Abstract
A field experiment was carried out to investigate the patterns of macrobenthic recolonization and to determine the effects of biodeposition on benthic communities at an intertidal oyster culture site in New Brunswick, Canada. Total organic deposition in azoic organic-free sediment trays was generally higher within the farm compared to reference sites. Two weeks after deployment of trays, total organic content had reached 1.1%. The abundance, species number, and diversity of the macrobenthic community were positively correlated with the total organic content in the experimental trays, but the correlations between community parameters and organic content were negative in the ambient sediment. The results suggest that organic matter in sediment may have positive effects on macrobenthic infauna at low levels as an additional food source but may be harmful to benthic animals at high levels. This study also indicates that location in the intertidal zone is a major parameter affecting the community structure of macrobenthic colonization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquaculture (finfish farming and shellfish culture) in coastal waters of many countries has increased dramatically in recent years, and concerns about impacts of culture on the seabed environment and benthic ecosystem have grown worldwide (Gowen and Bradbury 1987; Folke and Kautsky 1989, 1992; Beveridge et al. 1994; Wu 1995; Kaiser et al. 1998). Marine fish farming deposits a high organic and nutrient loading onto sediments, thereby changing benthic composition (Findlay et al. 1995; Tsutsumi 1995) or leading to anoxic conditions and depriving macrofauna in the vicinity (Gowen and Bradbury 1987; Tsutsumi et al. 1991; Beveridge et al. 1994; Wu et al. 1994). The responses of macrofaunal species to stress from organic loading have been well discussed (Grassle and Grassle 1974; Pearson and Rosenberg 1978; Ritz et al. 1989).
Shellfish culture is based on natural sources for food with no additional organic matter to the ecosystem. Suspension-feeding bivalves (e.g., oysters and mussels) filter phytoplankton and other seston and pack them into larger particles (feces and pseudofeces), which may cause local increase of biodeposition to the seabed (Kautsky and Evans 1987). This additional organic material may have an impact similar to the waste accumulated under marine finfish farms (Folke and Kautsky 1992). Some studies indicated that shellfish farming had substantially negative impacts on marine benthic environment and macrobenthic communities (Kaspar et al. 1985; Stenton-Dozey et al. 1999; Stenton-Dozey et al. 2001; Chamberlain et al. 2001; Christensen et al. 2003; Smith and Shackley 2004). Kaspar et al. (1985) found in New Zealand that the benthic infauna consisted of polychaetes, bivalves, crustaceans, and brittle stars at the reference site but contained only polychaetes in the mussel-farm sediment, in which the ammonium concentration was about twice as high as in the reference sediment. The study of Stenton-Dozey et al. (1999) in South Africa indicated that raft-culture of mussels had an impact on macrobenthic community structure. Smith and Shackley (2004) showed a significant change in the species composition of the benthic community, with a decrease in species number and total number of individuals within a year of commencement of a commercial mussel lay in UK. However, other studies, including three studies carried out in Eastern Canada, showed little or no harmful effects of shellfish culture on the benthic environment and macrobenthos (Grant et al. 1995; Crawford et al. 2003; Miron et al. 2005; Mallet et al. 2006; Da Costa and Nalesso 2006).
Benthic recolonization is an important aspect of community structure in aquaculture-impacted habitats but has not been emphasized in associated studies. Field experiments with sediment trays have been conducted for measuring sediment transport and deposition of organic matter (Grant 1985; Grant et al. 1997) and for investigating the patterns of macrobenthic recolonization (Turner et al. 1997; Lu and Wu 2000; 2007). Nevertheless, few studies have been conducted to link organic deposition on sediment to the response of colonizing macrobenthic community in tray experiments. The purposes of the present study are to investigate the pattern of macrobenthic recolonization at an intertidal oyster culture site and to test the hypotheses that oyster culture increases biodeposition on the bottom, and higher biodeposition from oyster culture has an impact on recolonization of macrobenthic infauna.
Materials and Methods
Study Site
A field experiment was carried out at an intertidal site with oyster culture (Crassostrea virginica) in St. Simon Bay, situated in the northern New Brunswick, Canada (47°43′ N, 64°47′ W) (Fig. 1). Oysters were grown in floating bags attached to long lines, which spread from the lower mid-tidal zone to the upper subtidal zone. The sediment type was fine-medium sand (79.8%; sand, 97.5%), and the seabed was covered densely by the sea grass Zostera marina in the low-tidal and subtidal zones. Seven stations were set up from the mid- to low-tidal zone (Fig. 2). Stations Z2 and Z4 were located in the mid-tidal zone, 5 and 15m away from the edge of the floating bags, respectively. Stations Z1, Z3, Z5, Z6, and Z7 were at the same tidal level in the low-tidal zone, of which Stations Z1 and Z3 were inside the oyster farm and Stations Z5, Z6, and Z7 were 5, 10, and 30m away from the edge of the floating bags, respectively.
Experimental Design
Sediment used for the experiments was taken from the intertidal zone of the study site. The sediment was washed with freshwater to remove detritus. The washed sediment was dried at 70°C and then burned in a muffle furnace at 500°C for 6h to remove organic matter. The organic-free sediment was put in rectangular iron trays (L, 9.20cm; W, 6.03cm; D, 3.49cm; surface area, 55.48cm2), which were fixed in sediment each with a 15-cm bolt so that the surface of tray was at the same level of the ambient sediment. Four trays were deployed at each station in June 2006 and retrieved after 14days (one tray was lost at Station Z2). Three replicate samples from the ambient sediment were also collected at each station at the same time, using a PVC corer (diameter, 11.5cm; surface area, 103.87cm2). One subsample of top 1-cm sediment was collected with a plastic syringe from each replicate sample for measurement of organic matter.
Sample Treatment
Sediment samples were washed through a 0.5-mm sieve, and the residues retained on the sieve were preserved in a 10% formalin–seawater solution in the field. In the laboratory, benthic animals were sorted out from the residues under a binocular dissecting microscope, identified to the lowest possible taxonomic levels, and counted.
Organic content in sediment was determined by incinerating a dried sediment sample at 500°C for 6h (Buchanan, 1984).
Data Processing
Species diversity of each sample was calculated using the Shannon–Wiener index (H′) (Shannon and Weaver 1963):
where S = the number of species in the sample, N = the total number of individuals and Ni = the number of individuals in the ith species (i = 1 to S). For comparison between benthic samples with different sizes (i.e., tray vs. ambient), species richness was calculated using Margalef’s index (d) (Margalef 1963):
where S = species number and N = total number of individuals. Similarities between benthic samples were calculated using the Bray–Curtis coefficient (Bray and Curtis 1957), and data were fourth-root transformed before computing the coefficient. Hierarchical agglomerative clustering and non-metric multi-dimensional scaling (MDS) were used to assess differences in community structure between samples. Data were normalized to same sampling area when MDS was used to compare differences between the tray and ambient samples. A group-average linked method was used in clustering. Analysis of similarities (ANOSIM) was carried out to test differences in community composition among samples groups (Clarke and Warwick 2001). A one-way analysis of variance (ANOVA) was employed to test differences in organic content and community parameters (abundance, species number, and species diversity) among stations, and Tukey’s post-hoc test was carried out to test differences between each pair of stations. The D’Agostino and Pearson omnibus normality test was used to test data normality for community parameters before ANOVA. Logarithm transformation was employed when the data were not conformable to normality. Organic content data were arcsin square-root-transformed prior to ANOVA to conform with data normality (Zar 1999). A two-tailed t test was used to test differences in the community parameters between the tray and ambient sediments.
Results
Organic Content
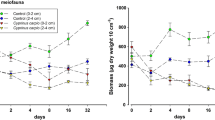
No significant difference in organic content of ambient sediment was found between the beginning and the end of the experiment at all stations. Organic deposition onto the tray sediment varied at different stations, with the highest mean value of organic content (1.10%) recorded at Z3 and the lowest mean value (0.34%) found at Z6. The mean organic content at Z1 was only 0.63%, probably due to its location close to the edge of farm. In the ambient sediment, the mean organic content ranged between 4.55% at Z3 and 1.52% at Z4 (Fig. 3). The differences in organic content among stations were highly significant (one-way ANOVA, p < 0.0001) for both tray and ambient sediments. The organic content was significantly higher at Z3 than at other stations (p < 0.01, Tukey’s post-hoc test), and no significant differences were found between the other six stations both in tray and ambient sediments.
Macrobenthic Community in the Trays
The mean values in abundance and species number of macrobenthic community were both highest (154 animals and 14.3 species/tray) at Z2 and lowest (13.3 animals and six species/tray) at Z5, while the mean value of species diversity (H′) was highest (1.95) at Z1 and the lowest (1.30) at Z6 (Fig. 4). The high abundance at Z2 was mainly attributed to high numbers of the oligochaete Tubificidae sp. and the bivalve Gemma gemma. The highest number of species at Z2 may be due to the fact that the station was located at an ecotone region between the mid-tidal and low-tidal zones. The differences in these parameters among stations were significant (one-way ANOVA, p < 0.05)
A total of 35 species, including 13 species of polychaetes, 11 species of crustaceans, ten species of molluscs, and one species of oligochaete, were recorded in the tray samples. The gastropod Bittium alternatum was the most dominant species at the low-tidal stations, except at Z3 where it was replaced by the polychaete Heteromastus filiformis, while the oligochaete Tubificidae sp. and the bivalve G. gemma dominated at the mid-tidal stations (Table 1). B. alternatum was mainly found at the low-tidal stations, while Tubificidae sp. and G. gemma largely colonized at the mid-tidal stations (Fig. 5).
Hierarchical clustering of benthic samples demonstrates that four groups, A (Z4), B (Z2), C (Z3), and D (Z1, 5–7), can be clearly divided, based on the Bray–Curtis similarities (Fig. 6). The MDS plot also shows the differences between the groups (Fig. 7). ANOSIM analyses for the four groups show significant difference in community structure among the groups (global R = 0.781, p = 0.001), and no significant differences were found between Stations Z1, Z5, Z6, and Z7 within group D. The mid-tidal communities were significantly different from the low-tidal ones, implying the tidal effects on recolonization of intertidal infauna. It is worth noting that the benthic community at Z3 was separated from those at other low-tidal stations.
Comparisons Between the Tray and Ambient Communities
The number of species usually increases with sample size. Due to different surface areas between the tray and ambient samples, species richness (Margalef’s index d), rather than species number, was used for comparison. Generally speaking, the abundance, species richness, and diversity were lower in the trays than in the ambient at all stations except Z3, where the parameters were significantly higher (p < 0.05, t test) in the trays (Fig. 8). The differences in community structure between the tray and ambient sediments were also obvious at all stations (Fig. 9).
Relationship Between Benthic Community and Organic Matter
As tidal levels have significant impacts on macrobenthic infauna, only the data from the five low-tidal stations (Z1, Z3, and Z5–Z7) were used for the analyses of relationship between faunal parameters and organic matter.
The abundance, species number, and diversity increased significantly with the increase of total organic content in the tray sediment (Fig. 10), but the parameters were decreased with organic content in the ambient sediment (Fig. 11). These results suggest that organic matter in sediment may have positive effects on macrobenthic infauna at low levels (<1.5%), probably as an additional food source, but could be harmful to benthic animals at higher levels due to organic enrichment.
Figure 12 display the MDS plots of benthic samples at the five low-tidal stations with superimposed bubbles of different size, representing relative values of organic content. Points with higher organic content group at the lower right corner in the plots, suggesting that organic matter in sediment also had important impacts on community structure of macrobenthos both in the experimental trays and in the ambient sediment. Higher stress level (>0.2) for the MDS plot (Fig. 12a) suggests that the points may not be well placed in this two-dimensional ordination.
Discussion
The highest values in species number and abundance of the tray community were found at Station Z2, which is located at an ecotone region between the mid- and low-tidal zones. An ecotone is generally considered as a transition region where two diverse communities meet. It normally contains members of both communities, and both the species number and the abundances of some species are higher in the ecotone than on either side (Pearson and Rosenberg 1978). In this case, the mid-tidal zone is different from the low-tidal zone in the environmental factors such as submerging time, temperature and salinity during low tide, and wave and current conditions. As a result, the community composition was significantly different between the two zones, as shown by multivariate analyses. The colonizing community at Z2 contained species from both tidal zones with higher abundances of some species (e.g., the oligochaete Tubificidae sp. and the bivalve G. gemma) than either zone.
Kautsky and Evans (1987) indicated that suspension-feeding bivalves (e.g., oysters and mussels) remove large amounts of small suspended particles that would otherwise stay in suspension and increase local deposition of organic matter as settling feces near the suspension cultures. Deposition of organic matter onto the seabed was different at different locations, and the highest organic content was found inside the oyster farm in the present study, suggesting the contribution of biodeposition from the oyster culture. In addition to vertical deposition, the organic matter in tray sediment may also partially come from horizontal transport of ambient sediment (Grant 1985; Grant et al. 1997).
Organic matter deposited as feces may represent a significant proportion of the energy potentially available to benthic invertebrates as a food resource (Stuart et al. 1982). In this study, the abundance, species number, and diversity of macrobenthos were positively correlated with organic content in the tray sediment, suggesting the important role of biodeposition at low levels as a food source during the colonization of benthic infauna. Indeed, the deposit feeder H. filiformis dominated the tray sediment with the highest biodeposition. The organic content in sediment favored the recruits of some species, thus changed the community structure of macrofauna.
Nevertheless, high biodeposition from shellfish farming may result in accumulation of organic matter in sediment, which may cause oxygen depletion, increased sulfate reduction, and low macrofauna diversity (Dahlback and Gunnarsson 1981; Kaspar et al. 1985; Christensen et al. 2003; Stenton-Dozey et al. 1999; Stenton-Dozey et al. 2001). Smith and Shackley (2004) found a significant change in the species composition of macrobenthic community with a decrease in abundance and species number 1 year after the mussel culture at a commercial mussel lay. In our study, the abundance, species number, and diversity of macrobenthic community showed negative correlations with organic content in the ambient sediment, implying the adverse impacts of organic enrichment.
Mallet et al. (2006) found no indication of organic enrichment in sediment and no negative effects of oyster culture on macrofauna in the same bay where our study was carried out. This is probably due to the little differences in sedimentary organic content between the reference and the culture sites in their study.
References
Beveridge, M.C.M., L.G. Ross, and L.A. Kelly. 1994. Aquaculture and biodiversity. Ambio 23: 497–502.
Bray, J.R., and J.T. Curtis. 1957. An ordination of upland forest community of Southern Wisconsin. Ecological Monograph 27: 325–349.
Buchanan, J.B. 1984. Sediment analysis. In Methods for the study of marine benthos 2nd edn., eds. A. N. Holme and A. D. McIntyre , 41–65. Oxford: Blackwell.
Chamberlain, J., T.F. Fernandes, P. Read, T.D. Nickell, and I.M. Davies. 2001. Impacts of biodeposits from suspended mussel (Mytilus edulis L.) culture on the surrounding surficial sediment. ICES Journal of Marine Science 58: 411–416.
Christensen, P.B., R.N. Glud, T. Dalsgaard, and P. Gillespie. 2003. Impacts of longline mussel farming on oxygen and nitrogen dynamics and biological communities of coastal sediments. Aquaculture 218: 567–588.
Clarke, K.B., and R.M. Warwick. 2001. Changes in Marine Communities: an Approach to Statistical Analysis and Interpretation. 2Plymouth, United Kingdom: PRIMER-E.
Crawford, C.M., C.K.A. Macleod, and I.M. Mitchell. 2003. Effects of shellfish farming on the benthic environment. Aquaculture 224: 117–140.
Da Costa, K.G., and R.C. Nalesso. 2006. Effects of mussel farming on macrobenthic community structure in Southeastern Brazil. Aquaculture 258: 655–663.
Dahlback, B., and L.H. Gunnarsson. 1981. Sedimentation and sulfate reduction under a mussel culture. Marine Biology 63: 269–275.
Findlay, R.H., L. Watling, and L.M. Mayer. 1995. Environmental impact of salmon net-pen culture on marine benthic communities in Maine: a case study. Estuaries 18: 145–179.
Folke, C., and N. Kautsky. 1989. The role of ecosystem for a sustainable development of aquaculture. Ambio 18: 234–243.
Folke, C., and N. Kautsky. 1992. Aquaculture with environment: prospects for sustainability. Ocean Coastal Management 17: 5–24.
Gowen, R.J., and N.B. Bradbury. 1987. The ecological impact of salmonid farming in coastal waters: a review. Oceanography and Marine Biology: An Annual Review 25: 563–575.
Grant, J. 1985. A method for measuring horizontal transport of organic carbon over sediments. Canadian Journal of Fisheries and Aquatic Sciences 42: 595–602.
Grant, J., S.J. Turner, P. Legendre, T.M. Hume, and R.G. Bell. 1997. Patterns of sediment reworking and transport over small scales on an intertidal sandflat, Munukau Harbour, New Zealand. Journal of Experimental Marine Biology and Ecology 216: 33–50.
Grant, J., A. Hatcher, D.B. Scott, P. Pocklington, C.T. Schafer, and G.V. Winters. 1995. A multidisciplinary approach to evaluating impacts of shellfish aquaculture on benthic communities. Estuaries 18: 124–144.
Grassle, J.F., and J.P. Grassle. 1974. Opportunistic life histories and genetic systems in marine benthic polychaetes. Journal of Marine Research 32: 253–84.
Kaiser, M.J., I. Laing, S.D. Utting, and G.M. Burnell. 1998. Environmental impacts of bivalve mariculture. Journal of Shellfish Research 17: 59–66.
Kaspar, H.F., P.A. Gillepie, I.C. Boyer, and A.L. MacKenzie. 1985. Effects of mussel aquaculture on the nitrogen cycle and benthic communities in Kenepuru Sound, Marlborough Sounds, New Zealand. Marine Biology 85: 127–136.
Kautsky, N., and S. Evans. 1987. Role of biodeposition by Mytilus edulis in the circulation of matter and nutrients in a Baltic coastal ecosystem. Marine Ecology Progress Series 38: 201–212.
Lu, L., and R.S.S. Wu. 2000. An experimental study on recolonization and succession of marine macrobenthos in defaunated sediment. Marine Biology 136: 291–302.
Lu, L., and R.S.S. Wu. 2007. Seasonal effects on recolonization of macrobenthos in defaunated sediment: a series of field experiments. Journal of Experimental Marine Biology and Ecology 351: 199–210.
Margalef, D.R. 1963. On certain unifying principles in ecology. American Naturalist 97: 357–374.
Mallet, A.L., C.E. Carver, and T. Landry. 2006. Impact of suspended and off-bottom Eastern oyster culture on the benthic environment in eastern Canada. Aquaculture 255: 362–373.
Miron, G., T. Landry, P. Archambault, and B. Fronette. 2005. Effects of mussel culture husbandry practices on various benthic characteristics. Aquaculture 250: 138–154.
Pearson, T.H., and R. Rosenberg. 1978. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanography and Marine Biology: an Annual Review 16: 229–311.
Ritz, D.A., M.E. Levis, and M.A. Shen. 1989. Response to organic enrichment of infaunal macrobenthic communities under salmonid seacages. Marine Biology 103: 211–214.
Shannon, C.E., and W. Weaver. 1963. The Mathematical Theory of Communication. Urbana, IL: University of Illinois Press.
Smith, J., and S.E. Shackley. 2004. Effects of a commercial mussel Mytilus edulis lay on a sublittoral, soft sediment benthic community. Marine Ecology Progress Series 282: 185–191.
Stenton-Dozey, J.M.E., L.F. Jackson, and A.J. Busby. 1999. Impact of mussel culture on macrobenthic community structure in Saldanha Bay, South Africa. Marine Pollution Bulletin 39: 357–366.
Stenton-Dozey, J., T. Probyn, and A. Busby. 2001. Impact of mussel (Mytilus galloprovincialis) raft-culture on benthic macrofauna, in situ oxygen uptake, and nutrient fluxes in Saldanha Bay, South Africa. Canadian Journal of Fisheries and Aquatic Sciences 58: 1021–1031.
Stuart, V., R.C. Newell, and M.I. Lucas. 1982. Conversion of kelp debris and faecal material from the mussel Aulacomya ater by marine microorganisms. Marine Ecology Progress Series 7: 47–57.
Tsutsumi, H. 1995. Impact of fish pen culture on the benthic environment of a cove in South Japan. Estuaries 18: 108–115.
Tsutsumi, H., T. Kikuchi, M. Tanaka, T. Higashi, K. Imasaka, and M. Miyazaki. 1991. Benthic faunal succession in a cove organically polluted by fish farming. Marine Pollution Bulletin 23: 233–238.
Turner, S.J., J. Grant, R.D. Pridmore, J.E. Hewitt, M.R. Wilkinson, T.M. Hume, and D.J. Morrisey. 1997. Bedload and water-column transport and colonization precesses by mobile post-settlement macrofauna: Does infaunal density matter? Journal of Experimental Marine Biology and Ecology 216: 51–75.
Wu, R.S.S., K.S. Lam, D.W. MacKay, T.C. Lau, and V. Yam. 1994. Impact of marine fish farming on water quality and bottom sediment: a case study of the sub-tropical environment. Marine Environmental Research 38: 115–145.
Wu, R.S.S. 1995. The environmental impact of marine fish culture: towards a sustainable future. Marine Pollution Bulletin 31: 159–166.
Zar, J.H. 1999. Biostatistical Analysis. 4Upper Saddle River, New Jersey: Prentice Hall, 123pp.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, L., Grant, J. Recolonization of Intertidal Infauna in Relation to Organic Deposition at an Oyster Farm in Atlantic Canada—A Field Experiment. Estuaries and Coasts 31, 767–775 (2008). https://doi.org/10.1007/s12237-008-9059-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-008-9059-2