, Barbeau MA, McLellan NR (2020) A managed realignment in the upper Bay of Fundy: community dynamics during salt marsh restoration over 8 years in a megatidal, ice-influenced environment. Ecol Eng 149:105713
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115
Waldock R, Rees H, Matthiessen P, Pendle M (1999) Surveys of the benthic infauna of the Crouch Estuary (UK) in relation to TBT contamination. J Mar Biol Assoc UK 79:225–232
Weersing K, Toonen RJ (2009) Population genetics, larval dispersal, and connectivity in marine systems. Mar Ecol Prog Ser 393:1–12
Whiteway SA, Paine MD, Wells TA, DeBlois EM, Kilgour BW, Tracy E, Crowley RD, Williams UP, Janes GG (2014) Toxicity assessment in marine sediment for the Terra Nova environmental effects monitoring program (1997–2010). Deep Sea Res Part II 110:26–37
Williams GJ, Smith JE, Conklin EJ, Gove JM, Sala E, Sandin SA (2013) Benthic communities at two remote Pacific coral reefs: effects of reef habitat, depth, and wave energy gradients on spatial patterns. PeerJ 1:e81
Yool AJ, Grau SM, Hadfield MG, Jensen RA, Markell DA, Morse DE (1986) Excess potassium induces larval metamorphosis in four marine invertebrate species. Biol Bull 170:255–266
Yunker MB, Lachmuth CL, Cretney WJ, Fowler BR, Dangerfield N, White L, Ross PS (2011) Biota–sediment partitioning of aluminium smelter related PAHs and pulp mill related diterpenes by intertidal clams at Kitimat, British Columbia. Mar Environ Res 72:105–126
Zhang Y, Pe-Piper G, Piper DJ (2014) Sediment geochemistry as a provenance indicator: unravelling the cryptic signatures of polycyclic sources, climate change, tectonism and volcanism. Sedimentology 61:383–410
Acknowledgements
The authors would like to thank Shaun Allen, Deborah Parker, Jayleen Starr and Zoey Starr for their assistance during data collection. Anne Dudley is acknowledged for assisting ICP-OES analysis. Andy Dodson is acknowledged for assisting ICP-MS analysis.
Funding
Funding for this work was provided by the Royal Geographical Society (TGG and TS), Kitsumkalum First Nation (TGG), an NSERC Engage grant (TGG and FJ), and a Mitacs Elevate grant to TGG. Cassiar Cannery provided accommodations onsite and access to study locations.
Author information
Authors and Affiliations
Contributions
TGG and TM designed the study, collected data, and wrote the paper. KD and LC helped collect data and write the paper. AMAG, MMD, JK, HMT helped analyze the data and write the paper. JK and SC helped analyze samples and write the paper. FJ and SED helped design the study, provided supervision, and write the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
No animal welfare approval was required as we were working with common invertebrates.
Additional information
Responsible Editor: M. Hüttel.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gerwing, T.G., Gerwing, A.M.A., Davies, M.M. et al. Sediment geochemistry influences infaunal invertebrate community composition and population abundances. Mar Biol 170, 4 (2023). https://doi.org/10.1007/s00227-022-04151-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04151-7